Abstract
Epileptogenic zones (EZs), where epileptic seizures cease after resection, are localized by assessing the seizure-onset zone using ictal electroencephalography (EEG). Owing to the difficulty in capturing unpredictable seizures, biomarkers capable of identifying EZs from interictal EEG are anticipated. Recent studies using intracranial EEG have identified several potential candidate biomarkers for epileptogenicity. High-frequency oscillation (HFO) was initially expected to be a robust biomarker of abnormal excitatory activity in the ictogenic region. However, HFO-guided resection failed to improve seizure prognosis. Meanwhile, the regularity of low-gamma oscillations (30-80 Hz) indicates inhibitory interneurons' hypersynchronization, which could be used to localize the EZ. Besides resting-state EEG assessments, evoked potentials elicited by single-pulse electrical stimulation, such as corticocortical evoked potentials (CCEP), became valuable tools for assessing epileptogenic regions. CCEP responses recorded in the cortex remote from the stimulation site indicate functional connectivity, revealing increased internal connectivity within the ictogenic region and elevated inhibitory input from the non-involved regions to the ictogenic region. Conversely, large responses close to the stimulation site reflect local excitability, manifesting as an increased N1 amplitude and overriding HFO. Further research is required to establish whether these novel electrophysiological methods, either individually or in combination, can function as robust biomarkers of epileptogenicity and hold promise for improving seizure prognosis.
Keywords: epilepsy, epileptogenic zone, gamma oscillation, CCEP, Excitatory-Inhibitory balance
Introduction
Surgical intervention for epilepsy primarily aims for effective seizure control. The epileptogenic zone (EZ) encompasses a cerebral area where surgical resection results in seizure elimination and is reversely defined by prognostic outcomes after epilepsy surgery.1) Although a combination of neuroradiological investigations, including magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computerized tomography (SPECT), and other diagnostic modalities, such as magnetoencephalography (MEG), are commonly employed to approximate the EZ, the gold standard for EZ localization is the identification of the seizure-onset zone using electroencephalography (EEG) recordings during epileptic episodes2) (Fig. 1). Capturing multiple seizures is preferable for optimal precision in estimating EZ. However, the lengthy duration of video-EEG examinations presents challenges for patients and medical workers, and the absence of captured seizures during the recording periods can complicate diagnosis. Consequently, developing biomarkers capable of estimating the EZ using interictal EEG is in demand, regardless of the presence of seizures.
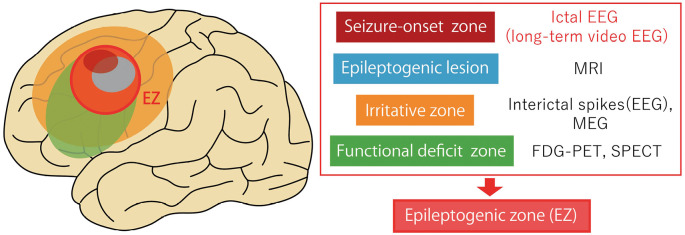
Fig. 1.
Localizing seizure-onset zone from ictal electroencephalography (EEG) is the gold standard for detecting the epileptogenic zone (EZ), combined with assessing the epileptogenic lesions (e.g., MRI), irritative zones (e.g., interictal spikes, MEG), and functional deficit zones (e.g., FDG-PET, SPECT).
MEG, magnetoencephalography; MRI, magnetic resonance imaging; FDG-PET, fluorodeoxyglucose-positron emission tomography; SPECT, single-photon emission computerized tomography
Intracranial EEG (iEEG), which employs subdural electrodes or stereoelectroencephalography (SEEG), can be utilized to assess brain activity with a high signal-to-noise ratio and fine spatiotemporal resolution.3) Moreover, iEEG enables the evaluation of the cortical activity evoked by electrical stimulation of the cerebral cortex or subcortical structures. In the current review, we focus on excitatory and inhibitory activities of the brain to identify epileptogenicity from the findings obtained by resting-state interictal EEG and evoked activity triggered by cortical electrical stimulation.
Potential and Limitation of High-Frequency Oscillation (HFO) as a Biomarker for Epileptogenicity
HFO has become a potential biomarker for assessing EZ using iEEG data during interictal periods; however, its effectiveness in improving the outcomes of resection surgery remains unclear. Advances in digital EEG techniques have facilitated rhythmic activity detection at frequencies higher than those within the gamma band, which have been reported to have a link to epileptogenic processes.4,5) Within the spectrum of HFOs, frequencies with ranges 80-200 Hz and 250-500 Hz are classified as “ripples” and "fast ripples,” respectively. The underlying microscopic mechanism of ripples depends on the synchronized excitation of neuronal networks comprising pyramidal cells and surrounding interneurons. Fast ripples are characterized by pathological action potential bursts originating from a population of pyramidal cells.6) These pathological HFOs may indicate the seizure-onset zone during ictal onset and reflect cortical hyperexcitability during interictal periods.
Contrary to the high expectations of a clear and robust biomarker for the EZ that could be derived from interictal iEEG, multiple studies have demonstrated that HFO-guided resection does not necessarily result in improved seizure outcomes.7-9) HFO-generating regions exhibit lower specificity for EZ8) and are no more effective predictors than interictal spikes.9) One possible explanation for this discrepancy is the challenge of distinguishing physiological HFOs linked to cognitive brain processes from pathological HFOs associated with epileptic activity. Consequently, HFOs are not deemed definitive biomarkers, as initially anticipated.
Gamma Oscillations as a Potential Biomarker for Cortical Inhibition
Considering the relationship between interictal brain activity and epileptogenicity, addressing why patients with epilepsy do not continuously experience seizures would be helpful. Although cortical excitatory and inhibitory imbalances have been proposed as explanations for seizure generation,10) this model suggests that sufficient inhibition of excitatory activity around epileptic tissues is maintained during the interictal period.11) Thus, the inhibitory nature of cortical activity can be a biomarker for assessing the EZ.
Cortical excitatory and inhibitory mechanisms operate at various levels, ranging from neurotransmitter to network levels. From a microscopic perspective, the excitatory activity of glutamate-mediated pyramidal neurons is regulated by surrounding interneuronal networks through GABAergic transmission.12) Parvalbumin-positive basket cells are central to this inhibitory mechanism; they form a local network with numerous inhibitory synapses on pyramidal cells.13) These basket cells, called fast-spiking interneurons, exert GABAergic activity to form gamma-band spikes (30-80 Hz).14) These individual potentials can be summated through extracellular recordings or local field potentials manifesting as gamma-band oscillations.
Although broadband high-frequency gamma activity, as observed in HFOs, signifies the excitatory activity of pyramidal cells, low-frequency gamma oscillations can be biomarkers for GABAergic inhibition.15)
Moreover, the spectral power of low-gamma activity exhibits potential specificity in identifying seizure-onset regions; however, it is insufficiently sensitive to accurately detect the EZ, even with intracranial EEG evaluation.16)
An alternative approach to evaluate cortical inhibition via gamma activity is quantifying gamma oscillation regularity (GOR), not spectral power, using entropy.17) Entropy gauges the complexity of time-series data, with low entropy indicating reduced information and enhanced regularity. The regularity of waveforms within a specific frequency band can be quantified using the sample entropy, calculated for individual bands and combined with cross-frequency analysis (multi-scale entropy).18) A reduction in multi-scale entropy within the gamma band signifies an augmentation in the regularity of gamma oscillations, potentially indicating the synchronization of inhibitory interneurons through GABAergic activity.19) Sato et al. reported increased GOR within the seizure-onset region during the interictal period. This increase in the GOR was evident in the seizure-onset region and the surrounding area during seizures.17) Moreover, Sato et al. demonstrated that an increased GOR during the interictal period is a valuable biomarker for the seizure-onset area in patients with focal cortical dysplasia.20) Furthermore, the epileptogenic region estimated from the GOR aligned with the results of traditional estimations of the EZ derived from ictal recordings, MRI, and nuclear medicine imaging.21) Accordingly, assessing GOR using multi-scale entropy during interictal periods could predict cortical inhibitory activity and be a biomarker for future EZ assessment.
Evaluation of Functional Connectivity by Corticocortical Evoked Potential (CCEP)
Besides resting-state interictal EEG signals, electrophysiological responses evoked by electrical stimulation of the brain have also been employed to indicate cortical connectivity and excitability; CCEP represents this approach. CCEP involves recording evoked potentials in nearby or distant brain regions through cortical-to-cortical propagation, achieved by applying single-pulse electrical stimulation (SPES) using chronically implanted subdural or stereoelectroencephalography (SEEG) electrodes. The recorded responses are summed to emphasize time-locked components, whose amplitudes or root mean squares represent the strength of the directed connectivity from the stimulation site to the target cortex (Fig. 2A).
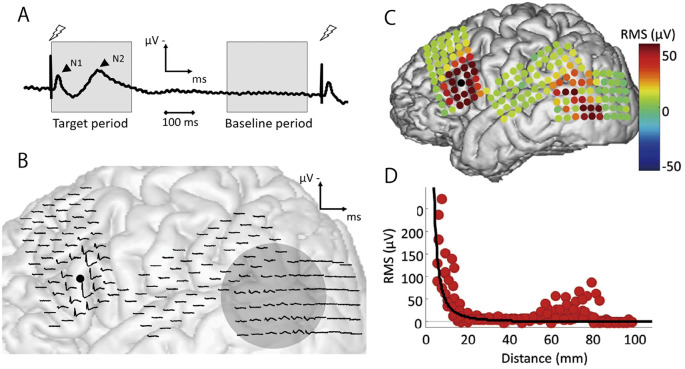
Fig. 2.
(A) An overview of corticocortical evoked potential (CCEP). A CCEP waveform comprises two distinct components: the early N1 and the late N2. (B) (C) The CCEP waveforms obtained through single-pulse electrical stimulation and their signal intensity measured as root mean square. A black dot depicts the point of stimulation. A remote response is observed in the posterior part of the lateral temporal cortex, indicating increased functional connectivity with the stimulated area. Electrodes surrounding the stimulating site elicit a large early peak, signifying cortical excitability. (D) CCEP signal intensity in the vicinity of the stimulation site decays according to the inverse square of the distance, indicating the contribution of the volume-conducted potential. Reprinted from Ref 26, with permission from the journal.
CCEP has been employed to evaluate functional connectivity between distinct cortical areas, including language and motor cortex networks.22,23) Moreover, CCEP has provided insights into abnormal networks within the epileptic brain, offering a perspective distinct from structural connectivity analyses under diffusion tensor imaging. Some CCEP studies have demonstrated increased internal functional connectivity within epileptogenic regions and employed CCEP-based internal connectivity as a biomarker to localize the EZ.24)
Recent developments of SEEG have focused on network-level connectivity rather than traditional methods focusing on specific cortical areas to evaluate epileptogenicity. Directed functional connectivity can be assessed using a short resting-state interictal SEEG by calculating the coherence of a specific frequency band from two remote regions. This approach revealed increased inward connectivity from the non-involved regions to the ictogenic region, in contrast to decreased outward connectivity in the opposite direction.25) This observation agrees with the increased internal connectivity reported in CCEP studies. Furthermore, CCEP can add valuable insights to resting-state connectivity analysis using the power spectral density approach, which can estimate the band-specific elevation or attenuation of neural activity evoked by SPES. A recent study has demonstrated that stimulating non-involved regions to induce inward input toward the seizure-onset region can reduce the theta-band response and enhance gamma activity.11) The theta-band signal intensity reflects the long-range integration of brain activity, whereas the gamma-band activity indicates local integration.26) Furthermore, gamma oscillations were found to be associated with inhibitory interneuronal activity. These findings suggest that enhanced inhibitory input from non-epileptogenic regions to the seizure-onset region could contribute to forming a local network architecture.
Evaluation of Cortical Excitability Using CCEP
In the preceding section, we addressed CCEP as the response detected distant from the location of electrical stimulation. These remote responses arise from the direct depolarization of the dendrites of pyramidal cells and interneurons, propagating to the remote cortex via oligo- or polysynaptic mechanisms.23) Conversely, high-voltage evoked responses can be observed near the stimulation site (Fig. 2B and C). Although conventional analyses often exclude responses recorded within 1.5-2 cm of the stimulus site as electrical stimulation artifacts, their electrophysiological relevance has been reported. Although the precise mechanism of CCEP in the vicinity of the stimulus site remains unclear, the observed N1 apex latency, longer than expected based on the inter-electrode distance, suggests that local jitter of oligosynaptic activity is evoked at the stimulus site.22) Consequently, a sizable signal originating immediately beneath the stimulus site generates a high-voltage volume-conducted potential (VCP) expanding to the surrounding area. The root mean square of the VCP is inversely proportional to the square of the distance from the stimulus site regardless of the anatomical structure26) (Fig. 2D). While conventional electrocorticographic examination assumes that the recorded potential reflects the activity of the neural populations immediately beneath the electrode, responses near the stimulation site may indirectly capture the VCP, which originates from a signal source immediately beneath the stimulation site and then attenuates based on distance. This model allows the estimation of the magnitude of the signal source based on responses captured by electrodes near the stimulation site.
Some CCEP studies have explored these local and large-scale responses. The N1 amplitude measured near the stimulation site was prominent when stimulating the ictal-onset zone.27,28) This response increases during the seizure aura,29) indicating local hyperexcitability at the stimulation site. The relationship between the SPES and HFO has also been explored. SPES, followed by ripples and fast ripples, can afford higher specificity than interictal spikes in localizing the seizure-onset zone.30) Furthermore, increased HFO overriding the N1 waveform of the CCEP as a nearby cortical response has been reported and proposed as a surrogate marker for abnormal cortical hyperexcitability.31) The enhanced local cortical excitability derived from SPES could be a novel modality for evaluating epileptogenicity.
Conclusion
The current review presents various methodologies for assessing epileptogenicity using interictal intracranial EEG, including HFOs, GOR, functional connectivity, and local excitability analysis using CCEP (Table 1). These electrophysiological approaches could unravel the mechanisms underlying seizure generation and become valuable epileptogenic biomarkers. These methods may offer a concise and accurate evaluation of the EZs without needing to capture epileptic seizures using video-EEG recordings.
Table 1.
Novel approaches to approximate the epileptogenic zone from interictal intracranial EEG
| Recording style | Potential biomarkers | The characteristics of the method |
|---|---|---|
| Resting-state EEG | High-frequency oscillation (HFO): 80-500 Hz | - HFO reflects cortical hyperexcitability. - HFO indicates the excitatory activity of pyramidal cells. |
| Resting-state EEG | Gamma oscillation regularity (GOR): 30-80 Hz | - Increased GOR (i.e., lower entropy) reflects cortical inhibition. - GOR indicates synchronization of inhibitory interneurons through GABAergic activity. |
| Evoked responses by SPES | CCEP connectivity (Remote region) | - Increased internal functional connectivity can be observed within the epileptogenic region. - Increased inhibitory input from the non-epileptogenic to ictogenic regions can also be observed. |
| Evoked responses by SPES | CCEP excitability (Neighboring region) | - Local cortical excitability is demonstrated as enhanced N1 amplitudes and increased HFOs overriding N1 waveforms close to the stimulation site. |
CCEP, corticocortical evoked potential; EEG, electroencephalography; HFO, High-frequency oscillation; GABA, gamma-aminobutyric acid; SPES, single-pulse electrical stimulation
However, as demonstrated in the context of HFOs, outcomes observed at the theoretical and laboratory levels may not persistently align with actual surgical results. Prospective studies to assess actual surgical outcomes using novel biomarkers for the EZ must determine the clinical applicability of these methods.
The integration of multiple modalities may afford additional benefits. Attempts to predict EZs accurately by combining multiple epileptogenicity assessment modalities have also been reported. Further research is required to develop robust epileptogenic biomarkers, either alone or in combination.
Conflicts of Interest Disclosure
The authors declare no conflicts of interest (COI). They completed a self-reported registration of the COI according to the criteria of the Japan Neurosurgical Society.
References
- 1). Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W: The epileptogenic zone: general principles. Epileptic Disord 8(Suppl 2): S1-S9, 2006 [PubMed] [Google Scholar]
- 2). Shih JJ, Fountain NB, Herman ST, et al. : Indications and methodology for video-electroencephalographic studies in the epilepsy monitoring unit. Epilepsia 59: 27-36, 2018 [DOI] [PubMed] [Google Scholar]
- 3). Lesser RP, Crone NE, Webber WRS: Subdural electrodes. Clin Neurophysiol 121: 1376-1392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Bragin A, Engel J Jr., Wilson CL, Fried I, Mathern GW: Hippocampal and entorhinal cortex high-frequency oscillations (100-500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 40: 127-137, 1999 [DOI] [PubMed] [Google Scholar]
- 5). Urrestarazu E, Chander R, Dubeau F, Gotman J: Interictal high-frequency oscillations (100-500 Hz) in the intracerebral EEG of epileptic patients. Brain 130: 2354-2366, 2007 [DOI] [PubMed] [Google Scholar]
- 6). Bragin A, Engel J Jr., Wilson CL, Fried I, Buzsáki G: High-frequency oscillations in human brain. Hippocampus 9: 137-142, 1999 [DOI] [PubMed] [Google Scholar]
- 7). Gloss D, Nevitt SJ, Staba R: The role of high-frequency oscillations in epilepsy surgery planning. Cochrane Database Syst Rev 10: CD010235, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Jacobs J, Wu JY, Perucca P, et al. : Removing high-frequency oscillations: a prospective multicenter study on seizure outcome. Neurology 91: e1040-e1052, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Zweiphenning W, Klooster MAV, van Klink NEC, et al. : Intraoperative electrocorticography using high-frequency oscillations or spikes to tailor epilepsy surgery in the Netherlands (the HFO trial): a randomised, single-blind, adaptive non-inferiority trial. Lancet Neurol 21: 982-993, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Engel J, Jr.: Excitation and inhibition in epilepsy. Can J Neurol Sci 23: 167-174, 1996 [DOI] [PubMed] [Google Scholar]
- 11). Johnson GW, Doss DJ, Morgan VL, et al. : The interictal suppression hypothesis in focal epilepsy: network-level supporting evidence. Brain 146: 2828-2845, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Bradford HF: Glutamate, GABA and epilepsy. Prog Neurobiol 47: 477-511, 1995 [DOI] [PubMed] [Google Scholar]
- 13). Buzsáki G, Leung LW, Vanderwolf CH: Cellular bases of hippocampal EEG in the behaving rat. Brain Res 287: 139-171, 1983 [DOI] [PubMed] [Google Scholar]
- 14). Cardin JA, Carlén M, Meletis K, et al. : Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663-667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Sohal VS, Zhang F, Yizhar O, Deisseroth K: Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698-702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Ren L, Kucewicz MT, Cimbalnik J, et al. : Gamma oscillations precede interictal epileptiform spikes in the seizure onset zone. Neurology 84: 602-608, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Sato Y, Wong SM, Iimura Y, Ochi A, Doesburg SM, Otsubo H: Spatiotemporal changes in regularity of gamma oscillations contribute to focal ictogenesis. Sci Rep 7: 9362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Richman JS, Moorman JR: Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278: H2039-H2049, 2000 [DOI] [PubMed] [Google Scholar]
- 19). Medvedev AV, Murro AM, Meador KJ: Abnormal interictal gamma activity may manifest a seizure onset zone in temporal lobe epilepsy. Int J Neural Syst 21: 103-114, 2011 [DOI] [PubMed] [Google Scholar]
- 20). Sato Y, Ochi A, Mizutani T, Otsubo H: Low entropy of interictal gamma oscillations is a biomarker of the seizure onset zone in focal cortical dysplasia type II. Epilepsy Behav 96: 155-159, 2019 [DOI] [PubMed] [Google Scholar]
- 21). Sato Y, Tsuji Y, Yamazaki M, et al. : Interictal high gamma oscillation regularity as a marker for presurgical epileptogenic zone localization. Oper Neurosurg (Hagerstown) 23: 164-173, 2022 [DOI] [PubMed] [Google Scholar]
- 22). Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Lüders HO: Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain 130: 181-197, 2007 [DOI] [PubMed] [Google Scholar]
- 23). Matsumoto R, Nair DR, LaPresto E, et al. : Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain 127: 2316-2330, 2004 [DOI] [PubMed] [Google Scholar]
- 24). Zhang N, Zhang B, Rajah GB, et al. : The effectiveness of cortico-cortical evoked potential in detecting seizure onset zones. Neurol Res 40: 480-490, 2018 [DOI] [PubMed] [Google Scholar]
- 25). Narasimhan S, Kundassery KB, Gupta K, et al. : Seizure-onset regions demonstrate high inward directed connectivity during resting-state: an SEEG study in focal epilepsy. Epilepsia 61: 2534-2544, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Shimada S, Kunii N, Kawai K, et al. : Impact of volume-conducted potential in interpretation of cortico-cortical evoked potential: detailed analysis of high-resolution electrocorticography using two mathematical approaches. Clin Neurophysiol 128: 549-557, 2017 [DOI] [PubMed] [Google Scholar]
- 27). Enatsu R, Piao Z, O'Connor T, et al. : Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: a cortico-cortical evoked potential study. Clin Neurophysiol 123: 252-260, 2012 [DOI] [PubMed] [Google Scholar]
- 28). Iwasaki M, Enatsu R, Matsumoto R, et al. : Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord 12: 292-302, 2010 [DOI] [PubMed] [Google Scholar]
- 29). Matsumoto R, Kinoshita M, Taki J, et al. : In vivo epileptogenicity of focal cortical dysplasia: a direct cortical paired stimulation study. Epilepsia 46: 1744-1749, 2005 [DOI] [PubMed] [Google Scholar]
- 30). van 'T Klooster MA, Zijlmans M, Leijten FS, Ferrier CH, van Putten MJ, Huiskamp GJ: Time-frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain 134: 2855-2866, 2011 [DOI] [PubMed] [Google Scholar]
- 31). Kobayashi K, Matsumoto R, Matsuhashi M, et al. : High frequency activity overriding cortico-cortical evoked potentials reflects altered excitability in the human epileptic focus. Clin Neurophysiol 128: 1673-1681, 2017 [DOI] [PubMed] [Google Scholar]