Key Points
-
•
Blast-reduction strategies in MPN-AP/BP most commonly result in reversion to chronic phase MPN with significant residual disease burden.
-
•
Mutational burden remains high even after intensive AML-type therapy and does not correlate with clinicopathological response.
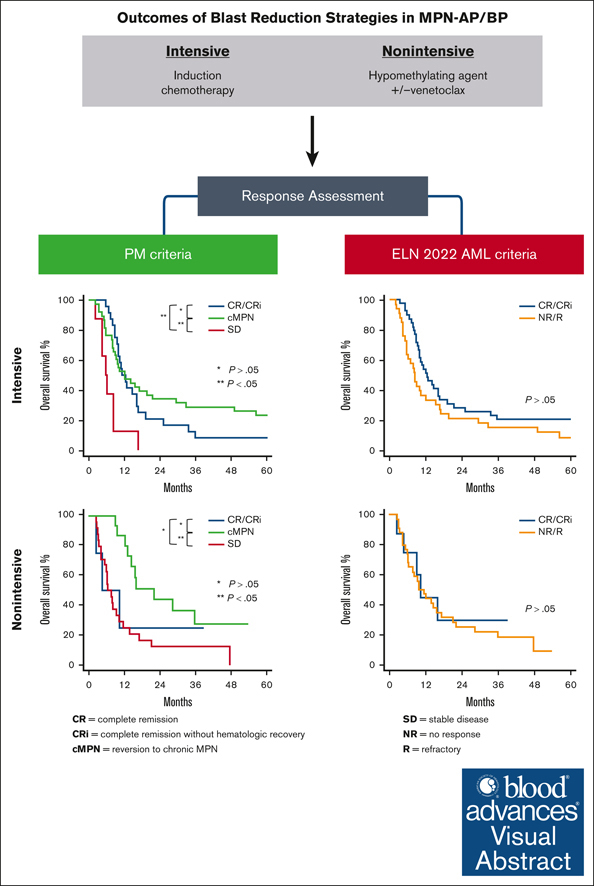
Visual Abstract
Abstract
Transformation of BCR::ABL1-negative myeloproliferative neoplasms (MPN) to an accelerated or blast phase is associated with poor outcomes. The efficacy of acute myeloid leukemia (AML)–type intensive and nonintensive hypomethylating agent–based regimens is not well studied. We therefore performed a retrospective analysis of patients with MPN-AP/BP (N = 138) treated with intensive (N = 81) and nonintensive (N = 57) blast-reduction strategies. We used clinically relatable response criteria developed at the Princess Margaret Cancer Centre. The overall best response, comprising complete remission (CR), complete remission with incomplete hematologic recovery (CRi), and reversion to chronic phase MPN (cMPN), in the intensive and nonintensive groups was 77% (62 of 81) and 39% (21 of 54), respectively. Similar overall best response rates were observed in patients receiving induction with daunorubicin combined with cytarabine arabinoside (daunorubicin + ara-C) (74% [23 of 31]) or FLAG-IDA/NOVE-HiDAC (78% [39 of 50], P = .78). However, patients receiving daunorubicin + ara-C more often required second inductions (29% [9 of 31] vs 4% [2 of 50], P = .002). Most responses in the entire cohort were reversions to cMPN (55 of 83 [66%]). CR and CRi comprised 30% (25 of 83) and 4% (3 of 83) of responses, respectively. Mutations in TP53 (overall response [OR] 8.2 [95% confidence interval [CI] 2.01, 37.1], P = .004) and RAS pathway (OR 5.1 [95%CI 1.2, 23.7], P = .03) were associated with inferior treatment response for intensively treated patients, and poorer performance status (Eastern Cooperative Oncology Group) was associated with inferior treatment response in both intensively (OR 10.4 [95% CI 2.0, 78.5], P = .009) and nonintensively treated groups (OR 12 [95% CI 2.04, 230.3], P = .02). In patients with paired samples before and after therapy (N = 26), there was a significant residual mutation burden remaining irrespective of response to blast-reduction therapy.
Introduction
BCR::ABL1-negative myeloproliferative neoplasms (MPN) are a group of clonal hematopoietic disorders characterized by driver mutations, which constitutively activate the JAK-STAT pathway. Common to these disorders is the variable risk of progression to an accelerated (AP; blasts 10%-19%) or blast phase (BP; blasts ≥20%), characterized by an increase in peripheral blood (PB) or bone marrow (BM) blasts.1,2 The risk of transformation is highest in myelofibrosis (MF), followed by polycythemia vera (PV) and essential thrombocythemia (ET).3, 4, 5, 6 Transformation is associated with decreased survival to less than 2 years for AP and 3 to 5 months for BP.7,8 Allogeneic hematopoietic cell transplantation (HCT) is the only therapy associated with improvement in long-term survival.7,9,10 As in de novo acute myeloid leukemia (AML), HCT is usually only pursued after achieving disease control. AML-type treatment strategies such as induction chemotherapy are commonly used for blast reduction, but in older, more frail patients, less-intensive approaches such as hypomethylating agents (HMA) or more recently, HMA combinations like azacitidine and venetoclax (AZAVEN) have been reported.11, 12, 13 However, the optimal blast-reduction strategy and depth of disease clearance required before HCT are unknown.
Several factors complicate the pre-HCT treatment course of MPN-AP/BP. Lower response rates are observed with AML therapy in MPN-AP/BP than in de novo AML, likely because of more aggressive disease biology as characterized by adverse karyotype and high-risk mutations like TP53.10 Moreover, a lack of standardized response criteria to evaluate the treatment of MPN-AP/BP poses challenges for understanding treatment efficacy between reported studies. Application of standardized AML response criteria, such as those proposed in the revised guidelines by the European Leukemia Net (ELN) for AML, may not be directly applicable to patients with MPN for several reasons. First, patients with MPNs often have inaspirable BMs, precluding the enumeration of marrow blasts by morphology and flow cytometry, necessitating CD34 or CD117 immunohistochemistry of the trephine biopsy for quantification. Furthermore, treatment of MPN-AP/BP may result in the reversion of the disease to chronic-phase MPNs (cMPN) both histologically and clinically. Marrow fibrosis and features of the underlying MPN commonly persist after AML-type treatment. Patients may have ongoing extramedullary hematopoiesis with persistence of circulating blasts, which essentially precludes assigning complete remission (CR) by strict ELN criteria. Further, patients who were transfusion-dependent or had other cytopenias before progression to MPN-AP/BP may not see improvement in these parameters after blast reduction. It is also unclear if patients who achieve BM morphological remission but have persistent splenomegaly because of extramedullary hematopoiesis would be considered to have extramedullary disease by ELN criteria and therefore deemed to be nonresponders. Many centers base HCT eligibility on ELN response criteria, which likely excludes many patients who may potentially benefit from a consolidative HCT. Although dedicated consensus response criteria for MPN-AP/BP were developed in 2012,14 they have not been readily adopted in clinical practice or in reported prospective clinical trials.15, 16, 17
To this end, we aim to retrospectively evaluate the efficacy of various AML-type blast-reduction strategies in patients with MPN-AP/BP treated at the Princess Margaret Cancer Centre. Specifically, we compared standard induction therapy with daunorubicin + ara-C vs higher-dose ara-C–based combinations, namely fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) or mitoxantrone, etoposide, and high-dose cytarabine (NOVE-HIDAC), and lower-intensity strategies, including single-agent HMA and HMA-venetoclax combinations. We evaluated and compared responses using criteria developed at Princess Margaret Cancer Centre (PM-criteria) as well as ELN 2022 AML response criteria.18 In an exploratory analysis, in patients with available paired samples, we also evaluated the mutational burden before and after blast-reduction treatment.
Methods
Study design and population
This was a single-center retrospective cohort study. All patients with BCR::ABL1-negative MPNs in AP/BP assessed at Princess Margaret Cancer Centre between January 1998 and April 2022 were identified from the program database. To be included in the study, patients required (1) a confirmed diagnosis of BCR::ABL1-negative MPN, including ET, PV, primary MF, post-ET MF, post-PV MF, or MPN-unclassifiable; (2) evidence of transformation to AP (10%-19% blasts in the PB or BM) or BP (≥20% blasts in the PB or BM, or biopsy proven granulocytic sarcoma); and (3) treatment with intensive induction chemotherapy or nonintensive therapy. A chart review was performed to obtain pertinent clinical information.
The University Health Network Research Ethics Board approved the study (REB CAPCR 16-5169). All patients provided written informed consent for the collection of their PB or BM samples as part of the University Health Network Hematologic Malignancy Tissue Bank (REB CAPCR 01-0573).
Therapy
Patients who were treated with AML-type induction chemotherapy including daunorubicin + ara-C and FLAG-IDA or NOVE-HiDAC (supplemental Table 1) were considered to have received an intensive blast-reduction strategy; the latter 2 were grouped together as higher-dose cytarabine-containing (HD-AraC) regimens. Nonintensive therapy consisted of HMA monotherapy with azacitidine (AZA) or decitabine or AZA in combination with venetoclax (AZAVEN) (supplemental Table 1).
Response definitions
Response to blast reduction was assessed using both PM criteria and ELN 2022 criteria (Table 1). CR and CR with incomplete hematologic recovery (CRi) are defined similarly in both criteria. The PM criteria include reversion to cMPN as a clinically meaningful response, with <10% circulating and/or BM blasts and/or clinical evidence of cMPN, as previously described.7 Patients with stable disease (SD) or progressive disease were considered nonresponders.
Table 1.
Comparison of Princess Margaret Cancer Centre–defined treatment response criteria to ELN 2022 response criteria
| PM-criteria responses | ELN 2022 responses | ||
|---|---|---|---|
| CR | No circulating blasts and/or BM blast <5% Platelets >100 × 109/L and ANC >1 × 109/L |
CR | BM blasts <5%; absence of circulating blasts; absence of extramedullary disease; ANC ≥1.0 × 109/L (1 000/μL); platelet count ≥100 × 109/L (100 000/μL) |
| CRi | No circulating blasts BM blast <5% Platelets <100 × 109/L or ANC <1 × 109/L acceptable |
CRi | All CR criteria except for residual neutropenia < .0 × 109/L (1 000/μL) or thrombocytopenia <100 × 109/L (100 000/μL) |
| Reversion to cMPN | PB blasts <10% or BM blasts 5%-9% BM blasts <5% and MPN features on BM biopsy (not just fibrosis) |
CR with partial hematologic recovery (CRh) | ANC ≥0.5 × 109/L (500/μL) and platelet count ≥50 × 109/L (50 000/μL), otherwise all other CR criteria met |
| Platelets <100 × 109/L and/or ANC <1 × 109/L acceptable (also clinical features ie, hepatosplenomegaly, thrombocytosis, leukocytosis) | Morphologic leukemia free state (MLFS) | BM blasts <5%; absence of circulating blasts; absence of extramedullary disease; no hematologic recovery required | |
| Partial response (PR) | All hematologic criteria of CR; decrease of BM blast percentage to 5%-25%; and decrease of pretreatment BM blast percentage by at least 50% | ||
| SD | SD No significant change in blast count from baseline and increase in PB/BM blasts <50% from baseline |
No response | Patients evaluable for response but not meeting the criteria for CR, CRh, CRi, MLFS, or PR are categorized as having no response prior to the response landmark. Patients failing to achieve response by the designated landmark are designated as having refractory disease |
| Progressive disease (PD) | Increase in PB/BM blasts ≥50% New extramedullary disease |
Refractory disease | No CR, CRh, or CRi at the response landmark, ie, after 2 courses of intensive induction treatment or a defined landmark, eg, 180 d after commencing less-intensive therapy |
| Relapse | PB/BM blasts ≥5% after initial CR/CRi PB/BM blasts ≥10% after initial cMPN |
Relapse | BM blasts ≥5%; or reappearance of blasts in the blood in at least 2 PB samples at least 1 wk apart; or development of extramedullary disease |
| Early death (ED) | Death within first 30 d of induction chemotherapy or HMA treatment | ED | Death from any cause within a timeframe relevant for the therapy being investigated (eg, 30 and 60 d from commencing therapy) |
ANC, absolute neutrophil count; MLFS, morphologic leukemia free state.
Definition of transplant eligibility
At our center, the eligibility criteria for HCT include patients who have a suitable matched sibling, unrelated, or haploidentical donor and achieve at least CR/CRi or cMPN. Although there is no defined upper age limit for candidacy for HCT, HCT is usually restricted to patients 70 years of age or younger.
Molecular analysis
Targeted next-generation sequencing (NGS) was performed on DNA extracted from PB or BM samples. NGS was carried out using 1 of 2 NGS panels: (1) the Trusight Myeloid amplicon-based panel (Illumina, 54 myeloid genes, supplemental Table 6) for samples collected before April 2018, or (2) a custom hybridization capture-based panel (Oxford Gene Technologies, 49 myeloid genes, supplemental Table 7) for samples collected from April 2018 onward. Sequencing reads were processed as previously described.10,19,20 Variants not meeting laboratory-defined quality metrics (read depth <100, population frequency >1% in the gnomAD database, or allele frequency <2%) were removed from further analysis. Variants were classified as pathogenic/likely pathogenic, or variants of uncertain significance as previously described,10,19,20 with the latter excluded from downstream analyses. For paired sample analysis, only genes present on both panels were included.
End points and statistical methods
The primary end point of the study was the overall best response (CR + CRi + cMPN) after blast reduction therapy. Other end points of interest were overall survival (OS) and the proportion of patients undergoing HCT. Baseline characteristics were described using the median and range for continuous variables and frequencies and proportions for categorical variables. The t test and Mann-Whitney tests were used to examine the association between continuous variables and treatment groups, for normally and nonnormally distributed variables, respectively. The Fisher exact test was used to examine whether categorical variables were associated with the treatment groups. Univariate and multivariable logistic regression models were conducted to assess the association between the clinical variables and response to blast reduction. Odds ratios and corresponding 95% confidence intervals (CI) were estimated for each variable.
OS, defined as time from transformation to AP/BP until death or last follow-up, was evaluated using the Kaplan-Meier method. A landmark analysis was used to compare OS between patients who were eligible for HCT (≤70 years with CR/CRi or cMPN) and did not undergo transplantation but who survived at least 155 days (the median time from transformation to AP/BP to HCT) with those who underwent HCT. In addition, univariate and multivariable Cox regression models were used to investigate the association of study variables with survival. The hazard ratio of the clinical variables with corresponding 95% CIs was estimated for each variable.
All statistical tests were two-sided tests, and a P value ≤ .05 was considered for statistical significance. All the statistical analyses were conducted using SAS v9.4, R v4.2.1, and STATA 16.1.
Results
Study population and baseline characteristics
From the institutional databases, 138 patients were identified who met eligibility criteria. Eighty-one patients received intensive induction chemotherapy, and 57 received HMA-based therapy (36 patients received HMA alone [35 AZA; 1 decitabine] and 18 received AZAVEN). Baseline characteristics of patients treated with these 2 blast reduction strategies is shown in Table 2. The nonintensive group had a higher proportion of patients with older age, worse Eastern Cooperative Oncology Group (ECOG) performance status, and lower serum albumin. Patients with AP disease were also more common in this group, leading to the observed lower median PB and BM blast percentages.
Table 2.
Baseline characteristics of MPN-AP/BP cohort at time of transformation
| Variable (%) | Whole cohort (N = 138) | Intensive (AML-type induction) (N = 81) |
Nonintensive (HMA or AzaVen) (N = 57) |
P value (intensive vs nonintensive) |
|---|---|---|---|---|
| Age at transformation, median [range], y | 64 [22-87] | 60 [22-76] | 70 [42-87] | <.001 |
| Sex | .16 | |||
| Male | 87 (63) | 47 (58) | 40 (70) | |
| Female | 51 (37) | 34 (42) | 17 (30) | |
| Transformation type | <.001 | |||
| AP | 34 (25) | 9 (11) | 25 (44) | |
| BP | 104 (75) | 72 (89) | 32 (56) | |
| Chronic MPN diagnosis∗ | .16 | |||
| PV/ET | 35 (25) | 23 (28) | 12 (21) | |
| MPN-U | 15 (11) | 12 (15) | 3 (5) | |
| PMF | 36 (26) | 20 (25) | 16 (28) | |
| PPV/PET-MF | 52 (38) | 26 (32) | 26 (46) | |
| Number of prior MPN treatments in chronic phase | N = 137 | N = 80 | N = 57 | .16 |
| 0-1 | 80 (58) | 51 (64) | 29 (51) | |
| ≥2 | 57 (42) | 29 (36) | 28 (49) | |
| ECOG | N = 126 | N = 74 | N = 52 | .03 |
| 0-1 | 106 (84) | 67 (91) | 39 (75) | |
| ≥2 | 20 (16) | 7 (9) | 13 (25) | |
| Palpable spleen length (BCM) at time of AP/BP | N = 126 | N = 71 | N = 55 | .80 |
| Not palpable | 47 (37) | 24 (34) | 23 (42) | |
| < 10 cm | 30 (24) | 17 (24) | 13 (24) | |
| ≥10 cm | 38 (30) | 23 (32) | 15 (27) | |
| Splenectomy | 11 (9) | 7 (10) | 4 (7) | |
| Laboratory parameters†, median (range) [number] | ||||
| Hb, g/L | 87 (51-177) [n = 132] | 87 (51-177) [n = 77] | 87 (51-134) [55] | .58 |
| WBC, ×109/L | 12.2 (1-213) [n = 134] | 12.8 (1.1-213) [n = 79] | 11.5 (1-126) [n = 55] | .56 |
| ANC, ×109/L | 3.8 (0-91) [n = 123] | 3.8 (0-91) [n = 70] | 3.6 (0.2-79) [n = 53] | .77 |
| Platelets, ×109/L | 90 (6-1918) [n = 133] | 73 (6-865) [n = 78] | 109 (13-1918) [n = 55] | .01 |
| PB blasts, % | 16 (0-83) [n = 134] | 19.5 (0-83) [n = 78] | 12 (0-83) [n = 56] | .03 |
| BM blasts, % | 25 (3-90) [n = 113] | 31 (4-90) [n = 67] | 20 (3-89) [n = 46] | .03 |
| LDH, U/L | 598 (43-5893) [n = 127] | 681 (144-5893) [n = 74] | 553 (43-4000) [n = 53] | .15 |
| Albumin, g/L | 39 (27-47) [n = 124] | 38 (27-47) [n = 74] | 42 (30-45) [n = 50] | .03 |
| ELN 2022 RISK | N = 121 | N = 69 | N = 52 | .14 |
| Adverse | 72 (60) | 37 (54) | 35 (67) | |
| Nonadverse | 49 (40) | 32 (46) | 17 (33) | |
| MPN driver mutations | N = 101 | N = 53 | N = 48 | .29 |
| JAK2/MPL | 66 (65) | 31 (58) | 35 (73) | |
| CALR | 12 (12) | 7 (13) | 5 (10) | |
| Triple negative | 23 (23) | 15 (29) | 8 (17) | |
| Genes mutated in ≥5% of patients | N = 101 | N = 53 | N = 48 | |
| ASXL1 | 29 (29) | 10 (19) | 19 (40) | .03 |
| TET2 | 26 (26) | 18 (34) | 8 (17) | .07 |
| SRSF2 | 26 (26) | 10 (19) | 16 (33) | .11 |
| IDH1/2 | 22 (22) | 11 (21) | 11 (23) | .81 |
| TP53 | 21 (21) | 13 (25) | 8 (17) | .46 |
| RUNX1 | 19 (19) | 8 (15) | 11 (23) | .45 |
| DNMT3A | 16 (16) | 7 (13) | 9 (19) | .59 |
| NRAS | 13 (13) | 5 (9) | 8 (17) | .38 |
| EZH2 | 10 (10) | 4 (8) | 6 (12) | .51 |
| STAG2 | 12 (12) | 5 (9) | 7 (15) | .54 |
| PTPN11 | 7(7) | 1 (2) | 6 (12) | .05 |
| CEBPA | 6 (6) | 4 (8) | 2 (4) | .68 |
| SETBP1 | 6 (6) | 4 (8) | 2 (4) | .68 |
| U2AF1 | 6 (6) | 0 | 6 (12) | .01 |
| CBL | 5 (5) | 1 (2) | 4 (8) | .19 |
| SF3B1 | 6 (6) | 4 (8) | 2 (4) | .68 |
| ZRSR2 | 5 (5) | 2 (4) | 3 (6) | .67 |
| Mutations by pathway | N = 101 | N = 53 | N = 48 | |
| JAK-STAT pathway‡ | 80 (79) | 38 (72) | 42 (88) | .08 |
| RAS pathway§ | 23 (23) | 10 (19) | 13 (27) | .4 |
| RNA splicing‖ | 40 (40) | 15 (28) | 25 (52) | .02 |
| Epigenetic regulation¶ | 67 (66) | 35 (66) | 32 (67) | 1.0 |
| Transcription factors# | 32 (32) | 15 (28) | 17 (35) | .5 |
| Number of mutations | N = 101 | N = 53 | N = 48 | .04 |
| 0-1 | 17 (17) | 12 (23) | 5 (10) | |
| 2-3 | 36 (36) | 22 (42) | 14 (29) | |
| ≥4 | 48 (48) | 19 (36) | 29 (60) |
BCM, below costal margin; LDH, lactate dehydrogenase; MPN-U, MPN unclassifiable; PET-MF, post–ET MF; PMF, primary MF; PPV-MF, post–PV MF. Bold values are statistically significant.
Before leukemic transformation.
One patient received decitabine and the remainder received azacitidine.
JAK2, MPL, CALR, CSF3R.
CBL, NRAS, PTPN11, KIT, KRAS.
SF3B1, SRSF2, U2AF1, ZRSR2.
ASXL1, BCOR, DNMT3A, EZH2, IDH1/2, SETBP1, TET2.
CEBPA, CUX1, ETV6, GATA2, IKZF1, PHF6, RUNX1, WT1.
Treatment response
Of the 81 patients treated with intensive induction chemotherapy, 31 received daunorubicin + ara-C and 50 received a HD-AraC regimen. Using PM-defined criteria (Table 1), we observed a nonstatistically significant trend toward more responses to the first course of induction (CR/CRi/CMPN) with HD-AraC regimens vs daunorubicin + ara-C (Table 3). Notably, more patients initially treated with daunorubicin + ara-C were given a reinduction compared with those starting with a HD-AraC regimen (Table 3, P = .002). After the induction phase (initial induction and first reinduction), similar responses were seen regardless of the initial chemotherapy regimen.
Table 3.
Treatment response with intensive and nonintensive blast-reduction therapies
| OUTCOME | Regimen |
P value (daunorubicin + AraC vs HD-AraC) (Fisher exact) |
Regimen |
P value (HMA vs AzaVen) |
||
|---|---|---|---|---|---|---|
| Daunorubicin + AraC (N = 31) |
HD-AraC regimen (N = 50) |
|||||
| HMA (N = 36) | AzaVen (N = 18) | |||||
| Response after first induction (%) | ||||||
| CR | 7 (23) | 12 (24) | ||||
| CRi | 0 | 2 (4) | ||||
| cMPN | 11 (35) | 24 (48) | .15 | – | – | – |
| SD | 8 (26) | 7 (14) | ||||
| PD | 3 (10) | 0 | ||||
| Early death | 2 (6) | 5 (10) | ||||
| Overall response after first induction (%) | ||||||
| Response (CR/CRi/cMPN) | 18 (58) | 38 (76) | – | – | – | |
| Nonresponse (SD, PD, early death) |
13 (42) | 12 (24) | .14 | |||
| Second chemotherapy induction given (%) | ||||||
| Yes | 9 (29)∗ | 2 (4)† | .002 | – | – | – |
| No | 22 (71) | 48 (96) | ||||
| Best response (%) | ||||||
| CR | 9 (29) | 12 (24) | .38 | 1 (3) | 3 (17) | |
| CRi | 1 (3) | 2 (4) | 0 | 0 | ||
| cMPN | 13 (42) | 25 (50) | 11 (31) | 6 (33) | .14 | |
| SD | 3 (10) | 6 (12) | 16 (44) | 9 (50) | ||
| PD | 3 (10) | 0 | 5 (14) | 0 | ||
| Early death | 2 (6) | 5 (10) | 3 (8) | 0 | ||
| Overall best response (%) | ||||||
| Response (CR/CRi/cMPN) | 23 (74) | 39 (78) | .79 | 12 (33) | 9 (50) | .26 |
| Nonresponse (SD, PD, early death) | 8 (26) | 11 (22) | 24 (67) | 9 (50) | ||
PD, progressive disease. Bold values are statistically significant.
Patients initially treated with 3+7 and achieving cMPN (n = 1), SD (n = 6), and PD (n = 2); 8 received HD-AraC–based regimen for reinduction; 1 unknown; responses were CR (n = 2), cMPN (n = 3), CRi (n = 1), SD (n = 1), PD (n = 2).
Patients initially treated with FLAG-IDA/NOVE-HiDAC and achieving SD (n = 2); both patients received alternate HD-AraC regimen achieving cMPN (n = 1) and CRi (n = 1).
Among 57 patients treated with lower-intensity approaches, treatment responses were evaluable in 54, including 36 patients treated with single-agent HMA and 18 treated with AZAVEN. Responses were observed in 33% of patients (12 of 36) treated with HMA alone, with most of those responses resulting in reversion to cMPN (11 of 12). For patients treated with AZAVEN, 50% of patients (9 of 18) responded (Table 3).
The overall best response rates for the intensively and nonintensively treated groups were 77% (62 of 81, 24 CR/CRi, and 38 cMPN) and 39% (21 of 54, 4 CR/Cri, 17 cMPN), respectively.
Predictors of treatment response
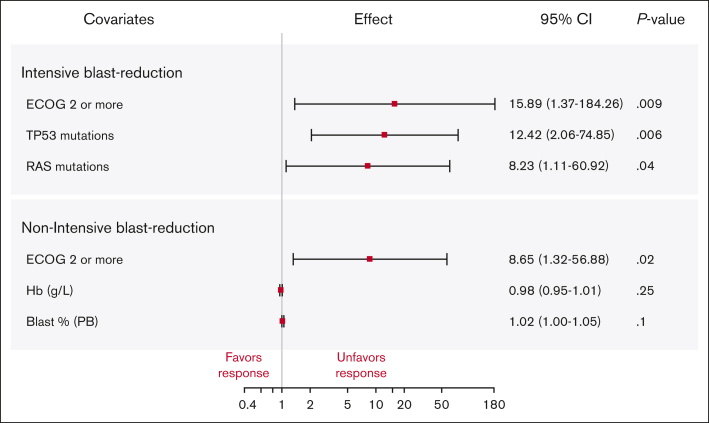
Among patients receiving an intensive blast-reduction strategy, ECOG performance status and mutations in the RAS pathway genes or TP53 were associated with treatment failure by univariate analysis (UVA) (supplemental Table 2); significance was maintained in multivariable analysis (MVA) (Figure 1).
Figure 1.
Multivariable logistic regression analysis of response to blast-reduction therapy among patients treated with intensive and nonintensive strategies. Variables statistically significant in UVA were chosen for multivariable logistic regression. The odds ratios are depicted as the effect size with the corresponding 95% CIs.
In the nonintensively treated group, variables significantly associated with treatment response in UVA were ECOG performance status, hemoglobin, and percent of blasts in the PB (supplemental Table 3); only ECOG remained significant in the MVA (Figure 1).
Allogeneic stem cell transplant
To assess the optimal pre-HCT blast-reduction strategy, we compared the proportion of patients undergoing HCT after a first-line intensive or nonintensive blast-reduction strategy (supplemental Table 4). We limited this comparison to patients ≤70 years of age and those who achieved a response to first-line blast reduction (including those receiving 2 inductions). As such, in the intensive group, 73% of patients (59 of 81) were eligible for HCT, 68% (21 of 31) of patients treated with daunorubicin + ara-C, and 76% (38 of 50) of patients treated with the HD-AraC protocol (P = .45). Of these, a total of 54% of patients (32 of 59) underwent HCT, 48% (10 of 21) in the daunorubicin + ara-C group, and 58% (22 of 38) in the HD-AraC group (P = .59). Early relapse (32%; 19 of 59) was the main reason for not proceeding with HCT in patients who were otherwise eligible. A donor was identified for all but 1 patient.
Similarly, in the nonintensive group, 17% (6 of 36) and 28% (5 of 18) of patients treated with HMA-alone or AZAVEN (P = .48), respectively, would have been eligible for HCT. Sixty-seven percent (4 of 6) of patients treated with HMA and 100% (5 of 5) of patients treated with AZAVEN proceeded to HCT (P = .45).
There were no statistically significant differences in the median time to HCT (from treatment start) between intensive and nonintensive strategies (145 days [54-455] vs 102 days [51-260], P = .41) nor between the induction regimens (supplemental Table 4). There was no survival difference between blast-reduction strategies among patients undergoing HCT (supplemental Figure 1).
Reclassification of study-defined response using ELN 2022 criteria
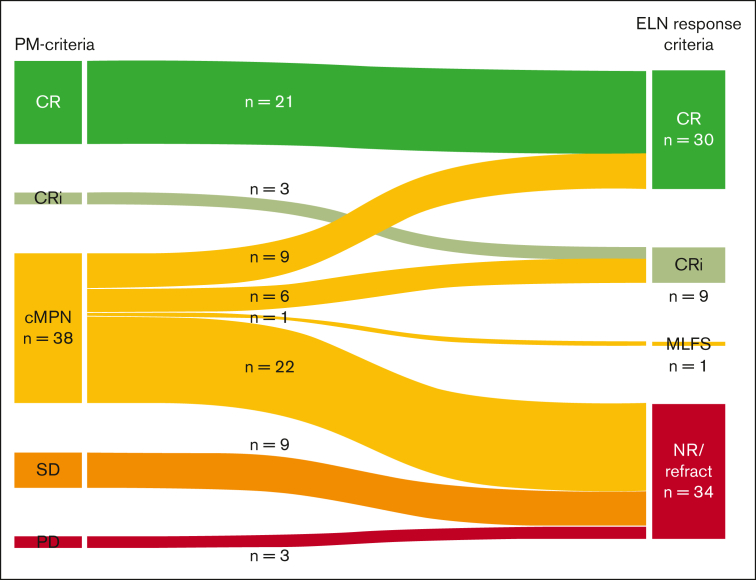
To understand how PM response criteria compare to traditional AML response criteria, we reclassified treatment responses according to ELN 2022 AML response criteria18 (Figure 2) in intensively treated patients. All CR and CRi classifications remained consistent. Twelve patients with SD and progressive disease were reclassified as having no response by the ELN 2022 classification. Most notably, of the 38 patients with cMPN, 9 (24%) and 6 (16%) were reclassified to CR and CRi, respectively, based on BM blast clearance to less than 5% and absence of circulating blasts. As these patients had residual histologic features of MPN on BM biopsy, they were classified as cMPN by PM response criteria. ELN 2022 AML response criteria do not address the issue of residual MPN features on remission BMs and thus do not exclude a CR or CRi designation in these cases. Of the remaining patients with cMPN, 1 (3%) was reclassified to morphologic leukemia free state (MLFS), whereas 22 (58%) were deemed to have no response (N = 20) or to be refractory (N = 2). Nonresponse was assigned because of the persistence of circulating blasts and/or 5% to 9% BM blasts in 20 of these patients.
Figure 2.
Reclassification of responses to induction chemotherapy by ELN 2022 criteria. The Sankey diagram shows comparison of response assessment to intensive blast-reduction therapy by PM criteria (left) and how this change when responses are reclassified by ELN 2022 criteria (right). Responses categorized as CR and CRi by PM criteria remain CR and CRi by ELN 2022 criteria, respectively. cMPN responses were reclassified as indicated to CR, CRi, MFLS, or no response/refractory (NR/R). SD and PD by PM criteria are NR/R by ELN 2022 criteria. MLFS, morphologic leukemia-free state.
Survival
The median follow-up time for patients remaining alive at last follow-up was 40.3 months. Median OS after transformation to MPN-AP and MPN-BP was 21.9 months (95% CI, 12.2-95.1) and 8.8 months (95% CI, 7.97-10.27), respectively.
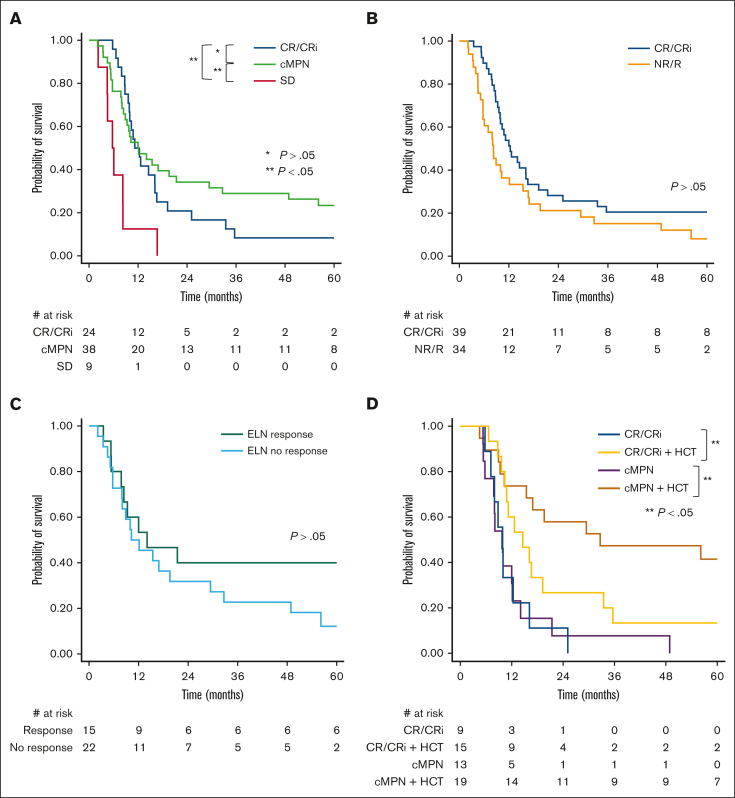
Among patients treated intensively, there was no difference in survival between patients achieving cMPN vs CR/CRi as the best response by PM criteria (Figure 3A). However, survival was improved in responders (CR/CRi/cMPN) compared with those who achieved SD. In a landmark analysis, we show that the survival benefit is limited to responders who are consolidated with HCT after blast reduction therapy (Figure 3D). When ELN 2022 AML response criteria were used to distinguish between responders and nonresponders, no survival difference was observed between these groups (Figure 3B). Finally, a survival difference was not observed when cMPN responders by PM criteria were reclassified by ELN 2022 criteria to either responders or nonresponders (Figure 3C).
Figure 3.
Kaplan-Meier survival analysis among patients treated with intensive blast-reduction strategies. (A) Comparison of OS among patients achieving CR/CRi, cMPN, or SD as overall best response. (B) Comparison of OS by ELN 2022 response criteria; CR/CRi vs NR/R. (C) Comparison of OS among patients with cMPN reclassified to either responders or nonresponders by ELN 2022 criteria. (D) Landmark Kaplan-Meier survival analysis comparing patients under age 71 who underwent HCT and patients who achieved CR/CRi or cMPN by PM criteria after either intensive or nonintensive blast-reduction strategy and survived at least 155 days (median time to HCT) after transformation to AP/BP but who did not undergo HCT.
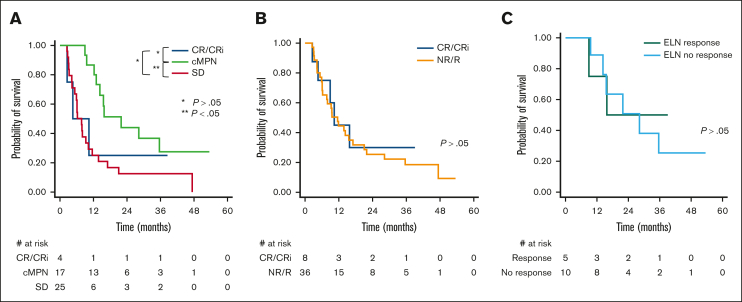
Among those treated nonintensively, a statistically significant difference in survival was observed in those achieving cMPN vs SD by PM criteria (Figure 4A). When ELN criteria were used to assign responses, no difference in survival was observed among responders vs nonresponders (Figure 4B). Similarly, when patients classified as cMPN by PM criteria were reclassified to either responder or nonresponder by ELN, no difference in OS was observed (Figure 4C).
Figure 4.
Kaplan-Meier survival analysis among patients treated nonintensive blast-reduction strategies. (A) Comparison of OS among patients achieving CR/CRi, cMPN, or SD as best response. (B) Comparison of OS by ELN 2022 response criteria; CR/CRi vs NR/R. (C) Comparison of OS among patients with cMPN reclassified to either responders or nonresponders by ELN 2022 criteria.
In UVA, treatment response, transformation type (AP vs BP), ECOG performance, PB and BM blast percentage, hemoglobin, platelet count, albumin, and ELN risk, TP53 mutations, RAS-pathway mutations, and number of mutations were all associated with survival (supplemental Table 5). In MVA of clinical variables, treatment response and transformation type remained significant. Among genetic and molecular factors, TP53 and the number of mutations remained significant.
TP53 mutations
Among 21 patients with mutations in TP53, 13 were treated with an intensive strategy (2 daunorubicin + AraC; 11 HD-AraC), resulting in CR and cMPN in 3 patients each, whereas 5 had SD and 2 patients had early death. Three were treated with AZAVEN and 5 were treated with AZA alone; responses were observed in only 2 patients (1 cMPN and 1 CR in AZAVEN). Among the 6 patients who underwent transplantation with TP53 mutations, 2 underwent HCT after achieving only SD after blast reduction, and 1 patient underwent HCT after an early relapse. HCT did not appear to provide a survival benefit for these patients with TP53 mutations (supplemental Figure 2).
Paired sample analysis
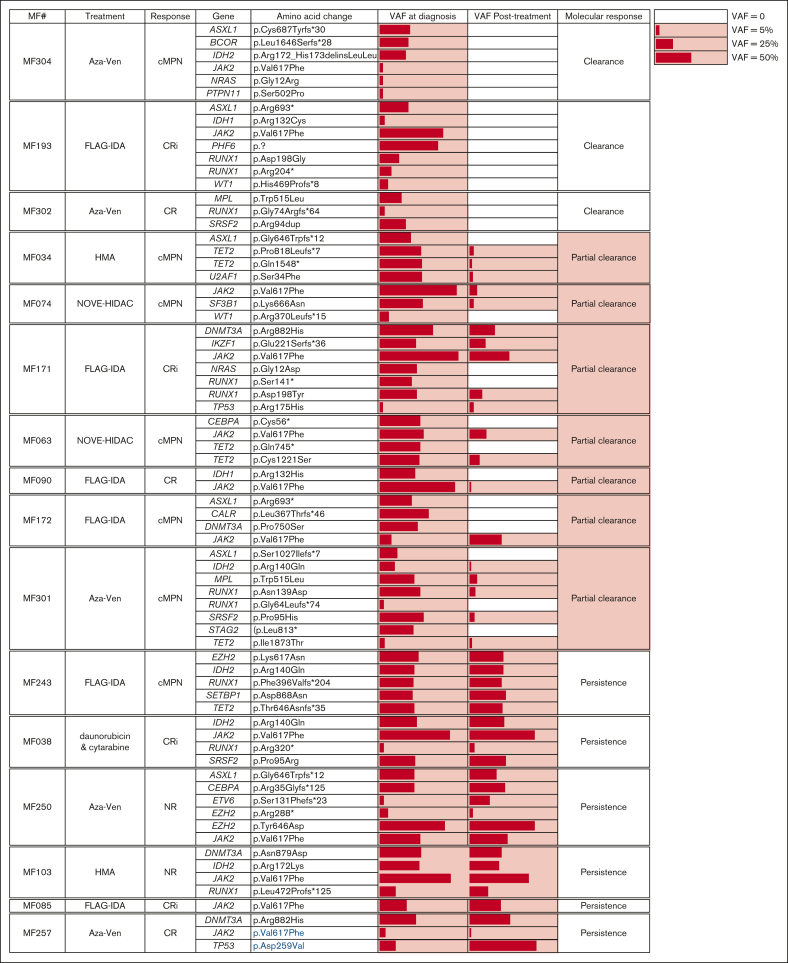
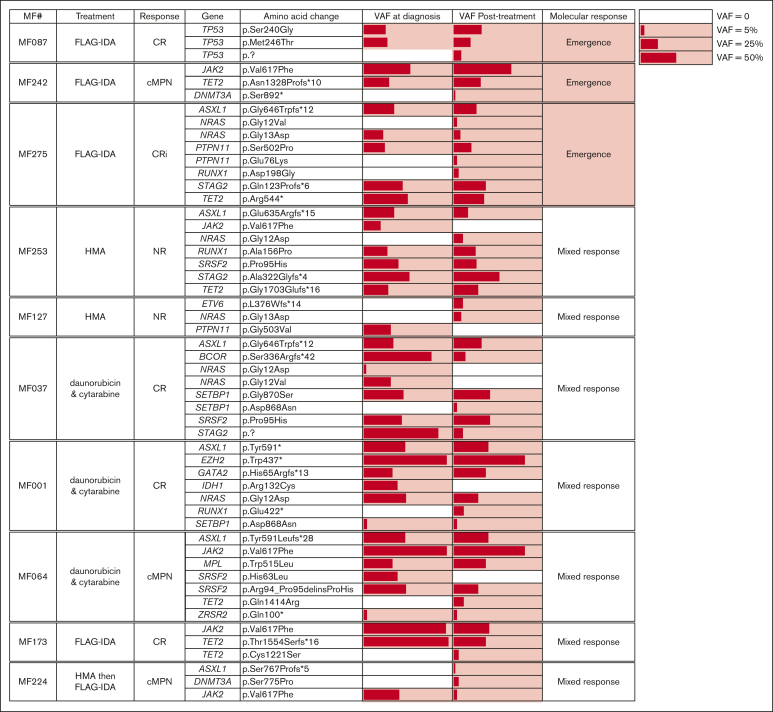
To gain insight into the molecular responses to blast-reduction strategies and their association with PM-defined response criteria, mutation burden at the time of MPN-AP/BP diagnosis was compared with posttreatment time points (Figure 5). This analysis included 17 patients treated with induction chemotherapy (samples collected ∼30 days after induction), 5 patients treated with AZAVEN (samples collected after 1-2 cycles), and 5 patients treated with HMA monotherapy (samples collected after a variable number of cycles, ranging from 3-13). Molecular responses were defined as “clearance” (all variants cleared, n = 3), “partial clearance” (clearance of at least 1, but not all AP/BP variants, n = 7), “persistence” (all variants from AP/BP remain detectable, n = 6), “emergence” (all variants from AP/BP remain detectable, with emergence of a variant after treatment not initially detected at diagnosis, n = 3), and “mixed response” (loss of some AP/BP variants coupled to emergence of novel variants, n = 7).
Figure 5.
Paired NGS sample analysis at time of MPN-AP/BP diagnosis and after blast reduction. Mutation-positive fractions are shaded in red, with the length of the bars proportional to the variant allele frequency (VAF). Patients are sorted by their molecular response pattern after treatment. Post–blast-reduction samples were taken after the indicated treatment.
In general, mutational burden remained high despite blast-reduction therapy, with full or partial clearance seen in only 38% (10 of 26) of patients. Notably, the mutations that persisted after treatment were not limited to genes previously associated with clonal remissions in AML (DNMT3A, TET2, ASXL1; DTA genes).21, 22, 23, 24
Aside from nonresponders (N = 4) who exclusively showed either mutational persistence or a mixed response, there were no other clear associations between molecular response patterns and PM-defined treatment responses. For example, among the 12 patients with CR/Cri, each of the molecular response patterns were observed (2 clearance, 2 partial clearance, 3 persistence, 2 emergence, and 3 mixed response). A similar distribution was noted in patients who achieved cMPN. Together, these findings indicate that mutational burden does not align with the observed clinicopathologic response after blast reduction treatment in patients with AP/BP.
Finally, for 5 patients, mutational analysis was also performed on samples collected after HCT, whereas in BM remission (supplemental Figure 3). AP/BP mutations persisted after initial blast reduction therapy in these individuals; however, after transplant, full mutational clearance was observed for all patients who had samples available for analysis.
Discussion
The transformation of MPN to AP/BP portends poor long-term survival. There is no standard treatment approach for MPN-AP/BP. In this study, we evaluated the outcomes of AML-type intensive and nonintensive blast-reduction strategies in these patients. As there are no validated response criteria for MPN-AP/BP, we applied a set of clinically relatable criteria developed at Princess Margaret Cancer Centre. These were developed out of practical challenges associated with applying AML response criteria, such as ELN 2022, and our desire to establish criteria for proceeding to HCT. Unlike de novo AML, patients with MPN-AP/BP frequently revert to a state resembling cMPN after blast-reduction treatment. Persistent cMPN features included splenomegaly, cytopenias/cytoses, 5% to 9% BM blasts, and/or low levels of circulating blasts. The presence of such features can lead to the classification of many patients as nonresponders by ELN criteria, which in turn may influence decisions about subsequent therapy, including HCT. Indeed, when applying the ELN 2022 response criteria, more than half of cMPN responders per PM criteria were reclassified as nonresponders, primarily based on the residual blast count of 5% to 9% and/or the persistence of circulating blasts. Of note, we show that, together with CR/CRi responders, patients who revert to a cMPN state after blast reduction have improved survival if consolidated with HCT. Although improvement in OS was observed in patients deemed to have a response compared with nonresponders by PM criteria, no difference in survival was discerned by the ELN 2022 response assignment.
Our response criteria for MPN-AP/BP share features with those previously proposed by Mascarenhas et al,14 which also were designed to facilitate response assignment in the setting of an underlying MPN. These criteria have not been broadly adopted, especially in routine clinical practice. Contributing to this lack of adoption may be the need for full cytogenetic and molecular data as part of response assessment; these are not standard of care at many centers outside of clinical trials. Our criteria can be readily applied in the real-world clinical setting and in retrospective and prospective studies. Further, PM response criteria capture clinical benefits of therapies and serve as markers of clinically important outcomes, similar to the recent revision of response criteria in myelodysplastic syndrome as described in the consensus proposal for revised International Working Group 2023 response criteria.25
Using PM response criteria, we show that 77% (62 of 81) of patients who underwent induction chemotherapy achieved a response. However, only 30% (24 of 81) achieved CR/CRi, whereas the remaining 47% (38 of 81) reverted to a cMPN. This is similar to reported response rates to induction chemotherapy, ranging from 30% to 60%.7,26, 27, 28 When HD-AraC induction was used upfront, fewer patients required a second induction, consistent with the known utility of HD-AraC in overcoming resistance as observed in relapsed/refractory AML. The risk of early death (≤30 days from treatment) was 9% (7 of 81), comparing favorably with previous reports of early deaths as high as 15% to 33%.8,29 In patients treated with lower-intensity strategies, the overall response rate was 39% (21 of 54); AZAVEN produced a higher response rate than HMA alone, in keeping with the 40% to 50% response rate observed by others.11, 12, 13 In our cohort, lower-intensity blast-reduction strategies were preferentially used in older patients with poorer performance status and among those with AP disease.
Failure to achieve response with an intensive blast-reduction strategy was associated with pretreatment poorer ECOG performance status, TP53, and RAS pathway mutations. Interestingly, the ELN risk category was not associated with the response to blast reduction in MVA. Similarly, response to induction chemotherapy was independent of abnormal karyotype in the Mayo cohort.27 RAS pathway mutations are also associated with shortened OS and an increased risk of AP/BP in chronic phase MF.30 The association of TP53 mutations with the failure of intensive blast-reduction strategies is in keeping with the known chemotherapy resistance imparted by TP53 dysfunction.19
The current study confirms our previous findings that consolidation with HCT after blast reduction is required to achieve long-term survival benefit in MPN-AP/BP,7 in a larger cohort of patients. Although it is largely regarded that pre-HCT CR/CRi improves post-HCT outcomes, available reports are conflicting.1,7,9,27,31,32 Here, we show that the survival benefit imparted by HCT extends to patients who revert to cMPN after blast reduction. Notably, a recent analysis of Center for International Blood and Marrow Transplant Research (CIBMTR) data among 177 patients with MPN-BP showed that outcomes in patients who underwent HCT with BM/PB blasts <5% were comparable with those with higher BM/PB blast counts, with the latter population likely including a significant proportion of patients who would be classified as cMPN.19 Moreover, we show that both intensive and nonintensive blast-reduction strategies can be used as a bridge to HCT in carefully selected patients. For example, similar proportions of eligible patients were able to undergo HCT across the treatment groups, and 4 of 6 patients who were eligible for HCT treated with AZA for AP disease went onto HCT. The observed HCT-associated survival benefit does not appear to extend to patients with TP53 mutations in our study.
In our paired sample sequencing analysis, we show that irrespective of response to blast reduction, the residual mutational burden remains high after therapy, and full mutational clearance was rarely seen. Like a previous CIBMTR study, a wide range of mutations remained detectable after therapy, including DTA genes and driver mutations as well as mutations in genes such as TP53, RUNX1, and RAS pathway members.19 Moreover, mutational burden remained high across individuals who achieved responses such as CR/CRi or cMPN, with many of these patients exhibiting persistence of all variants or the emergence of novel variants. By contrast, full clearance of the mutations was observed in patients who underwent HCT. The persistence of mutations after blast reduction likely reflects the inherent chemoresistance of the abnormal myeloid clone in AP/BP MPN and explains the high rates of relapse and poor outcomes when responses are not consolidated by HCT.7 Taken together, these data suggest that currently available blast-reduction modalities have limited anticlonal activity and thereby a limited capacity for disease modification without consolidative HCT.
Our findings must be interpreted within the context of our methodology. This was a retrospective, single-center study. As such, the potentially beneficial use of our response criteria described here requires prospective validation in independent cohorts. Further, because of the retrospective design, the response evaluation was not comparable between all patients. For example, a proportion of patients treated nonintensively did not undergo BM analysis as part of their response assessment because of patient frailty or preference.
Taken together, our study highlights that both intensive and nonintensive blast-reduction modalities have limited disease-modifying and clonal clearance capability on their own, and consolidative HCT is required to impart long-term disease control and survival benefit. MPN-AP/BP represents an ongoing area of unmet need for the development of effective disease-modifying therapies. Moreover, the application of response criteria designed for use in de novo AML to evaluate the response of MPN-AP/BP to blast-reduction treatment is fraught with challenges. Responses often entail a reversion to the cMPN. The stringent pre-HCT response requirements of CR/CRi applied to de novo AML are not appropriate in patients with MPN-AP/BP treated with the currently available blast-reduction strategies. There is a need for the MPN community to revisit the criteria best suited for assigning responses and informing clinical decision-making in patients with MPN-AP/BP.
Conflict-of-interest disclosure: G.R.-C. participated in advisory boards with AbbVie, Taiho, Pfizer, Bristol Myers Squibb (BMS), and Astellas, and received honorarium from Pfizer and Astellas. D.M. has received research support from Novartis, Celgene/BMS, PharmaEssentia, and Takeda, and has participated in advisory boards and received honoraria from Novartis, Celgene/BMS, and Jazz. D.M. has participated in consultancy for Pfizer. A.T. has received honoraria from BD Biosciences and Amgen. A.D.S. has received research funding from Takeda Pharmaceuticals, BMS, and Medivir AB; received consulting fees/honorarium from Takeda, Novartis, Jazz, and Otsuka Pharmaceuticals; is named on a patent application for the use of double-negative T cells to treat AML; and is a member of the medical and scientific advisory board of the Leukemia and Lymphoma Society of Canada. V.G. reports consulting fees from AbbVie, BMS/Celgene, Constellation Biopharma, Novartis, Pfizer, and Sierra Oncology; honoraria from BMS/Celgene, Constellation Biopharma, and Novartis; and participation in the data safety monitoring board or advisory board for AbbVie, BMS/Celgene, Pfizer, and Roche. The remaining authors declare no competing financial interests.
Acknowledgments
This study was funded by a program project grant from the Elizabeth and Tony Comper Foundation and the Princess Margaret Cancer Foundation (V.G.). V.G. is also supported by the Barbara Baker Chair for leukemia and related disorders at the University Health Network, Toronto.
Authorship
Contribution: M.B.D., J.A.K., and V.G. designed and performed the research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; Y.S. and W.X. performed statistical analysis; J.-M.C.-C. provided and reviewed the molecular data; A.A. and M.D.M. provided data and tissue samples; V.C. collected data; A.A., A.B., A.C.S., A.D.S., A.V., A.T., D.M., G.R.-C., H.S., J.A.K., K.Y., M.B.D., M.D.M., S.C., and V.G. provided the study patients and reviewed the manuscript; and all authors approved the final version of the manuscript.
Footnotes
M.B.D. and J.A.K. contributed equally to this study.
For original, deidentified data, please contact the corresponding author, Vikas Gupta (vikas.gupta@uhn.ca).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Tam CS, Kantarjian H, Cortes J, et al. Dynamic model for predicting death within 12 months in patients with primary or post–polycythemia vera/essential thrombocythemia myelofibrosis. J Clin Oncol. 2009;27(33):5587–5593. doi: 10.1200/JCO.2009.22.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mudireddy M, Gangat N, Hanson CA, Ketterling RP, Pardanani A, Tefferi A. Validation of the WHO-defined 20% circulating blasts threshold for diagnosis of leukemic transformation in primary myelofibrosis. Blood Cancer J. 2018;8(6):57. doi: 10.1038/s41408-018-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–2513. doi: 10.1182/blood-2014-05-579136. quiz 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbui T, Thiele J, Passamonti F, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29(23):3179–3184. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- 5.Cervantes F, Tassies D, Salgado C, Rovira M, Pereira A, Rozman C. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta Haematol. 1991;85(3):124–127. doi: 10.1159/000204873. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy JA, Atenafu EG, Messner HA, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121(14):2725–2733. doi: 10.1182/blood-2012-10-464248. [DOI] [PubMed] [Google Scholar]
- 8.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105(3):973–977. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 9.Alchalby H, Zabelina T, Stubig T, et al. Allogeneic stem cell transplantation for myelofibrosis with leukemic transformation: a study from the Myeloproliferative Neoplasm Subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20(2):279–281. doi: 10.1016/j.bbmt.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 10.McNamara CJ, Panzarella T, Kennedy JA, et al. The mutational landscape of accelerated- and blast-phase myeloproliferative neoplasms impacts patient outcomes. Blood Adv. 2018;2(20):2658–2671. doi: 10.1182/bloodadvances.2018021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangat N, Guglielmelli P, Szuber N, et al. Venetoclax with azacitidine or decitabine in blast-phase myeloproliferative neoplasm: a multicenter series of 32 consecutive cases. Am J Hematol. 2021;96(7):781–789. doi: 10.1002/ajh.26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masarova L, DiNardo CD, Bose P, et al. Single-center experience with venetoclax combinations in patients with newly diagnosed and relapsed AML evolving from MPNs. Blood Adv. 2021;5(8):2156–2164. doi: 10.1182/bloodadvances.2020003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King AC, Weis TM, Derkach A, et al. Multicenter evaluation of efficacy and toxicity of venetoclax-based combinations in patients with accelerated and blast phase myeloproliferative neoplasms. Am J Hematol. 2022;97(1):E7–E10. doi: 10.1002/ajh.26381. [DOI] [PubMed] [Google Scholar]
- 14.Mascarenhas J, Heaney ML, Najfeld V, et al. Proposed criteria for response assessment in patients treated in clinical trials for myeloproliferative neoplasms in blast phase (MPN-BP): formal recommendations from the post-myeloproliferative neoplasm acute myeloid leukemia consortium. Leuk Res. 2012;36(12):1500–1504. doi: 10.1016/j.leukres.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose P, Verstovsek S, Cortes JE, et al. A phase 1/2 study of ruxolitinib and decitabine in patients with post-myeloproliferative neoplasm acute myeloid leukemia. Leukemia. 2020;34(9):2489–2492. doi: 10.1038/s41375-020-0778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascarenhas JO, Rampal RK, Kosiorek HE, et al. Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv. 2020;4(20):5246–5256. doi: 10.1182/bloodadvances.2020002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in accelerated and blast-phase myeloproliferative neoplasms. Blood Adv. 2018;2(24):3572–3580. doi: 10.1182/bloodadvances.2018019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Kennedy JA, Capo-Chichi JM, et al. Genetic factors rather than blast reduction determine outcomes of allogeneic HCT in BCR-ABL-negative MPN in blast phase. Blood Adv. 2020;4(21):5562–5573. doi: 10.1182/bloodadvances.2020002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel JY, McNamara C, Kennedy JA, et al. Impact of genomic alterations on outcomes in myelofibrosis patients undergoing JAK1/2 inhibitor therapy. Blood Adv. 2017;1(20):1729–1738. doi: 10.1182/bloodadvances.2017009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378(13):1189–1199. doi: 10.1056/NEJMoa1716863. [DOI] [PubMed] [Google Scholar]
- 22.Morita K, Kantarjian HM, Wang F, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36(18):1788–1797. doi: 10.1200/JCO.2017.77.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy T, Zou J, Daher-Reyes GS, et al. Impact of preleukemic mutations and their persistence on hematologic recovery after induction chemotherapy for AML. Blood Adv. 2019;3(15):2307–2311. doi: 10.1182/bloodadvances.2019000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg-Thurley M, Amler S, Goerlich D, et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia. 2018;32(7):1598–1608. doi: 10.1038/s41375-018-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeidan AM, Platzbecker U, Bewersdorf JP, et al. Consensus proposal for revised International Working Group 2023 response criteria for higher-risk myelodysplastic syndromes. Blood. 2023;141(17):2047–2061. doi: 10.1182/blood.2022018604. [DOI] [PubMed] [Google Scholar]
- 26.Mollard LM, Chauveau A, Boyer-Perrard F, et al. Outcome of Ph negative myeloproliferative neoplasms transforming to accelerated or leukemic phase. Leuk Lymphoma. 2018;59(12):2812–2820. doi: 10.1080/10428194.2018.1441408. [DOI] [PubMed] [Google Scholar]
- 27.Tefferi A, Mudireddy M, Mannelli F, et al. Blast phase myeloproliferative neoplasm: Mayo-AGIMM study of 410 patients from two separate cohorts. Leukemia. 2018;32(5):1200–1210. doi: 10.1038/s41375-018-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel AA, Yoon JJ, Johnston H, et al. Outcomes of patients with accelerated/blast-phase myeloproliferative neoplasms in the current era of myeloid therapies. Blood. 2022;140(suppl 1):6860–6862. [Google Scholar]
- 29.Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood. 2008;112(5):1628–1637. doi: 10.1182/blood-2008-02-138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos FPS, Getta B, Masarova L, et al. Prognostic impact of RAS-pathway mutations in patients with myelofibrosis. Leukemia. 2020;34(3):799–810. doi: 10.1038/s41375-019-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahu X, Chevallier P, Clavert A, et al. Allo-SCT for Philadelphia-negative myeloproliferative neoplasms in blast phase: a study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) Bone Marrow Transplant. 2014;49(6):756–760. doi: 10.1038/bmt.2014.31. [DOI] [PubMed] [Google Scholar]
- 32.Takagi S, Masuoka K, Uchida N, et al. Allogeneic hematopoietic cell transplantation for leukemic transformation preceded by Philadelphia chromosome-negative myeloproliferative neoplasms: a nationwide survey by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(12):2208–2213. doi: 10.1016/j.bbmt.2016.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.