Abstract
The movement of 14C-photosynthate in morning glory (Ipomea nil Roth, cu. Scarlet O'Hara) vines 2 to 5 meters long was followed by labeling a lone mature leaf with 14CO2 and monitoring the arrival rate of tracer at expanding sink leaves on branches along the stem. To a first approximation, the kinetic behavior of the translocation profiles resembled that which would be expected from movement at a single velocity (“plug flow”) without tracer loss from the translocation stream. There was no consistent indication of a velocity gradient along the vine length. The profile moved along the vine as a distinct asymmetrical peak which changes shape only slowly. The spatial distribution of tracer along the vine reasonably matched that predicted on the basis of the arrival kinetics at a sink, assuming plug flow with no tracer loss. These observations are in marked contrast to the kinetic behavior of any mechanism describable by diffusion equations.
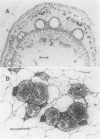
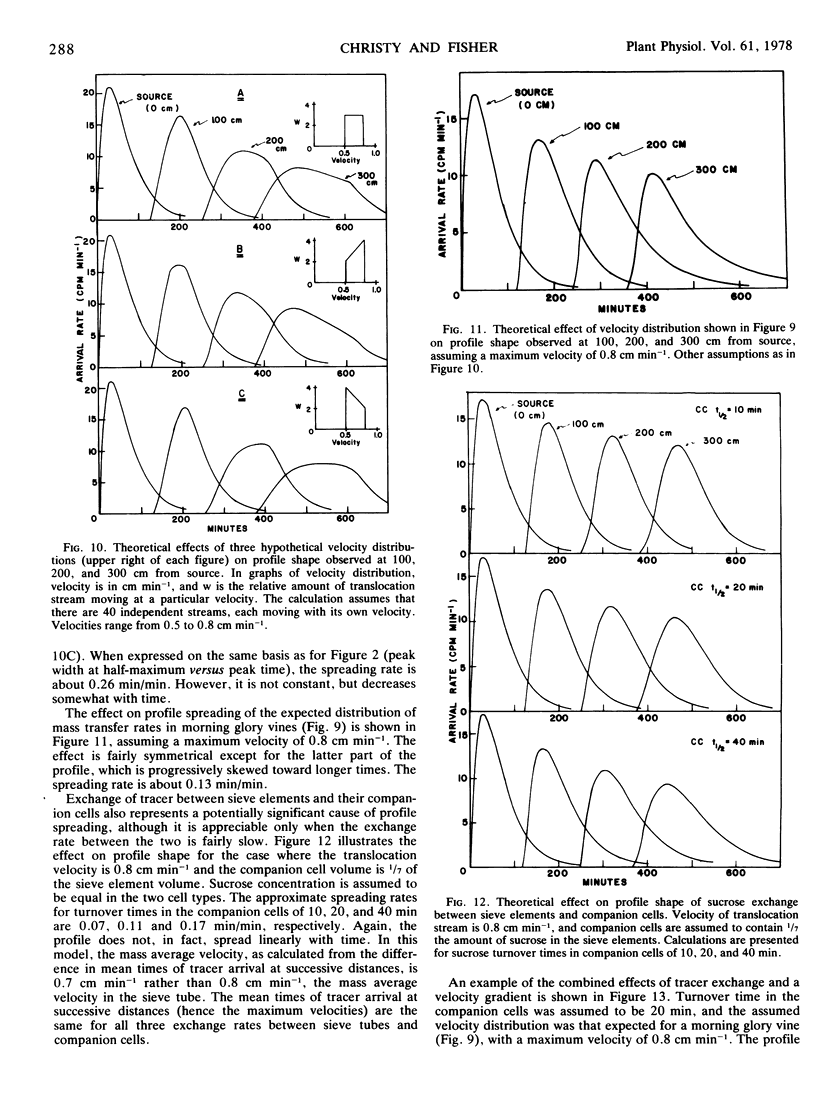
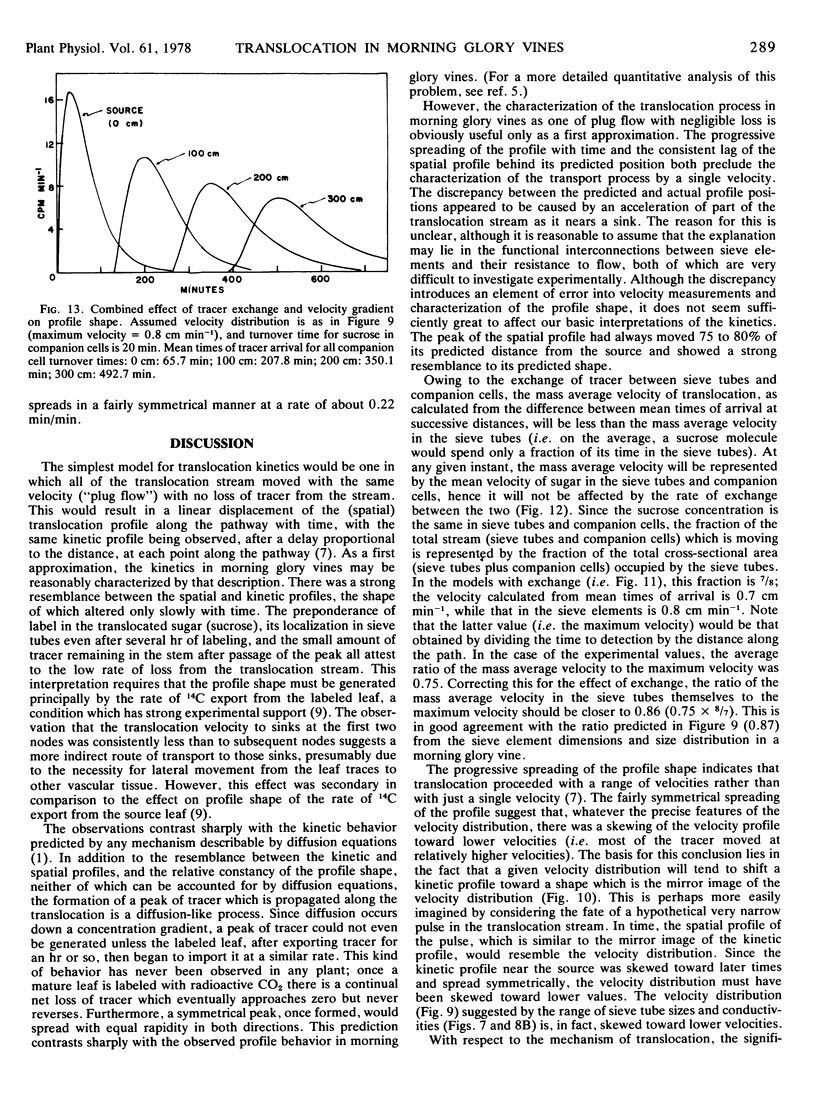
However, a progressive change in profile shape (a symmetrical widening) was observed, indicating a range of translocation velocities. A minimum of at least two factors must have contributed to the observed velocity gradient: the exchange of 14C between sieve elements and companion cells (demonstrated by microautoradiography) and the range of velocities in the several hundred sieve tubes which carried the translocation stream. Possible effects of these two factors on profile spreading were investigated by means of numerical models. The models are necessarily incomplete, due principally to uncertainties about the exchange rate between sieve elements and companion cells and the degree of functional connectivity between sieve tubes of different conductivities. However, most of the observed profile spreading may be reasonably attributed to the combined effects of those two factors.
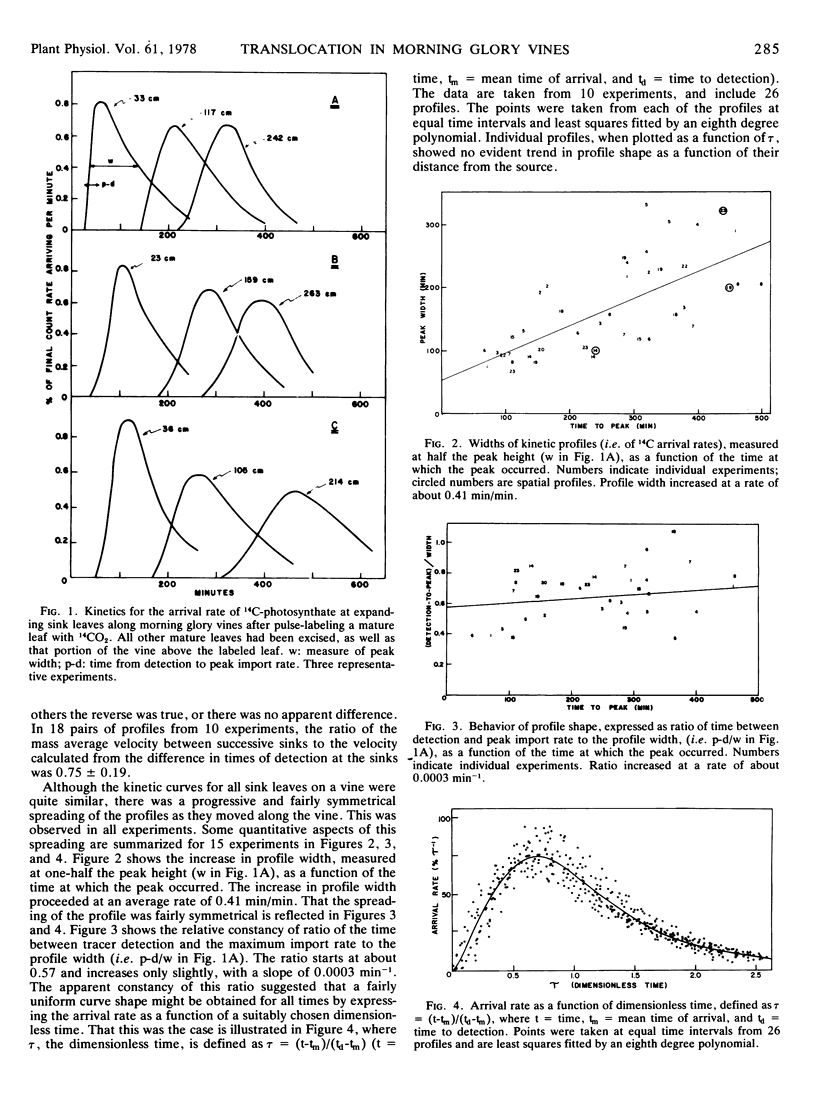
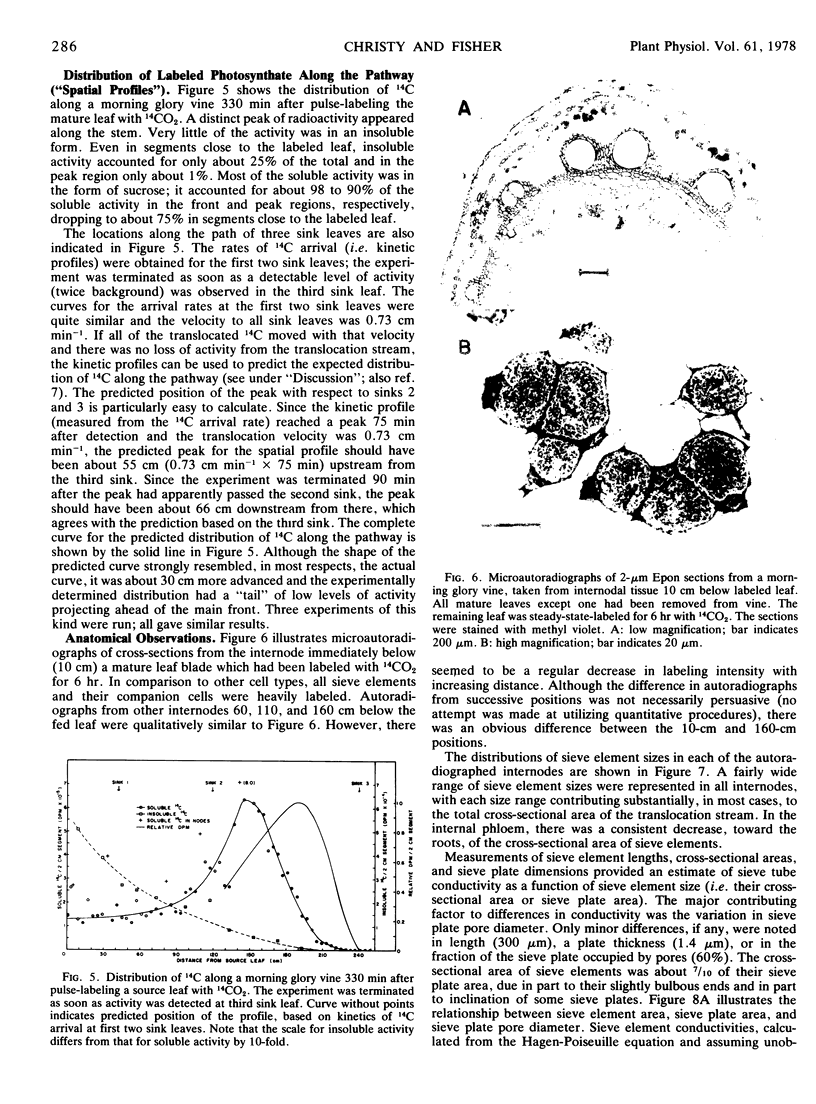
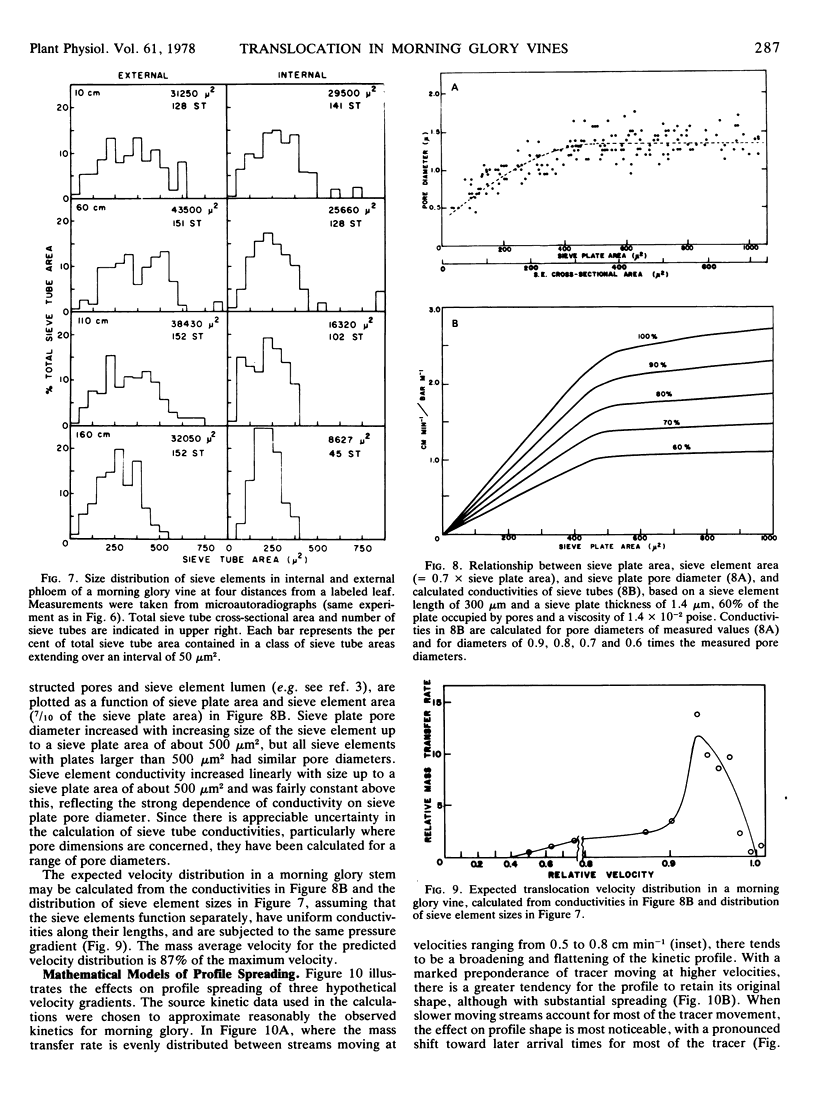
The mass average velocity of translocation (calculated from the mean times of 14C arrival at successive sink leaves) was about 75% of the maximum velocity (calculated from the times of initial detection at the same sink leaves), which was usually between 0.6 and 1 cm min−1. Owing to tracer exchange between sieve elements and companion cells, the mass average velocity of tracer in the sieve tubes was probably closer to 86% of the maximum velocity, a figure which agreed with a predicted velocity distribution based on calculated sieve tube conductivities and the size distribution of functional sieve tubes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A., Christy A. L., Coulson C. L., Ferrier J. M. Solution-flow in the Phloem: I. Theoretical considerations. Plant Physiol. 1972 May;49(5):685–689. doi: 10.1104/pp.49.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy A. L., Ferrier J. M. A Mathematical Treatment of Munch's Pressure-Flow Hypothesis of Phloem Translocation. Plant Physiol. 1973 Dec;52(6):531–538. doi: 10.1104/pp.52.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson C. L., Christy A. L., Cataldo D. A., Swanson C. A. Carbohydrate translocation in sugar beet petioles in relation to petiolar respiration and adenosine 5'-triphosphate. Plant Physiol. 1972 Jun;49(6):919–923. doi: 10.1104/pp.49.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Housley T. L., Christy A. L. Source pool kinetics for C-photosynthate translocation in morning glory and soybean. Plant Physiol. 1978 Feb;61(2):291–295. doi: 10.1104/pp.61.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Housley T. L. The Retention of Water-soluble Compounds during Freeze-Substitution and Microautoradiography. Plant Physiol. 1972 Feb;49(2):166–171. doi: 10.1104/pp.49.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. Structure of functional soybean sieve elements. Plant Physiol. 1975 Nov;56(5):555–569. doi: 10.1104/pp.56.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Sucrose Translocation in the Sugar Beet. Plant Physiol. 1965 Jul;40(4):685–690. doi: 10.1104/pp.40.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]