Key Points
Question
Is programmed death ligand 1 (PD-L1) associated with improved outcomes among patients with urothelial carcinoma treated with immune checkpoint inhibitors (ICIs)?
Findings
In this systematic review and meta-analysis of 14 phase 1 to 3 studies, an association emerged between PD-L1 status and overall response rate, favoring patients with PD-L1–positive tumors. There was a significant reduction in the risk of disease progression and death for patients with PD-L1–positive tumors compared with patients with PD-L1–negative tumors.
Meaning
This study suggests that PD-L1–positive tumors are associataed with improved prognosis among patients with metastatic urothelial carcinoma who recieve ICIs, but PD-L1 is not likely to be a predictive biomarker of ICI response.
Abstract
Importance
Immune checkpoint inhibitors (ICIs) have broadened the metastatic urothelial carcinoma (mUC) therapeutic scenario. The association of programmed death ligand 1 (PD-L1) with response and survival in patients treated with ICIs is still controversial.
Objectives
To evaluate the association of PD-L1 with response rate and overall survival among patients with mUC treated with ICIs.
Data Sources
PubMed, Embase, American Society of Clinical Oncology and European Society for Medical Oncology Meeting Libraries, and Web of Science were searched up to December 10, 2023.
Study Selection
Two authors independently screened the studies. Included studies were randomized and nonrandomized clinical trials enrolling patients with mUC receiving ICIs with available overall survival (OS), progression-free survival (PFS), or overall response rate (ORR) data, separated between patients with PD-L1–positive and –negative tumors.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was followed. Two reviewers independently extracted data. Fixed- or random-effects models were used depending on the heterogeneity among the studies.
Main Outcomes and Measures
Primary outcomes were odds ratios (ORs) for ORR and hazard ratios (HRs) for OS, comparing patients with PD-L1–positive tumors and patients with PD-L1–negative tumors. Secondary outcomes were the PFS HR between patients with PD-L1–positive and –negative tumors and OS HR between ICI arms and non-ICI arms of only randomized clinical trials.
Results
A total of 14 studies were selected, comprising 5271 patients treated with ICIs (2625 patients had PD-L1–positive tumors). The ORR was 13.8% to 78.6% in patients with PD-L1–positive tumors and 5.1% to 63.2% in patients with PD-L1–negative tumors, with an association between PD-L1 status and ORR favoring patients with PD-L1–positive tumors (OR, 1.94; 95% CI, 1.47-2.56; P < .001). Median OS ranged from 8.4 to 24.1 months in patients with PD-L1–positive tumors and from 6.0 to 19.1 months in patients with PD-L1–negative tumors. The pooled HR showed a significant reduction for patients with PD-L1–positive tumors compared with those with PD-L1–negative tumors in the risk of death (HR, 0.71; 95% CI, 0.57-0.89; P = .003) and risk of progression (HR, 0.55; 95% CI, 0.44-0.69; P < .001) when ICIs were administered. PD-L1 is not likely to be a predictive biomarker of ICI response.
Conclusions and Relevance
This systematic review and meta-analysis suggests that PD-L1 expression is associated with improved ORR, OS, and PFS for patients with mUC who receive ICIs, but it is unlikely to be useful as a predictive biomarker. Developing predictive biomarkers is essential to select patients most likely to benefit from ICIs and avoid toxic effects and financial burden with these agents.
This systematic review and meta-analysis evaluates the association of programmed death ligand 1 (PD-L1) with overall response rate and overall survival among patients with metastatic urothelial carcinoma treated with immune checkpoint inhibitors.
Introduction
Urothelial carcinoma (UC) accounts for almost 600 000 new cases and over 200 000 deaths per year, representing the ninth most common malignant neoplasm worldwide.1 Although approximately 70% of UCs are diagnosed at a nonmuscle-invasive stage, most patients develop distant metastases, with poor survival.2 In the metastatic setting, platinum-based chemotherapy represents the first-line standard of care. Although several chemotherapy agents have been studied, low response rates (8%-30%) and survival (7-10 months) have been reported after failure of platinum-based chemotherapy.3
Immune checkpoint inhibitors (ICIs) were added to the therapeutic armamentarium of metastatic UC (mUC) starting in 2016: 2 programmed cell death 1 (PD1) inhibitors (nivolumab and pembrolizumab) and 3 PD-ligand 1 (PD-L1) inhibitors (atezolizumab, durvalumab, and avelumab) were effective in mUC progressing after platinum-containing chemotherapy. However, in untreated patients, results were not satisfactory compared with platinum-based regimens. More recently, the JAVELIN Bladder 100 study changed the treatment paradigm of mUC after avelumab maintenance was associated with increased survival in patients who responded to platinum-based chemotherapy.4
Until now, identifying the best candidates for immunotherapy represents one of the most critical unmet needs in this field. Programmed death ligand 1 (B7-H1 or CD274) is 1 of the 2 ligands of PD1 and a member of the B7 family of type I transmembrane protein receptors.5 Historically, it has been the first biomarker associated with better outcomes with ICIs for different solid tumors. The role of PD-L1 expression as a biomarker for identifying patients with mUC who are most likely to benefit from ICIs is still controversial.6,7 Therefore, our systematic review and meta-analysis aimed to evaluate the association of PD-L1 with response rate and survival in patients with mUC.
Methods
Data Retrieval Strategies
We conducted a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.8 Embase, PubMed, American Society of Clinical Oncology and European Society for Medical Oncology Meeting library databases, and Web of Science were searched. Publications available up to December 10, 2023, were analyzed. The following terms were used: [urothelial cancer, urothelial carcinoma, bladder cancer, bladder carcinoma] and [avelumab, durvalumab, atezolizumab, nivolumab, pembrolizumab]. The search was restricted to the English language (eTable 1 in Supplement 1).
Population, Intervention, Comparison, and Outcomes
Patients with mUC treated with ICIs were included in our meta-analysis. To test the association of PD-L1 status with prognosis, the experimental group included patients with PD-L1–positive tumors, with criteria for PD-L1 positivity (cutoff and detection methods) established by the individual studies. This group was compared with PD-L1–negative patients. Overall survival (OS) and overall response rate (ORR) were the primary outcomes. Progression-free survival (PFS) was the secondary outcome, alongside OS between ICI arms and non-ICI arms. No correction for multiplicity was applied.
Inclusion Criteria
Two authors (B.A.M. and G.R.) independently screened the studies. Decisions regarding contentious studies were made in consultation with a third author (M.D.). The inclusion criteria were (1) studies enrolling patients with mUC; (2) use of ICIs as single agents or in combination; (3) availability of OS, PFS, or ORR data; and (4) data of the reported outcomes separated between patients with PD-L1–positive and PD-L1–negative tumors. Studies not reporting the selected outcome for patients with PD-L1–positive and PD-L1–negative tumors, observational studies, animal studies, and studies with a sample size of less than 10 patients were excluded. The analysis was limited to phase 1 to 3 clinical trials (eTable 2 in Supplement 1).
Data Extraction
Two authors (B.A.M. and G.R.) extracted the relevant data, including trial name and author, publication year, phase, and line of therapy; ICI type and dosage and presence of a control arm; PD-L1 assay and cutoff; total sample size, number of patients with PD-L1–positive and PD-L1–negative tumors, and primary outcomes; and ORR, OS, and PFS in the overall population, patients with PD-L1–positive tumors, and patients with PD-L1–negative tumors.
Statistical Analysis
Study quality was assessed using the Cochrane tools to assess the bias risk (ROB-2 [Risk of Bias 2] for randomized trials, ROBINS-I [Risk of Bias in Non-randomised Studies—of Interventions] for nonrandomized trials).9 The statistical analysis was performed with Revman, version 5.4 (Cochrane Training). The summary estimates were generated using the generic inverse variance and a fixed-effect model (Mantel-Haenszel method) or a random-effect model (DerSimonian-Laird method) depending on the absence or presence of heterogeneity. Statistical heterogeneity was assessed with the Q test and the I2 statistic.10,11 I2 values of 25%, 50%, and 75% were considered for low, moderate, and high heterogeneity, respectively.12 When I2 was less than 40%, the fixed-effects model was used; otherwise, the random-effects model was used. To test the association of PD-L1 status with prognosis, odds ratios (ORs) with 95% CIs for ORR and hazard ratios (HRs) for OS and PFS, comparing patients with PD-L1–positive tumors, and those with PD-L1–negative tumors were calculated for each study. A value of P < .05 was considered statistically significant, and all tests were 2-sided. We planned subgroup analyses according to the line of therapy (first, maintenance, second, and beyond), single agent vs combination (anti-PD1 or –PD-L1 plus anticytotoxic T-lymphocyte–associated protein 4 [CTLA4] or chemotherapy or enfortumab vedotin), and according to ICI mechanism of action (anti-PD1 or anti–PD-L1). A sensitivity analysis was performed to assess the stability of the global estimates by moving away 1 study at a time. Moreover, randomized clinical trials were included to assess HR for OS in patients with PD-L1–positive tumors treated with ICIs compared with other treatments in patients with PD-L1–negative tumors.
Results
Characteristics of the Included Studies
After the literature search and the inclusion and exclusion criteria screening, a total of 14 studies were selected (eFigure 1 in Supplement 1).13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 There were 7 phase 1 or 2 trials and 7 randomized phase 3 trials. IMvigor210 consisted of 2 cohorts: cohort 1 included pretreated patients and cohort 2 enrolled untreated patients.13 In 5 studies, ICIs were administered to pretreated patients.14,15,16,17,18,19 In 8 trials, ICIs were used as first-line treatment and, in 1 case, avelumab was administered as maintenance after at least stability to platinum-based frontline chemotherapy.20,21,22,23,24,25,26,27 A total of 8 studies used the anti-PD1 agents nivolumab (n = 3) or pembrolizumab (n = 5).14,15,16,17,22,23,24,26,27 In 6 studies, PD-L1 inhibitors were administered (avelumab, 2; atezolizumab, 2; or durvalumab, 2).13,18,19,20,21,25,26 In 2 studies, the anti-CTLA4 agents ipilimumab and tremelimumab were added to nivolumab (second line) and durvalumab (first line), respectively; in the first line, atezolizumab, nivolumab, and pembrolizumab were combined with chemotherapy in 3 studies, and pembrolizumab was combined with enfortumab vedotin in 1 study.17,23,24,25,26,27
Overall, 5271 patients were treated with ICIs; 2625 had PD-L1 positive tumors, representing 28.5% to 82.0% of the sample. Overall response rate was the primary end point in 5 studies; it was defined as the rate of complete responses and partial responses to treatment.13,15,16,17,18,20,22 Overall survival, defined as the time from starting treatment to the patient’s death, was the primary end point in 5 studies.14,21,23,25,26 In 5 studies, PFS, defined as the time from randomization to disease progression or patients’ death, whichever occurred first, was explored as a coprimary end point with OS14,21,23,26,27 (eTable 3 in Supplement 1). The main characteristics of included studies and efficacy data are listed in the Table.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28
Table. Characteristics of the Included Studies.
| Source | Trial name | Phase | Sample size | ORR (95% CI), % | mOS (95% CI), mo | PFS (95% CI), mo | PD-L1 cutoff, cell types (detection platform) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, No. | PD-L1 positive, No. (%) | PD-L1 negative, No. (%) | Overall | PD-L1 positive | PD-L1 negative | Overall | PD-L1 positive | PD-L1 negative | Overall | PD-L1 positive | PD-L1 negative | ||||
| Second-line therapy | |||||||||||||||
| Rosenberg et al,13 2016 | IMvigor 210 (cohort 2) | 2 | 310 | 207 (66.7) | 103 (33.3) | 14.8 (11-19) | 18.0 (13.0-24.0) | 8.0 (3.0-15.0) | 7.9 (6.6-9.3) | 8.8 (7.1-10.6) | NA | 2.1 (2.1-2.1) | 2.9 (2.1-4.1) | NA | 5%, IC (Ventana SP142) |
| Bellmunt et al,14 2017 | KEYNOTE-045 | 3 | 260 | 74 (28.5) | NA | 21.1 (16.4-26.5) | 21.6 (12.9-32.7) | NA | 10.3 (8.0-12.3) | NA | NA | 2.1 (2.0-2.2) | NA | NA | CPS ≥10, TC and IC (Dako 22C3) |
| Sharma et al,15 2017 | CheckMate 275 | 2 | 265 | 122 (46) | 143 (54) | 19.6 | 23.8 (16.5-32.3) | 16.1 (10.5-23.1) | 8.6 (6.1-11.3) | 11.9 (9.1-19.1) | 6.0 (4.4-8.1) | 1.9 (1.9-2.3) | 3.5 (1.9-3.7) | 1.9 (1.7–2.0) | 5%, amended to 1%, TC (Dako 28.8) |
| Sharma et al,16 2016 and Sharma et al,17 2019 | CheckMate 032 | 1/2 | 78 | 26 (33.3) | 43 (55.1) | N3: 25.6 (16.4-37.8) | 26.9 (11.6-47.8) | 25.6 (13.5-41.2) | 9.9 (7.3-21.1) | 12.9 (2.8-NR) | 10.4 (6.5-26.0) | 2.8 (1.5-5.3) | 2.7 (1.2-10.8) | 2.8 (1.4-5.9) | 1%, TC (Dako 28.8) |
| 104 | 31 (29.8) | 56 (53.8) | N3+I1: 26.9 (18.7-36.5) | 35.5 (19.2-54.6) | 25.0 (14.5-38.4) | 7.4 (5.6-11.0) | 10.8 (4.6-NR) | 7.4 (5.0-10.6) | 2.6 (1.4-3.9) | 3.4 (1.4-11.0) | 2.7 (1.4-3.9) | ||||
| 92 | 31 (33.7) | 42 (45.7) | N1+I3: 38.0 (28.1-48.8) | 58.1 (39.1-75.5) | 23.8 (12.1-39.5) | 15.3 (10.1-27.6) | 24.1 (10.2-NR) | 14.9 (5.6-27.6) | 4.9 (2.7-6.6) | 6.6 (3.8-27.6) | 4.3 (1.5-6.4) | ||||
| Powles et al,18 2017 | STUDY 1108 | 1/2 | 191 | 98 (51.3) | 79 (41.4) | 17.8 (12.7-24.0) | 27.6 (19.0-37.5) | 5.1 (1.4-12.5) | 18.2 (8.1-NR) | 20.0 (11.6-NR) | 8.1 (3.1-NR) | 1.5 (1.4-1.9) | 2.1 (1.4-2.8) | 1.4 (1.3-1.5) | 25%, TC/IC (Ventana SP263) |
| Patel et al,19 2018 | NCT01772004 (JAVELIN mUC EC) | 1b | 249 | 85 (34.1) | 135 (54.2) | 16.5 (12.1-21.8) | 23.8 (15.2-34.3) | 12.3 (7.2-19.2) | 7.0 (5.9-8.5) | 8.4 (6.0-11.3) | 6.5 (5.3-10.1) | 1.6 (1.4-2.7) | 2.2 (1.4-4.1) | 1.5 (1.4-2.4) | 5%, TC (Dako 73-10) |
| First-line therapy | |||||||||||||||
| Balar et al,20 2017 | IMvigor 210 (cohort 1) | 2 | 119 | 80 (67.0) | 39 (33.0) | 23.0 (16.0-31.0) | 24.0 (15.0-35.0) | 21.0 (9.0-36.0) | 15.9 (10.4-NR) | 12.3 (6.0-NR) | 19.1 (9.8-NR) | 2.7 (2.1-4.2) | 4.1 (2.3-11.8) | 2.6 (2.1-5.7) | 5%, IC (Ventana SP142) |
| Galsky et al,21 2020 | IMvigor 130 | 3 | 451 | 303 (67.0) | 148 (33.0) | Atezolizumab + CT: 47.0 (43.0-52.0) | NA | NA | 16 (13.9-18.9) | 23.6 | 14.2 | 8.2 (6.5-8.3) | 8.6 | 6.5 | 1% (IC1), 5% (IC2/3) (Ventana SP142) |
| 352 | 248 (68.0) | 114 (31.0) | Atezolizumab: 23.0 (19.0-28.0) | 34.0 (28.0-50.0) | NA | 15.7 (13.1-17.8) | NR (17.7-NR) | 13.5 (11.1-16.4) | NA | NA | NA | NA | |||
| Balar et al,22 2017 | KEYNOTE-052 | 2 | 317 | 219 (82.0) | 46 (17.0) | 28.6 (24.1-33.5) | 47.3 (37.7-57.0) | 20.3 (15.5-25.8) | 11.3 (9.7-13.1) | 18.5 (12.2-28.5) | 9.7 (7.6-11.5) | 2.2 (2.1-3.4) | NA | NA | CPS ≥10%, TC and IC (Dako 22C3) |
| Powles et al,23 2021 | KEYNOTE-361 | 3 | 351 | 160 (52.0) | 147 (48.0) | Pembrolizumab + CT: 54.7 | NA | NA | 17.0 (14.5-19.5) | NA | NA | 8.3 (7.5-8.5) | NA | NA | CPS ≥10%, TC and IC (Dako 22C3) |
| 307 | 159 (45.0) | 192 (55.0) | Pembrolizumab: 30.3 | NA | NA | 15.6 (12.1-17.9) | 16.1 (13.6-19.9) | NA | 3.9 | NA | NA | NA | |||
| Rosenberg et al,24 2020 | EV-103 | 1b/2 | 45 | 14 (31.1) | 19 (42.2) | 73.3 | 78.6 | 63.2 | NR | NA | NA | 12.3 | NA | NA | CPS ≥10%, TC and IC (Dako 22C3) |
| Powles et al,25 2020 | DANUBE | 3 | 346 | 209 (60.0) | 137 (40.0) | Durvalumab: 26.9 | 29.1 | 23.8 | 13.2 (10.3-15) | 14.4 (10.4-17.3) | 10.9 (8.0-14.8) | 2.3 (1.9-3.5) | 2.4 (1.9-3.7) | 1.9 | 25% TC or 25% IC + 1% TC (Ventana SP263) |
| 342 | 205 (60.0) | 137 (40.0) | Durvalumab + tremelimumab: 36.6 | 47.0 | 21.5 | 15.1 (13.1-18) | 17.9 (14.8-24.2) | 11.8 (8.9-15.8) | 3.7 (3.4-3.8) | 4.1 (3.6-5.7) | 2.9 | NA | |||
| van der Heijden et al,26 2023 | CheckMate 901 | 3 | 304 | 111 (36.5) | 193 (63.5) | 57.6 | NA | NA | 21.7 (18.6-26.4) | NA | NA | 7.9 (7.6-9.5) | NA | NA | 1%, TC (Dako 28.8) |
| Powles et al,27 2023 | EV-302/ KEYNOTE-A39 | 3 | 438 | 254 (58.0) | 184 (42.0) | 67.7 (63.1-72.1) | NA | NA | 31.5 (25.4-NR) | NA | NA | 12.5 (10.4-16.6) | 31.5 (25.4-NR) | NR (22.3-NR) | CPS ≥10, TC and IC (Dako 22C3) |
| First-line maintenance | |||||||||||||||
| Powles et al,28 2020 | JAVELIN Bladder 100 | 3 | 350 | 189 (54.0) | 139 (40.0) | 9.7 (6.8-13.3) | 13.8 (9.2-19.5) | NA | 21.4 (18.9-26.1) | NR (20.3-NR) | 18.8 (13.3-22.5) | 3.7 (3.5-5.5) | 5.7 (3.7-7.4) | 3.0 (2.0-3.7) | 25%, TC/IC (Ventana SP263) |
Abbreviations: CPS, combined positive score; CT, chemotherapy; IC, immune cells; mOS, median overall survival; NA, not available; NR, not reached; N1+I3, nivolumab 1 mg/kg + ipilimumab 3 mg/kg; N3, nivolumab 3 mg/kg; N3+I1, nivolumab 3 mg/kg + ipilimumab 1 mg/kg; ORR, overall response rate; PD-L1, programmed death ligand 1; PFS, progression-free survival; TC, tumor cells.
ORR of Patients With PD-L1–Positive vs PD-L1–Negative Tumors
Data for ORR in patients with PD-L1–positive vs PD-L1–negative tumors were available in 10 studies.13,14,15,16,17,18,19,20,22,24,25,26 Among the 3068 treated patients, 1590 had PD-L1 positive tumors and 1125 had PD-L1 negative tumors; information regarding PD-L1 status was unavailable in 353 cases. The ORR ranged from 13.8% to 78.6% in patients with PD-L1–positive tumors and from 5.1% to 63.2% in patients with PD-L1–negative tumors (Table).13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28
We found an association between PD-L1 status and ORR favoring patients with PD-L1–positive tumors (OR, 1.94, 95% CI, 1.47-2.56; P < .001; random-effects) (Figure 1). Low heterogeneity was observed among the studies (I2 = 45%; P = .03).
Figure 1. Overall Response Rate for Patients With Programmed Death Ligand 1 (PD-L1)–Positive vs PD-L1–Negative Tumors.
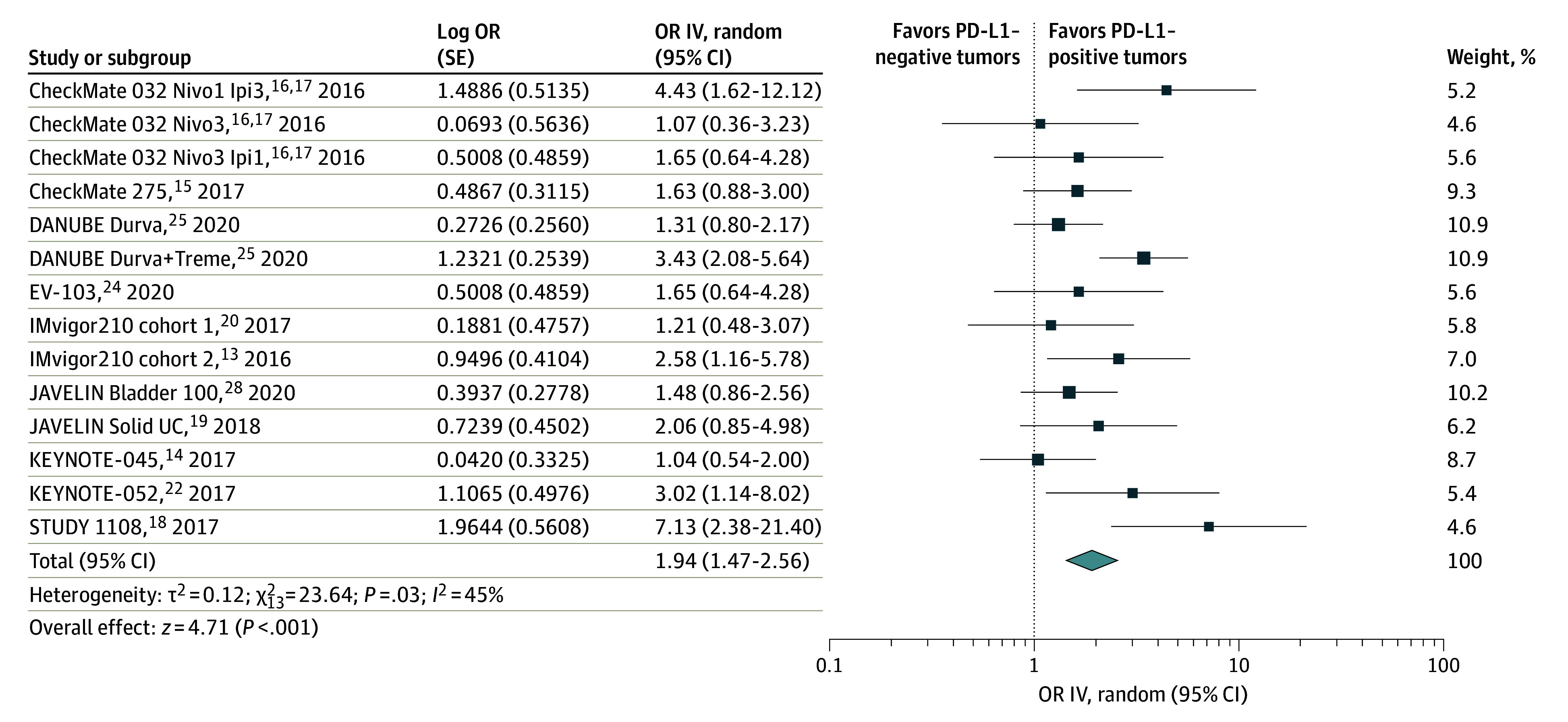
The diamond indicates the pooled estimate, derived from the generic inverse variance (IV) and a random-effects model. Durva indicates durvalumab; Ipi, ipilimumab; Nivo, nivolumab; OR, odds ratio; and Treme, tremelimumab.
OS of Patients With PD-L1–Positive vs PD-L1–Negative Tumors
Data for OS were available from 12 studies and 4909 patients, of whom 2267 had PD-L1–positive and 1888 had PD-L1–negative tumors.13,14,15,16,17,18,19,20,21,23,25,26,27,28 Among patients with PD-L1–positive tumors, median OS ranged from 8.4 to 24.1 months. Among patients with PD-L1–negative tumors, median OS ranged from 6.0 to 19.1 months (Table).13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28
The pooled HR showed a significant reduction in the risk of death for patients with PD-L1–positive tumors compared with those with PD-L1–negative tumors (HR, 0.71; 95% CI, 0.57-0.89; P = .003; random-effects) (Figure 2). There was a moderate heterogeneity among the studies (I2 = 61%; P < .001).
Figure 2. Overall Survival of Patients With Programmed Death Ligand 1 (PD-L1)–Positive vs PD-L1–Negative Tumors.
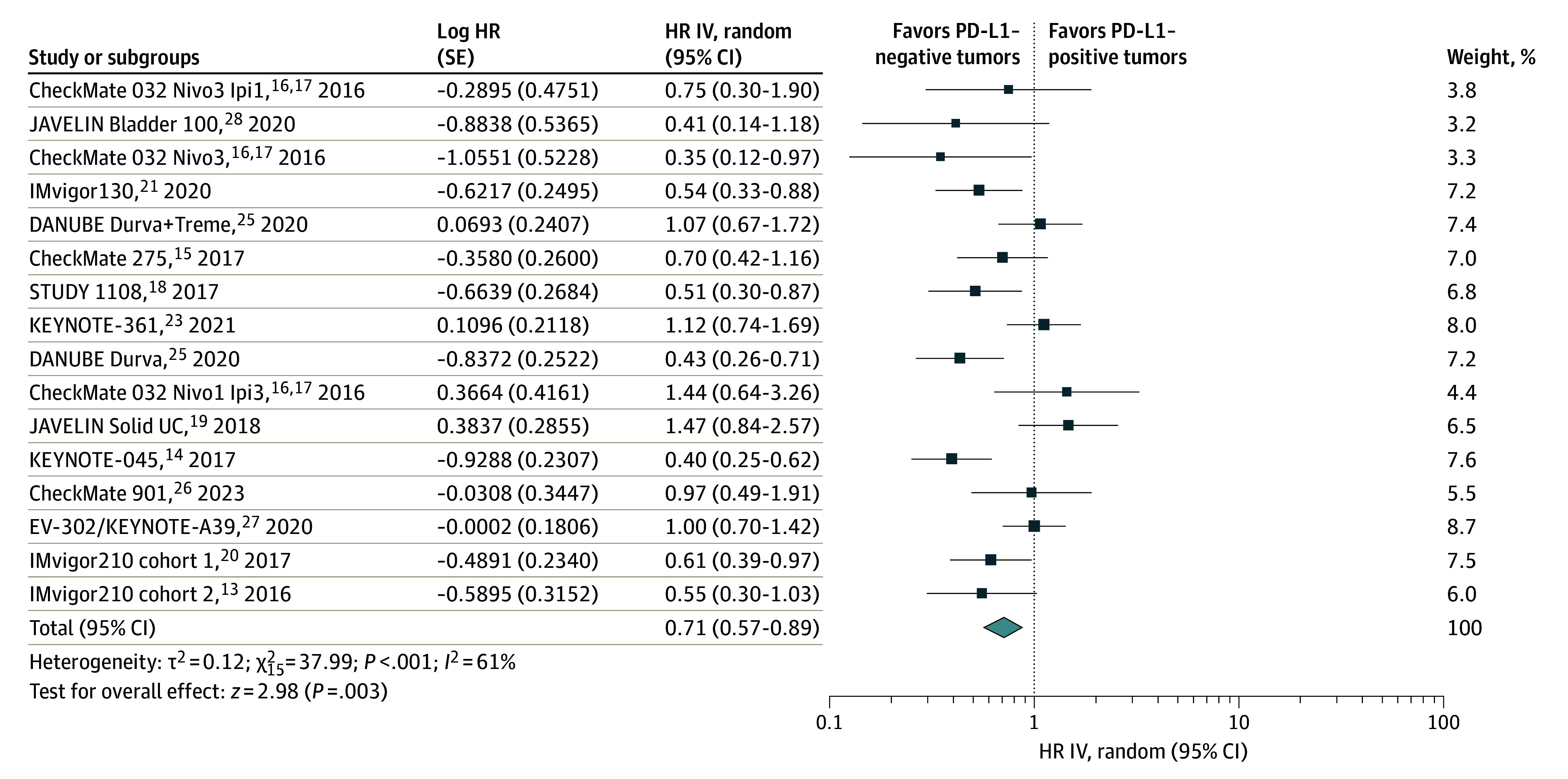
The diamond indicates the pooled estimate, derived from the generic inverse variance (IV) and a random-effects model. Durva indicates durvalumab; HR, hazard ratio; Ipi, ipilimumab; Nivo, nivolumab; and Treme, tremelimumab.
PFS of Patients With PD-L1–Positive vs PD-L1–Negative Tumors
Six studies reported PFS for a total of 1638 patients (884 with PD-L1 positive tumors and 754 with PD-L1 negative tumors).16,17,18,19,26,27,28 Compared with patients with PD-L1–negative tumors, those with PD-L1–positive tumors had improved PFS (HR, 0.55; 95% CI, 0.44-0.69; P < .001; fixed-effects) (Figure 3). No heterogeneity was observed (I2 = 0%; P = .62).
Figure 3. Progression-Free Survival of Patients With Programmed Death Ligand 1 (PD-L1)–Positive vs PD-L1–Negative Tumors.
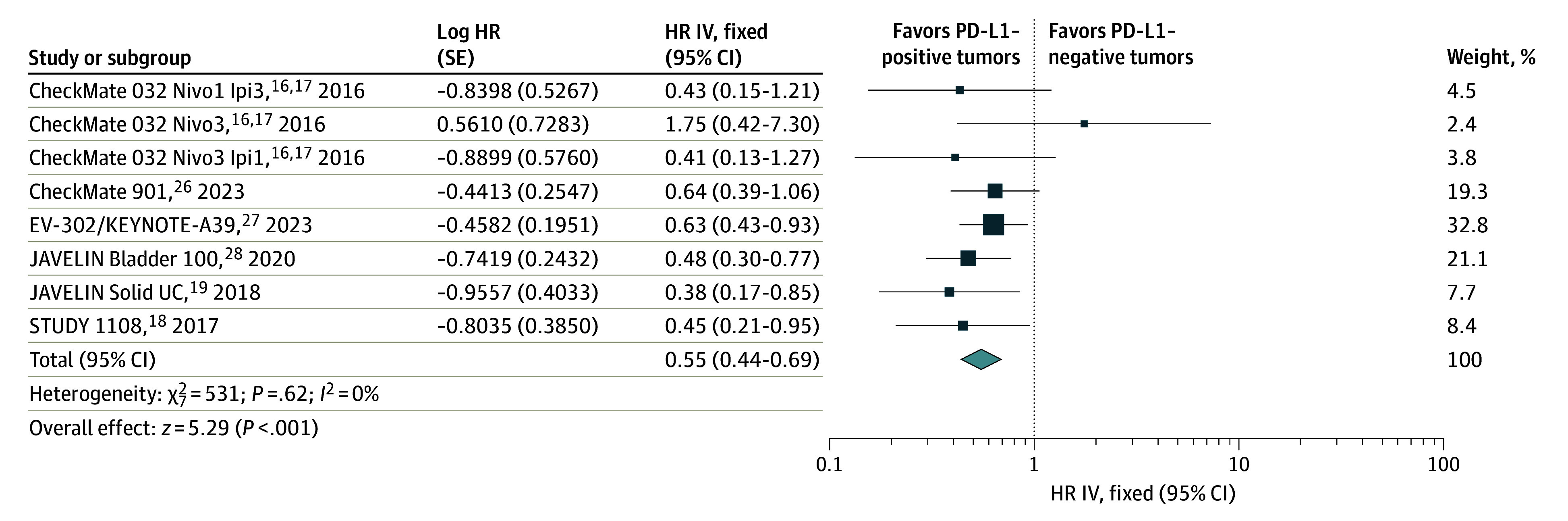
The diamond indicates the pooled estimate, derived from the generic inverse variance (IV) and a fixed-effects model. HR indicates hazard ratio; Ipi, ipilimumab; and Nivo, nivolumab.
Subgroup Analyses
We performed subgroup analyses for ORR and OS to test the source of heterogeneity, considering ICIs’ mechanisms of action, lines of therapy, and single agents vs combination (eTables 4 and 5 in Supplement 1). No differences were found in terms of ICIs’ mechanisms of action (ORR for anti-PD1 vs anti–PD-L1: OR, 1.83 [95% CI, 1.39-2.40]; P = .69; OS for anti-PD1 vs anti–PD-L1: OR, 0.68 [95% CI, 0.55-0.84]; P = .92), lines of therapy (ORR for first vs second vs maintenance: OR, 1.83 [95% CI, 1.39-2.40]; P = .73; OS for first vs second vs maintenance: 0.68 [95% CI, 0.55-0.84]; P = .07 for OS), or single agents vs combinations (ORR: OR, 1.94 [95% CI, 1.47-2.56]; P = .08; OS: OR, 0.69 [95% CI, 0.55-0.86]; P = .29).
Association of PD-L1 With OS Between ICIs and Non-ICIs in mUC
We subsequently aimed to analyze the association of PD-L1 with OS between ICIs and non-ICIs from randomized clinical trials. Six studies were selected comparing ICIs with different treatments (chemotherapy or best supportive care) in the control arm. No differences emerged in the PD-L1–positive compared with the PD-L1–negative subgroup (HR, 0.79 [95% CI, 0.69-0.91]; P = .93) for survival with ICIs compared with other treatments (Figure 4).
Figure 4. Overall Survival of Patients With Metastatic Urothelial Carcinoma Receiving Immune Checkpoint Inhibitors (ICIs) vs Non-ICIs by Programmed Death Ligand 1 (PD-L1) Status.
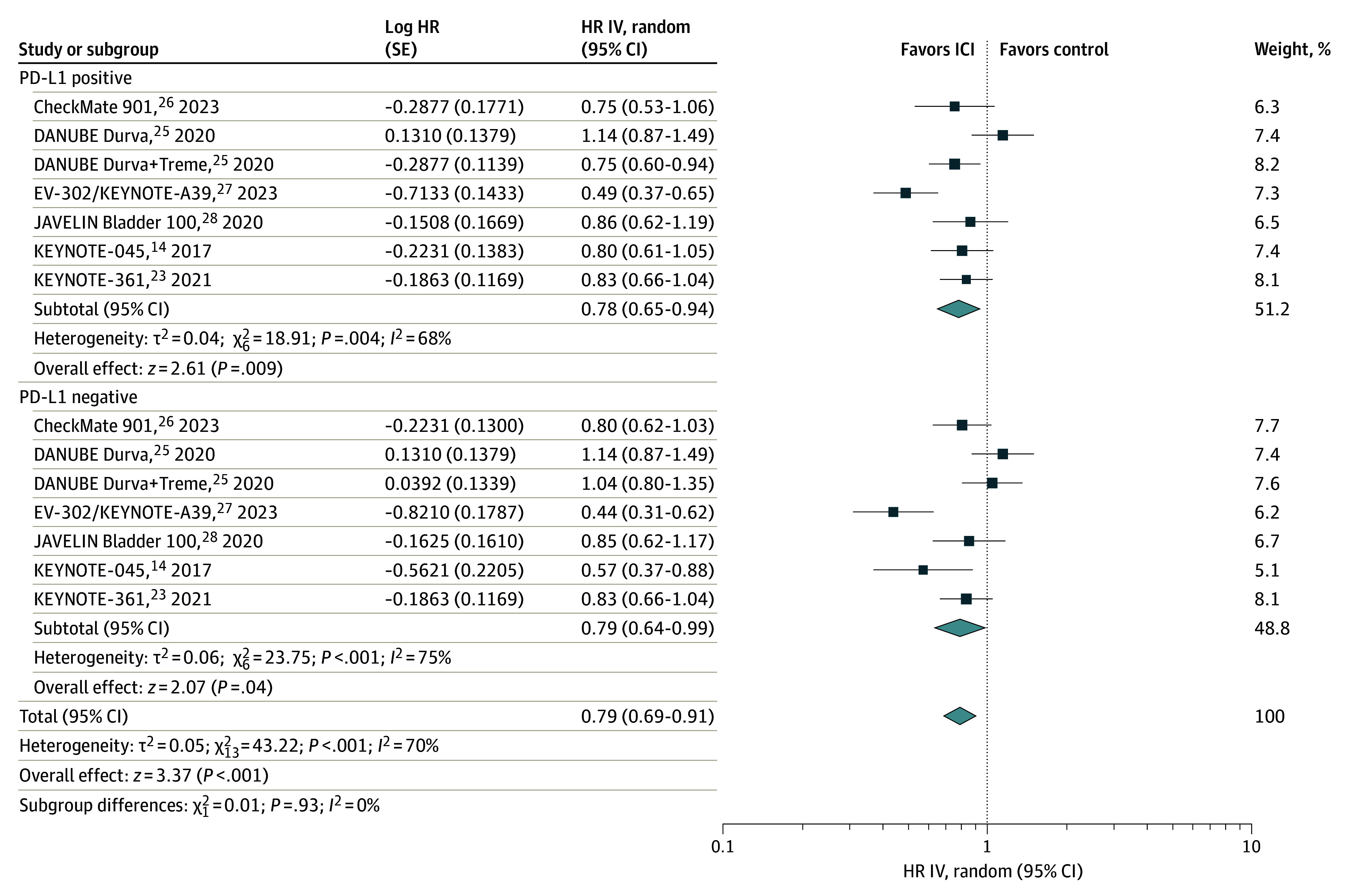
The diamonds indicate the pooled estimates, derived from the generic inverse variance (IV) and a random-effects model. Durva indicates durvalumab; HR, hazard ratio; and Treme, tremelimumab.
Risk of Bias of the Included Studies
Globally, the quality of the studies was high. Among the nonrandomized studies, the principal bias depended on missing data, whereas performance biases were most frequently detected in randomized clinical trials (eFigure 2A and B in Supplement 1).
Overall, there was a low asymmetry in the distribution of the published studies (eFigure 3A and B in Supplement 1). We performed a sensitivity analysis to test the association of the single studies with the overall results. The global estimates were not changed after removing every single study at a time (eFigure 4A-C in Supplement 1).
Discussion
The results of our systematic review and meta-analysis demonstrate a positive association of PD-L1 with ORR, OS, and PFS in patients with mUC treated with ICIs. However, our results suggest that PD-L1 is not a valid predictive biomarker for patients’ selection in this tumor type.
Urothelial carcinoma accounts for approximately 3% of tumors worldwide. For over 2 decades, platinum-based chemotherapy represented the standard of care in first-line therapy.29,30 Failure to respond to platinum-based first-line chemotherapy often implies a significant physical dysfunction and quality of life impairment. As a result, only 1 of 3 patients reaches further lines of treatment, with single agents ensuring a median survival of approximately 6 months and ORR of less than 10%.31,32 The introduction of ICIs heralds a new era for mUC treatment.4,33 First, pembrolizumab improved survival over chemotherapy in pretreated patients.4,13,14,15,16,17,18,19,33 Subsequently, in the US, pembrolizumab was authorized as first-line treatment for patients with mUC and PD-L1–positive tumors who were ineligible for cisplatin or for any patients with mUC who were ineligible for platinum, regardless of PD-L1 expression.4,20,21,22,23,33,34 In 2020, the US Food and Drug Administration (FDA) granted breakthrough approval to the first-line combination of pembrolizumab and the antibody-drug conjugate enfortumab vedotin for patients who were ineligible for cisplatin, and avelumab was approved by the FDA and European Medical Agency as maintenance treatment after reaching at least disease stability with platinum-based chemotherapy.4,24,26
Developing biomarkers with a prognostic role for the outcomes of patients with mUC is still an unmet need. This issue is particularly relevant if we address the costs of such agents. Programmed death ligand 1 expression has been reported in 20% to 30% of patients with UC, often associated with higher disease stages.35 Moreover, PD-L1 positivity, especially at higher levels of expression, is negatively prognostic for OS and disease-free survival in UC, particularly bladder cancer.36,37 In other reports, high PD-L1 expression on immune cells has been linked to a more favorable prognosis.37 Our systematic review and meta-analysis results for ORR, OS, and PFS indicate a positive prognostic role of PD-L1 when ICIs are administered. This correlation seems independent of the type of ICI, the use of ICIs as single agents or in association with other drugs, and the treatment line (eTables 4 and 5 in Supplement 1).
However, approximately 1 in 5 PD-L1–negative patients were also responsive to ICIs. We cannot ignore these data, especially considering response rates and survival that are far longer than with chemotherapeutic agents in second-line or maintenance settings (Table).13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 Therefore, despite the association with prognosis, it seems unlikely to propose PD-L1 as the only marker to guide the use of ICIs in mUC. However, novel biomarkers such as tumor mutational burden, The Cancer Genome Atlas groups, and genetic and immunologic classifications should be considered. In this regard, fibroblast growth factor receptor (FGFR)–targeting agents were purposed to be potentially synergistic with ICIs, as the FGFR pathway interacts with innate and adaptive immunity.38 Initially, trials have investigated sequential FGFR inhibition after progression with ICI treatment, demonstrating higher responses associated with UC tumor microenvironment modifications, especially on the lymphocyte side.39,40 A good prognostic role has been associated with FGFR, prevalent in the luminal-papillary UC subtype with a less general aggressiveness but a worse response to chemotherapy and lower PD-L1 levels.38,40 Over the prognostic role, such targets offer additive efficacy combined with ICIs; a valid example is the antibody-drug conjugate enfortumab vedotin targeting Nectin-4—a tumor-associated antigen expressed by 97% of UC—that showed an ORR of more than 73% in combination with pembrolizumab in the EV-103 trial, receiving the FDA breakthrough therapy designation for cisplatin-ineligible naive patients with mUC, and is a practice-changing candidate after the EV-302/KEYNOTE-A39 results.28,41,42
Limitations
Our meta-analysis has some limitations. A significant limitation is the heterogeneity between the studies. First and foremost, the studies assayed PD-L1 using tumor cells, immune cells, or both to assess PD-L1, and even the cutoff for defining PD-L1 positivity differed broadly. Four PD-L1 assays have been used in mUC clinical trials (Table).13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 STUDY 1108 with durvalumab used the Ventana SP263 system, considering PD-L1 positivity on tumor or immune cells (cutoff, 25%), with a difference for ORR between patients with PD-L1–negative tumors that reached only 5.1% and a difference for ORR between patients with PD-L1–positive tumors of 27.8%; similarly, OS ranged from 8.1 to 20.0 months between patients with PD-L1–negative tumors and those with PD-L1–positive tumors.18 The association was even more relevant in first-line treatment, with an ORR of 47% for durvalumab plus tremelimumab in patients with PD-L1–positive tumors in the DANUBE trial.25 With the same test in the maintenance setting, a more significant effect was observed in patients with PD-L1–positive tumors (median OS not reached), and survival was improved compared with best supportive care in PD-L1–negative patients (18.8 months).26 IMvigor210 and IMvigor130 used the Ventana SP142 platform, evaluating the qualitative immunohistochemical expression of PD-L1 on tumor-infiltrating immune cells, with a cutoff of 5% in IMvigor210 and 1% in Imvigor130. Whereas PD-L1 positivity correlated with a higher ORR in second-line treatment, similar percentages were detected in first-line treatment between patients with PD-L1–positive and PD-L1–negative tumors.13,20,21 In the CheckMate studies, assessing PD-L1 through the Dako 28.8 system considering expression on tumor cells, the ORR and OS improvement was more relevant in patients with PD-L1–positive tumors with nivolumab combination treatments.15,16,17,27 Responses were almost doubled in patients with PD-L1–positive tumors compared with those with PD-L1–negative tumors after ICIs when Dako platforms (22C3, with combined proportion score >10% on tumor and immune cells; 73-10, cutoff 5% on tumor cells) were considered, but the effect on OS was not so relevant with second-line avelumab compared with first-line pembrolizumab.19,22,23 The efficacy results were outperforming for the first-line combination of pembrolizumab and enfortumab vedotin, independent from PD-L1 status.28
The lack of standardization and variability of expression for PD-L1 among tumor samples may have limited our analysis. However, we did not split studies according to the PD-L1 assay, as it has already been reported that a good correlation exists between the platforms and detection methods.43,44,45 This is useful even in the case of multiple test use between laboratories. Another possible limitation could be the time from archival tissue used to detect PD-L1 and the ICI administration. We included both treatment-naive and pretreated patients receiving chemotherapy in the metastatic or perioperative setting. It has already been evidenced that chemotherapy could alter PD-L1 expression.46 Moreover, different samples were used for PD-L1 analysis in the selected trials. For example, the CheckMate 032 trial allowed fresh or archived specimens within 3 months from starting nivolumab treatment, whereas KEYNOTE-045 did not apply restrictions on the samples’ age.14,16,17 A further limitation could be the different PD-L1 expression between primary and metastatic sites or the intratumoral heterogeneity of PD-L1, influenced by factors such as the tumor microenvironment.47 Besides the surface expression, other variables, such as tumor dimension or posttranslational modifications of PD-L1, such as the N-glycosylation, could influence receptor detectability.48 The use of more accurate techniques, such as the evaluation of circulating tumor cells, could help overcome the limitations of the actual PD-L1 testing and allow real-time and longitudinal monitoring of this biomarker.
Another significant limitation is the exclusion of other studies performed in this setting, as data were not grouped for PD-L1 status (eFigure 1 in Supplement 1). Furthermore, most studies were not randomized, lowering the quality of our evidence, and the meta-analysis was carried out with aggregate rather than individual patients’ data. Finally, further data will come from studies in the adjuvant setting; in the CheckMate 274, PD-L1 confirms a prognostic role for disease-free survival both in the intention-to-treat population and in patients with PD-L1–positive tumors (disease-free survival at 6 months: 74.9% in the intention-to-treat population; HR, 0.70 [95% CI, 0.55-0.90]; 74.5% in patients with PD-L1–positive tumors; HR, 0.55 [95% CI, 0.35-0.85]).49
Conclusions
Our systematic review and meta-analysis demonstrates that PD-L1 expression is associated with improved ORR, OS, and PFS in patients with mUC who receive ICIs targeting PD1 and PD-L1. Results are significant for ICIs used both as first-line and second-line treatment. No predictive role of PD-L1 is indicated by our results. The development of predictive biomarkers is of utmost importance to select patients most likely to benefit from ICIs, avoiding toxic effects and financial burden with this type of treatment and justifying routine biomarkers analysis in the clinical practice.
eTable 1. Search Strings for the Used Electronic Databases
eTable 2. PICOS Structure for Study Selection
eFigure 1. PRISMA Flowchart of the Selection Process
eTable 3. Baseline Characteristics of the Included Studies
eTable 4. Subgroup Analyses for ORR
eTable 5. Subgroup Analyses for OS
eFigure 2. Risk of Bias Tools for (A) Not-Randomized (ROBINS-I) and (B) Randomized Studies (ROB-2)
eFigure 3. Funnel Plot of Selected Studies for (A) ORR, (B) OS, (C) PFS
eFigure 4. Sensitivity Analysis for (A) ORR, (B) OS, and (C) PFS by Removing One Study at a Time
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96-108. doi: 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Cathomas R, Lorch A, Bruins HM, et al. ; EAU Muscle-invasive, Metastatic Bladder Cancer Guidelines Panel . The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81(1):95-103. doi: 10.1016/j.eururo.2021.09.026 [DOI] [PubMed] [Google Scholar]
- 4.Maiorano BA, De Giorgi U, Ciardiello D, et al. Immune-checkpoint inhibitors in advanced bladder cancer: seize the day. Biomedicines. 2022;10(2):411. doi: 10.3390/biomedicines10020411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26(4):812-817. doi: 10.1093/annonc/mdv009 [DOI] [PubMed] [Google Scholar]
- 7.Ghate K, Amir E, Kuksis M, et al. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: a meta-analysis. Cancer Treat Rev. 2019;76:51-56. doi: 10.1016/j.ctrv.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. doi: 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 11.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101-129. doi: 10.2307/3001666 [DOI] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators . Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590-1598. doi: 10.1016/S1470-2045(16)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Siefker-Radtke A, de Braud F, et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol. 2019;37(19):1608-1616. doi: 10.1200/JCO.19.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64. doi: 10.1016/S1470-2045(17)30900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balar AV, Galsky MD, Rosenberg JE, et al. ; IMvigor210 Study Group . Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galsky MD, Arija JÁA, Bamias A, et al. ; IMvigor130 Study Group . Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547-1557. doi: 10.1016/S0140-6736(20)30230-0 [DOI] [PubMed] [Google Scholar]
- 22.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492. doi: 10.1016/S1470-2045(17)30616-2 [DOI] [PubMed] [Google Scholar]
- 23.Powles T, Csőszi T, Özgüroğlu M, et al. ; KEYNOTE-361 Investigators . Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931-945. doi: 10.1016/S1470-2045(21)00152-2 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg JE, Flaig TW, Friedlander TW, et al. Study EV-103: preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2020;38:441. doi: 10.1200/JCO.2020.38.6_suppl.441 [DOI] [Google Scholar]
- 25.Powles T, van der Heijden MS, Castellano D, et al. ; DANUBE study investigators . Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574-1588. doi: 10.1016/S1470-2045(20)30541-6 [DOI] [PubMed] [Google Scholar]
- 26.van der Heijden MS, Sonpavde G, Powles T, et al. ; CheckMate 901 Trial Investigators . Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389(19):1778-1789. doi: 10.1056/NEJMoa2309863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powles T, Perez Valderrama B, Gupta S, et al. EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (chemo) in previously untreated locally advanced or metastatic urothelial carcinoma (Ia/mUC). Ann Oncol. 2023;34(suppl 2):S1340. doi: 10.1016/j.annonc.2023.10.106 [DOI] [Google Scholar]
- 28.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. doi: 10.1056/NEJMoa2002788 [DOI] [PubMed] [Google Scholar]
- 29.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068-3077. doi: 10.1200/JCO.2000.18.17.3068 [DOI] [PubMed] [Google Scholar]
- 30.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191-199. doi: 10.1200/JCO.2011.37.3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taarnhøj GA, Johansen C, Pappot H. Quality of life in bladder cancer patients receiving medical oncological treatment; a systematic review of the literature. Health Qual Life Outcomes. 2019;17(1):20. doi: 10.1186/s12955-018-1077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454-4461. doi: 10.1200/JCO.2008.20.5534 [DOI] [PubMed] [Google Scholar]
- 33.Roviello G, Catalano M, Santi R, et al. Immune checkpoint inhibitors in urothelial bladder cancer: state of the art and future perspectives. Cancers (Basel). 2021;13(17):4411. doi: 10.3390/cancers13174411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzman DL, Agrawal S, Ning YM, et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist. 2019;24(4):563-569. doi: 10.1634/theoncologist.2018-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173-1182. doi: 10.1007/s00262-006-0266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen Y, Chen Y, Duan X, et al. The clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Clin Exp Med. 2019;19(4):407-416. doi: 10.1007/s10238-019-00572-9 [DOI] [PubMed] [Google Scholar]
- 37.Zhong Q, Shou J, Ying J, et al. High PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor in urothelial carcinoma. Future Oncol. 2021;17(22):2893-2905. doi: 10.2217/fon-2021-0092 [DOI] [PubMed] [Google Scholar]
- 38.Kacew A, Sweis RF. FGFR3 alterations in the era of immunotherapy for urothelial bladder cancer. Front Immunol. 2020;11:575258. doi: 10.3389/fimmu.2020.575258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loriot Y, Necchi A, Park SH, et al. ; BLC2001 Study Group . Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338-348. doi: 10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Zhang N, Shao J, Wang T, Wang X. Multi-omics perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration in urothelial cancer. J Cancer. 2019;10(3):697-707. doi: 10.7150/jca.28494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heath EI, Rosenberg JE. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol. 2021;18(2):93-103. doi: 10.1038/s41585-020-00394-5 [DOI] [PubMed] [Google Scholar]
- 42.Maiorano BA, Catalano M, Maiello E, Roviello G. Enfortumab vedotin in metastatic urothelial carcinoma: the solution eventually? Front Oncol. 2023;13:1254906. doi: 10.3389/fonc.2023.1254906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. 2017;3(2):256-259. doi: 10.1001/jamaoncol.2016.3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tretiakova M, Fulton R, Kocherginsky M, et al. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod Pathol. 2018;31(4):623-632. doi: 10.1038/modpathol.2017.188 [DOI] [PubMed] [Google Scholar]
- 45.Zajac M, Scott M, Ratcliffe M, et al. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagn Pathol. 2019;14(1):99. doi: 10.1186/s13000-019-0873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDaniel AS, Alva A, Zhan T, et al. Expression of PDL1 (B7-H1) before and after neoadjuvant chemotherapy in urothelial carcinoma. Eur Urol Focus. 2016;1(3):265-268. doi: 10.1016/j.euf.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 47.Kluger HM, Zito CR, Barr ML, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015;21(13):3052-3060. doi: 10.1158/1078-0432.CCR-14-3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YN, Lee HH, Hsu JL, Yu D, Hung MC. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J Biomed Sci. 2020;27(1):77. doi: 10.1186/s12929-020-00670-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102-2114. doi: 10.1056/NEJMoa2034442 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strings for the Used Electronic Databases
eTable 2. PICOS Structure for Study Selection
eFigure 1. PRISMA Flowchart of the Selection Process
eTable 3. Baseline Characteristics of the Included Studies
eTable 4. Subgroup Analyses for ORR
eTable 5. Subgroup Analyses for OS
eFigure 2. Risk of Bias Tools for (A) Not-Randomized (ROBINS-I) and (B) Randomized Studies (ROB-2)
eFigure 3. Funnel Plot of Selected Studies for (A) ORR, (B) OS, (C) PFS
eFigure 4. Sensitivity Analysis for (A) ORR, (B) OS, and (C) PFS by Removing One Study at a Time
Nonauthor Collaborators
Data Sharing Statement


