Abstract
Studies in many systems have led to the model that the human β-globin locus control region (LCR) regulates the transcription, chromatin structure, and replication properties of the β-globin locus. However the precise mechanisms of this regulation are unknown. We have developed strategies to use homologous recombination in a tissue culture system to examine how the LCR regulates the locus in its natural chromosomal environment. Our results show that when the functional components of the LCR, as defined by transfection and transgenic studies, are deleted from the endogenous β-globin locus in an erythroid background, transcription of all β-globin genes is abolished in every cell. However, formation of the remaining hypersensitive site(s) of the LCR and the presence of a DNase I-sensitive structure of the β-globin locus are not affected by the deletion. In contrast, deletion of 5′HS5 of the LCR, which has been suggested to serve as an insulator, has only a minor effect on β-globin transcription and does not influence the chromatin structure of the locus. These results show that the LCR as currently defined is not necessary to keep the locus in an “open” conformation in erythroid cells and that even in an erythroid environment an open locus is not sufficient to permit transcription of the β-like globin genes.
The human β-globin locus provides a model system to study the interplay between chromatin structure and transcriptional regulation. The locus is located on chromosome 11 (11p15.5) and contains five developmentally regulated erythroid cell-specific genes arranged in the order in which they are expressed during development (5′-ɛ-Gγ-Aγ-δ-β-3′) and an upstream regulatory region characterized by five DNase I-hypersensitive sites (HSs; see Fig. 1). By convention, these 5′HSs are denoted 5′HS1, 5′HS2, etc., with 5′HS1 being the most 3′ and located closest to the ɛ-globin gene. The importance of the upstream regulatory region was established by an analysis of the effects of a naturally occurring deletion which removes 5′HS2 to 5′HS5 and 25 kb of additional sequences 5′ of the HSs (Hispanic thalassemia). The chromosome carrying the deletion was transferred from lymphocytes of the thalassemic patient into murine erythroleukemia (MEL) cells to create the thalassemia line T-MEL. It was found that the mutation abolishes transcription of the adult human β-globin gene, prevents formation of 5′HS1 and other HSs throughout the locus, and renders the chromatin of the locus resistant to DNase I, indicative of a “closed” chromatin structure (19). In addition, the replication timing of the locus is changed from early to late in S phase (19) and a different origin of replication is used, even though the normal origin lies more than 50 kb from the site of the deletion (1, 36). The region containing the five HSs was termed the locus control region (LCR), because of its global effects on the locus. In transgenic mice, the LCR permits the expression of linked genes in all lines independent of the integration site (26), and regions with LCR properties have now been found in numerous other genes (reviewed in reference 35).
FIG. 1.
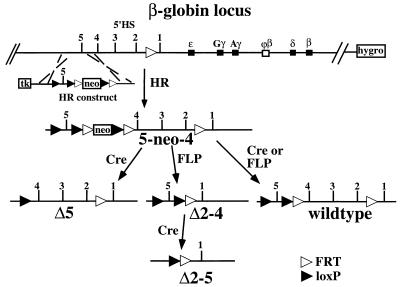
Homologous recombination in DT40 cells on a modified human chromosome 11 with the human β-globin locus. The genes of the β-globin locus and the upstream HSs are indicated, as well as the modification introduced into the locus in an earlier study (13) (see the text). Homologous recombination creates the 5-neo-4 mutation. After transfer of this chromosome into MEL cells, transient expression of the respective site-specific recombinases creates the indicated mutations. Abbreviations: neo, neomycin resistance gene; hygro, hygromycin B resistance gene; tk, thymidine kinase gene for negative selection. FLP recombinase recognition sites are depicted as open triangles; Cre recombinase recognition sites are depicted as solid triangles.
The properties of the β-globin LCR make it an interesting paradigm to study the global regulation of gene expression over large distances and the role that modulation of chromatin structure plays in transcriptional regulation. A variety of functional assays, including transient- and stable-transfection assays in cell lines and transgenic analyses, have been used in attempts to elucidate the function of the β-globin LCR and its component HSs. In general, these studies have demonstrated that the LCR and its component HSs demonstrate much higher activity in erythroid cells than in other cell types (5, 18, 44, 47, 63) and that for any given activity, the full LCR is more active than any individual component, suggesting interaction or additive effects among the individual sites (11, 18, 46, 55, 60). In addition, 5′HS2 and the full LCR are qualitatively equivalent: both function as classical enhancers in transient-transfection assays, increase the number of expressing clones when stably integrated in cell lines, and confer position independence and high-level expression in transgenic mice (5, 18, 41, 45, 58, 60, 63). In contrast to 5′HS2, both 5′HS3 and 5′HS4 have weak or no enhancer activity in transient-transfection assays but are active in colony assays when stably integrated and in transgenic mice (20, 30, 52, 63). Neither 5′HS1 nor 5′HS5 has appreciable activity in these transfection/transgenic-mouse assays (9, 21, 32). The failure of 5′HS1 to demonstrate activity in these assays is consistent with the lack of phenotypes associated with naturally occurring deletions of 5′HS1 in humans (37) and mice (3a).
Although 5′HS5 does not demonstrate enhancer activity in transfection assays, it may play a functional role in the β-globin locus. Hypersensitivity at 5′HS5 is found in many nonerythroid cell lines, whereas 5′HS1 to 5′HS4 are predominantly erythroid cell specific (12, 61), and 5′HS5 is contained on a 2.5-kb restriction fragment which was characterized as one of the potential matrix attachment regions within the β-globin locus (33). In addition 5′HS5 increases the tendency for position-independent transcription of a linked reporter (32, 68) and it has also been reported to block the activation of a promoter when placed between the promoter and an enhancer (39). It has therefore been suggested that 5′HS5 serves as an insulator which shields the locus from neighboring chromatin. This role has also been postulated for HS4 of the chicken β-globin locus, which resembles human 5′HS5 in its broad tissue distribution and its relative position within the locus (8).
While transfection and transgenic-mouse experiments have provided important information about the possible functions of the LCR, endogenous genes are regulated in defined chromosomal locations. Thus, analysis of unintegrated or randomly integrated constructs may overestimate, underestimate, or miss aspects of the activities of cis-regulatory elements, particularly those which influence chromatin, replication, and transcription over large distances. To examine how the LCR components interact to regulate the endogenous β-globin locus, we have developed homologous-recombination (HR) strategies for the mutational analysis of the endogenous human β-globin LCR in cell lines. Specifically, we studied the consequences of deleting those HSs of the LCR which are deleted in the Hispanic thalassemia deletion, as well as the consequences of the deletion of 5′HS5 on the chromatin structure and transcription of the β-globin locus. The results of these experiments show that in an erythroid background, the LCR is not required to maintain the open DNase I-sensitive chromatin structure of the locus but is essential for expression of all β-globin genes.
MATERIALS AND METHODS
Cell lines and culture conditions.
The chicken pre-B-cell line DT40 was cultured in Dulbecco’s modified Eagle’s medium (MEM) with 10% bovine calf serum, 10% tryptone phosphate buffer, and 1% chicken serum. To select for the presence of marked human chromosome 11, hygromycin B (Boehringer Mannheim) was added to 500 μg/ml.
MEL cells were cultured in Dulbecco’s MEM with 10% bovine calf serum, and GM979 cells were cultured in Dulbecco’s MEM with 10% fetal calf serum. To select for maintenance of human chromosome 11, hygromycin B was added to 750 μg/ml.
Plasmid constructs and homologous recombination.
The human chromosome 11 used in this study is the wild-type chromosome which was introduced into MEL cells from lymphocytes of a thalassemia patient, creating the N-MEL line (19). The chromosome expresses β-globin, and all the HSs of the β-globin locus are present. The chromosome was marked in the β-globin locus and transferred into DT40, where it was modified as described by Dieken et al. (13) and reviewed in Results. The homologous recombination construct we used to create deletions within the LCR is illustrated in Fig. 1. It contains the XhoI (−30.0 relative to the ɛ-globin gene)-EcoRI (−19.5) fragment 5′ of 5′HS5 of the β-globin as the 5′ region of homology, with a 34-bp loxP site (56) inserted into the BglII site at −22.3. The 3′ region of homology consists of the β-globin sequences from the EcoRI site at −19.5 to the StyI site at −18.2 between 5′HS5 and 5′HS4. A pgk-promoter neomycin resistance gene cassette flanked by the loxP and 48-bp FRT sites (24, 57) was cloned into the position of the EcoRI site at −19.5. The HR construct also contains a thymidine kinase gene to select against nonhomologous integration events.
HR in DT40 was performed as described by Dieken et al. (13). Briefly, cells were transfected with the linearized homologous recombination construct, incubated in nonselective medium for 24 h, and then plated into microtiter plates in DT40 conditioned medium containing 1.5 mg of G418 per ml and 10 μM ganciclovir. Individual clones were expanded, and DNA was isolated and analyzed by Southern blotting.
Microcell-mediated chromosome transfer and site-specific recombination.
Microcell-mediated chromosome transfer from DT40 to MEL/GM979 cells was performed as described previously (13), except that 1.2 mg of G418 per ml was used to select for cells containing the marked chromosome 11.
The site-specific recombinases Cre and FLP were expressed in MEL/GM979 by using the vectors pMC-Cre (27) and an improved version of pOG44 (7a, 49), respectively. The recombinase expression plasmids were cotransfected with a lacZ reporter plasmid, and individual lacZ-positive cells were sorted into 96-well microtiter plates by using a B-D Vantage fluorescence-activated cell sorter apparatus. Clones were picked and plated in duplicate onto 24-well microtiter plates in medium containing either 750 μg of hygromycin B per ml or 1.2 mg of G418 per ml. DNA from G418-sensitive, hygromycin B-resistant cells was analyzed by Southern blotting.
Analysis of the human chromosome by fluorescence in situ hybridization.
Metaphase spreads from MEL or GM979 lines were prepared for in situ hybridization as described in reference 67. Human chromosome 11 paint (Coatasome 11; Oncor) was detected as described by the vendor. Hybridization to cosmid probes from the human 11p15 region was detected with fluorescently labeled tyramide reagents (NEN Life Sciences Products) as described in reference 64. In most lines, we saw hybridization against the Ha-ras probe (29), located approximately 5 Mb from the β-globin locus toward the telomere, and against the J5-3 probe (40), located approximately 5 Mb from the β-globin locus toward the centromere. The few lines that gave a signal with only one of these probes hybridized with both the N159-H3 probe (29), located approximately 1,300 kb from the β-globin locus toward the telomere, and the N169-F10 probe (29), located approximately 1,600 kb from the β-globin locus toward the centromere.
DNase I analysis.
DNase I digestion in situ for HS analysis was performed as follows. Approximately 107 cells were pelleted, resuspended in RSB buffer (10 mM Tris [pH 7.4], 10 mM NaCl, 5 mM MgCl2) containing 0.5% Nonidet P-40 (NP-40), and divided into 50-μl aliquots. An equal volume of RSB buffer without NP-40 containing 0 to 40 mg of DNase I (Sigma) per ml was added, and digestion was performed for 4 min at room temperature. An equal volume of stop solution (20 mM Tris [pH 7.4], 600 mM NaCl, 10 mM EDTA, 1% sodium dodecyl sulfate, 400 μg of proteinase K per ml) was added, and the lysates were incubated at 37°C overnight. DNA was isolated and analyzed.
For general DNase I sensitivity analysis, nuclei were isolated and digested with DNase I as described previously (19). The following probes were used: EB, a 0.5-kb EcoRI-BglII fragment 5′ of 5′HS5; RN, a 0.39-kb EcoRV-NdeI fragment 2 kb 3′ of 5′HS1; m5′LCR, a 0.7-kb HpaI-KpnI fragment 5′ of 5′HS6 of the murine β-globin LCR; hϕβ, a 7.0-kb EcoRI fragment from the human ϕβ-globin pseudogene; hmyoD, a 0.82-kb EcoRI fragment from the human myoD1 gene (generously provided by S. Tapscott); and 3′β, a 1.9-kb EcoRI-XbaI fragment 3′ of the human β-globin gene.
RT-PCR analyses.
Expression analysis with a reverse transcription-PCR (RT-PCR) assay was performed analogously to that described in references 13 and 16. Briefly, cells were induced with 4 mM HMBA for 4 days. RNA was isolated with Trizol (Gibco BRL), and approximately 400 ng of RNA was used in an RT reaction at 37°C for 30 min with random hexamer primers. The RT reaction mixtures were diluted 1:25, and 2 μl was used in a 25-μl PCR in the presence of [α-32P]dCTP (in addition to cold deoxynucleoside triphosphates) to coamplify human and murine DNAs. The following conditions were used: denaturation at 95°C for 2 min and then 95°C for 30 s, 63°C for 30 s, and 72°C for 1 min. For the HBG1/HBG2 primer pair, 21 cycles were used; for BHGAM1/BHGAM2 and EPEY1/EPEY2, 24 cycles were used.
The following primer pairs were used to analyze β-globin gene expression: HBG1 (GGT GGT CTA CCC TTG GAC CC) and HBG2 (GAT ACT TGT GGG CCA GGG CA) (a 343-bp PCR product was generated from human δ- and β-globin as well as murine βmaj and βmin; a 10-μl aliquot was digested with EcoRI in a 20-μl reaction volume, yielding human digestion fragments of 266 and 77 bp); BHGAM1 (GGA GGA GAA ACC CTG GGA AG) and BHGAM2 (CCC AGG AGC TTG AAG TTC TC) (murine βh1 and human γ-globin signals were coamplified; a 255-bp product was formed, and digestion with PvuII cut the human fragment into 195- and 60-bp fragments); and EPEY1 (TGG AGG TGA AGC CTT GGG) and EPEY2 (AGT CAG CAC CTT CTT GCC) (murine Ey and human ɛ globin were coamplified as a 140-bp fragment; the murine product was cleaved by RsaI into 110- and 30-bp fragments).
The PCR products were resolved on 5% nondenaturing polyacrylamide gels. The gels were dried and exposed to X-ray film as well as a PhosphorImager screen (Molecular Dynamics). Band intensities and background values in the scanned image file were quantitated with ImageQuant software, and background levels were subtracted from band intensities before calculation of ratios.
For analysis of adult β-globin expression in single cells and 10-cell pools, the cells were harvested on day 4 after induction, washed, and stained with propidium iodide; then single live cells were deposited into reaction tubes containing lysis buffer (0.04% NP-40, 1 U of RNasin [Promega] per μl) by using a B-D Vantage fluorescence-activated cell sorter apparatus. RT was carried out with primer HBG2 for first-strand cDNA synthesis. The first round of PCR amplifications was performed by the addition of primer HBG1 and Taq polymerase (Cetus). The following cycle conditions were used: denaturation at 95°C for 2 min, and then 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s for 35 cycles. The first PCR mixture was diluted 1/100, and an aliquot was used as template for a second round of PCR, carried out in the presence of [α-32P]dCTP (in addition to cold deoxynucleoside triphosphates) and the nested primer pair HBG3 and HBG4 (see below). The PCR conditions were as above, but only 26 cycles of amplification were performed. The products of the second PCR were digested to completion with EcoRI and analyzed as above. About 50% of the single cells analyzed in this way gave a strong PCR product, probably reflecting heterogeneity in the induction of the tissue culture cells. The HBG3 and HBG4 primer pair was as follows: HBG3 (CCT CAA GGG CAC CTT TGC C); HBG4 (GCC ACA CCA GCC ACC AC) (a 174 bp fragment was generated, and EcoRI digestion cut the human product into fragments of 124 and 50 bp).
RESULTS
Experimental strategy.
The DT40 shuttle system (13) was used to generate targeted mutations in the human β-globin LCR. In this system, human chromosome 11 is introduced into the chicken pre-B-cell line DT40, which exhibits highly efficient gene targeting. As shown previously (13), the chicken/human hybrids are viable, stable, and recombination proficient. The human β-globin genes are not expressed in DT40, and, of the LCR HSs, only 5′HS2 is formed, consistent with a closed chromatin structure (13a). Human chromosome 11 retains its potential to express the β-globin genes when it is transferred back into the erythroid background of MEL cells (13).
The human chromosome 11 used as a substrate for these experiments had been modified previously in DT40 by HR (13) (see Fig. 1). A hygromycin B resistance gene was inserted in the Ha-ras locus, located approximately 5 Mb away from the β-globin locus, to allow selection for the chromosome without influencing β-globin expression, and a recognition site for the site-specific recombinase FLP (FRT site) was introduced between 5′HS1 and 5′HS2 of the LCR. This mutation did not affect the expression of the β-globin gene when the chromosome was reintroduced into erythroid cells (13).
The HR construct used to create deletions within the LCR is depicted in Fig. 1. HR introduces a loxP recognition site for the site-specific recombinase Cre upstream of 5′HS5 and a neomycin resistance gene flanked by the FRT and loxP sites between 5′HS4 and 5′HS5 of the LCR without deleting any LCR sequences. In conjunction with the FRT site already in place between 5′HS1 and 5′HS2, the introduced recombinase sites allow excision of either 5′HS2 to 5′HS4, 5′HS2 to 5′HS5, or 5′HS5, together with the marker gene upon expression of the appropriate recombinase. In addition, a site-specific recombination event involving only the recombinase sites flanking the neomycin resistance gene will excise this gene alone and restore the wild-type structure of the LCR except for the addition of loxP and FRT sites. Removal of the selection marker is necessary, since we have shown that the insertion of an expressed gene into the β-globin LCR disturbs LCR function (15, 34).
After DT40 cells were transfected with the HR construct, 36 G418-resistant clones were obtained, of which 4 were correctly targeted as determined by Southern analysis. Only one of these clones contained the loxP site upstream of 5′HS5, indicating that the 5′ recombination event occurred preferentially in the smaller inner part of the 5′ homology.
Transfer of this 5-neo-4 chromosome into MEL and GM979 cells (see below) for expression analyses was performed by microcell fusion followed by selection against DT40 cells and for MEL cells which have acquired the human chromosome. Southern blotting was used to assess the presence of the human β-globin locus. We obtained several 5-neo-4 MEL/GM979 lines, one of which was used to generate the deletions in the human β-globin LCR described above by transient expression of the site-specific recombinases Cre and FLP. We analyzed neomycin-sensitive lines by Southern blotting (data not shown, but see Fig. 5) and isolated several lines carrying each of the desired mutations, including lines that had re-created the wild-type LCR through a recombination event removing only the neomycin resistance gene.
FIG. 5.
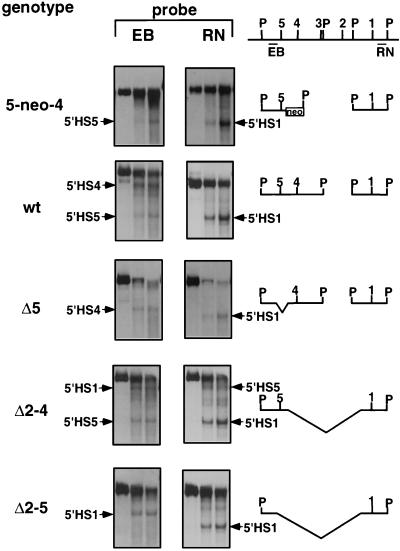
DNase I HS analysis of LCR mutations in MEL cells. Cells were digested with increasing concentrations of DNase I, and DNA was cut with PvuII and analyzed by hybridization against probes 5′ of 5′HS5 (left, EB) and 3′ of 5′HS1 (right, RN). The HSs detected in the different lines are indicated. Note that because of the deletions, the size of the PvuII band and the locations of the HSs differ between the lines. The fragments which can be observed in the different mutations with the two probes are indicated to the right, with P denoting PvuII sites. 5′HS3 is located so close to a PvuII site that it cannot be distinguished from the PvuII band by this probing strategy. The 5-neo-4 insertion leads to the insertion of a PvuII site between 5′HS5 and 5′HS4. All data shown are from one filter that had been hybridized sequentially to the EB and the RN probes. wt, wild type.
Breakage of the human chromosome and integration of human chromosome fragments into mouse chromosomes is often observed upon chromosome transfer into MEL cells (13, 38). Thus, the hybrid MEL lines obtained via the above strategy were analyzed by fluorescent in situ hybridization with probes from the chromosome 11p15 region and a chromosome 11 paint. In most lines, hybridization to human markers located more than 5,000 kb on either side of the β-globin locus was observed, and all lines contained a minimum of 1,300 kb of human chromosome 11 sequence on either side of the locus. All the lines were kept in hygromycin B to select against loss of the human chromosome fragment.
5′HS2 to 5′HS4 are required for expression of the adult β-globin gene.
We analyzed transcription of adult β-globin in MEL cells induced with hexamethylene bisacetamide (HMBA) by an RT-PCR assay that involves a primer pair which coamplifies the adult human (β-globin and δ-globin) and the adult mouse (β-major and β-minor) β-globin transcripts. The human and mouse PCR products can be distinguished by the presence of an EcoRI restriction site in the human products, and the uncut mouse PCR products serve as an internal control for induction efficiency, RNA input, and RT-PCR efficiency. The primers span an intron so that coamplified genomic DNA would lead to a larger PCR product.
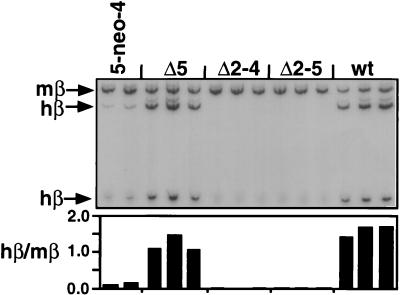
Each of the six independent lines obtained after transfer of the 5-neo-4 chromosome into MEL expresses adult β-globin, albeit in very small amounts (data not shown). One such line was used to generate wild-type and LCR deletion lines. All wild-type lines derived from this 5-neo-4 line by the excision of the selectable marker express the adult human β-globin gene at levels comparable to the high-level expression of the murine adult β-globin genes (Fig. 2). Thus, the low level of adult human β-globin expression in the 5-neo-4 lines is due to the insertion of the selection marker between 5′HS4 and 5′HS5, as observed previously when a selectable marker was introduced between 5′HS1 and 5′HS2 (15, 34). These results demonstrate that the insertion and subsequent removal of the selectable marker gene, the retention of FRT and loxP sites in the LCR, and the shuttle in and out of DT40 cells did not impair LCR function or β-globin expression. Furthermore, lines of identical genotype derived from the 5-neo-4 line through transient introduction of FLP and Cre vary only slightly in β-globin expression.
FIG. 2.
RT-PCR analysis of induced β-globin transcription in the LCR mutations in MEL cells. RT-PCR was performed with a primer pair which coamplifies the human and murine adult β-globin mRNA followed by restriction enzyme digestion specific for the human product and gel electrophoresis. Individual lines with the indicated genotype were analyzed (the second 5-neo-4 line shown is a subclone of the 5-neo-4 line used to create the LCR mutations that was transfected with FLP but did not carry out a recombination event). The ratio of human to mouse signal is shown below the autoradiograph. Note that the weak bands that appear above the main β-globin bands are proportional in their intensities to the correct bands. wt, wild type.
Deletion of 5′HS5 (Δ5) leads to a mild reduction of expression. In contrast, deletion of 5′HS2 to 5′HS4 (Δ2–4) and deletion of 5′HS2 to 5′HS5 (Δ2–5) have a dramatic effect on the transcription of the adult human β-globin gene. Both the Δ2–4 and the Δ2–5 deletions eliminate detectable adult human β-globin expression in each independent line we obtained.
5′HS2 to -4 are required for expression of the embryonic and the fetal β-like globin genes.
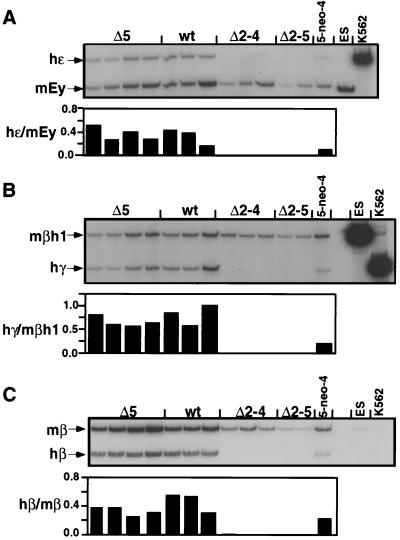
The MEL line used above and in the analysis of the Hispanic thalassemia mutation expresses only the adult genes of the human and murine β-globin locus. To test the effect of the LCR mutations on expression of the other β-like globin genes, we transferred the 5-neo-4 chromosome from DT40 into GM979 cells. This MEL subline expresses the murine embryonic Ey- and βh1-globin genes, in addition to the adult β-globin genes, and expression of human ɛ-, γ2-, and β-globin is activated when a human chromosome 11 is introduced into these cells (7, 69). Similarly to the RT-PCR assay described above, human embryonic ɛ-globin transcription was analyzed with a primer pair that amplifies both murine Ey- and human ɛ-globin, which were distinguished by a restriction cut in the murine PCR product (Fig. 3A). Likewise, murine βh1- and human Aγ-globin and Gγ-globin were coamplified with another primer pair and distinguished by a human-specific restriction site (Fig. 3B). RT-PCR analysis of adult β-globin expression was performed as described above (Fig. 3C).
FIG. 3.
RT-PCR analysis of induced β-globin transcription in GM979 cells. RT-PCR was performed as in Fig. 2 with primer pairs which coamplify the murine and human β-globin mRNAs followed by a species-specific restriction digest. (A) Coamplification of human ɛ-globin and mouse Ey. (B) Human γ-globin and mouse βh1 expression. (C) Adult human and murine β-globin expression. The ratio of human to murine signal after quantitation is shown below each lane. ES and K562 are controls for mouse- and human-specific signals and the restriction enzyme digestion. wt, wild type.
As in the MEL background, all four of the independent 5-neo-4 GM979 lines obtained after transfer of chromosome 11 from DT40 showed low-level human β-globin expression (data not shown), and one line was used to generate subclones with deletions in the LCR by site-specific recombination. Analysis of β-globin expression in the GM979 cells showed larger variations among individual lines with the same genotype than in the MEL cells (Fig. 3). A wide variation in the ratios of the various murine β-globin gene transcripts has been observed in GM979 subclones (69). Thus, the variations we observed most probably reflect line-to-line differences in the ratios of expression of the various murine and human β-globin genes in the GM979 background. Despite this variation, it is clear that compared to the wild-type lines, the 5-neo-4 insertion line showed a reduction in expression of all genes of the β-globin locus. As in the MEL lines, deletion of 5′HS2 to 5′HS4 (Δ2–4) or of 5′HS2 to 5′HS5 (Δ2–5) had a dramatic effect on human β-like globin gene expression. Both deletions result in the complete loss of human ɛ-, γ-, and adult β-globin expression (Fig. 3).
We also tested the effect of these deletions on uninduced β-globin expression. We found that in both the MEL and the GM979 backgrounds, the Δ2–4 and the Δ2–5 lines also failed to express human β-like globin in uninduced cells, while the wild-type cells expressed the human and murine β-like globin genes at a lower level than induced cells did (data not shown). Also, no human β-globin expression was seen in the Δ2–4 and the Δ2–5 lines in MEL or GM979 cells when they were induced with sodium butyrate, an inhibitor of histone deacetylase which stimulates human and murine β-globin gene expression in wild-type cells and murine β-globin gene expression in the Δ2–4 and the Δ2–5 lines (data not shown [see Discussion]).
In aggregate, comparison of the Δ5 lines to wild-type lines did not reveal a dramatic effect of the deletion of 5′HS5 on human β-like globin expression; however, the large variation among the individual wild-type and 5′HS5 deletion lines does not allow a precise assessment of the effect of this mutation in the GM979 cells.
Analysis of single cells and 10-cell pools shows no human β-globin expression in lines carrying the LCR-deleted chromosome.
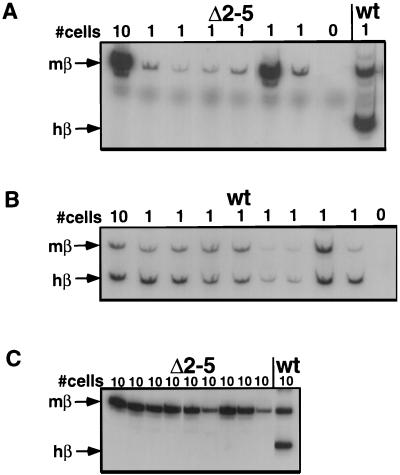
Recent data show that enhancers like the β-globin LCR do not act solely by increasing the level of transcription per cell; these elements may also increase the probability that a cell expresses a gene at a fixed level (46, 65, 66). Thus, it is possible that a small number of cells carrying the Δ2–4 or Δ2–5 deletion express wild-type levels of β-globin RNA (“jackpot cells”). To address this possibility, we analyzed the expression of human adult β-globin in sorted single MEL cells isolated by flow cytometry. We used an RT-PCR assay similar to the assays described above which involved two nested primer pairs which coamplify murine and human β-globin. As above, the human signal and the murine signal were distinguished by a restriction polymorphism and the uncut murine signal was used as an internal control. Representative examples of results obtained by analyzing single Δ2–5 and wild-type cells are shown in Fig. 4A and B. In cells carrying the wild-type chromosome, 31 of 50 single cells examined showed expression of both murine and human adult β-globin genes. In contrast, after deletion of 5′HS2 to 5′HS4 or 5′HS2 to 5′HS5, no human β-globin expression was detected in any of the 53 single cells analyzed, while mouse β-globin expression was observed in 26 cells. To analyze a larger number of cells, we analyzed β-globin transcription in 10-cell pools, reasoning that the sensitivity of the RT-PCR assay would allow detection of human β-globin signal even if only one of the 10 cells expressed marked amounts of adult human β-globin. Representative results of these experiments are shown in Fig. 4C. No human β-globin signal was observed in any of the 44 10-cell pools from the Δ2–4 or Δ2–5 cells we analyzed, whereas mouse β-globin transcripts were observed in 43 of 44 10-cell pools from these lines and 5 of 5 wild-type 10-cell pools analyzed showed both human and murine β-globin transcripts. In summary, we found no evidence that any cell of the Δ2–4 or Δ2–5 genotype expresses adult human β-globin at or near wild-type levels. In fact, we did not detect any human β-globin RNA in any single cell or 10-cell pool of these genotypes.
FIG. 4.
Single-cell PCR analysis of LCR mutations in MEL cells. Sorted induced MEL cells in the numbers indicated at the top were analyzed in an RT-PCR assay that was able to detect β-globin transcription from single cells. Adult murine and human β-globin mRNAs are amplified and distinguished by restriction enzyme digestion as indicated on the left. (A) Analysis of single MEL cells carrying the Δ2–5 deletion. (B) Analysis of single MEL cells with wild-type (wt) human chromosome 11. (C) Analysis of 10-cell pools carrying the Δ2–5 deletion.
The open chromatin structure of the β-globin locus is maintained in the absence of 5′HS2 to 5′HS5.
We next determined if the deletions within the LCR inhibit the formation of the remaining HSs of the LCR and if the loss of human β-globin expression correlates with the loss of DNase I hypersensitivity in the LCR.
Digestion of MEL cells in situ with DNase I and Southern blot analysis with probes on either side of the deletions demonstrates that the remaining LCR HSs are formed at comparable intensities to those in wild-type cells in the deletions which inactivate β-globin expression (e.g., 5′HS1 and 5′HS5 in the Δ2–4 deletion and 5′HS1 in the Δ2–5 deletion) (Fig. 5). Deletion of 5′HS5 has no effect on the formation of 5′HS4 and 5′HS1, and likewise insertion of the neo gene between 5′HS4 and 5′HS5 has no effect on the formation of 5′HS5 or 5′HS1. The same results were obtained with the GM979 lines (data not shown). We also analyzed the formation of a developmentally stable HS at the 3′ end of the locus, 21.8 kb 3′ to the β-globin gene (3′ β-globin +21.8 site), which is absent in the T-MEL line (19). We found that this HS was formed at a comparable intensity in the Δ2–5 background to that in a wild-type line (data not shown).
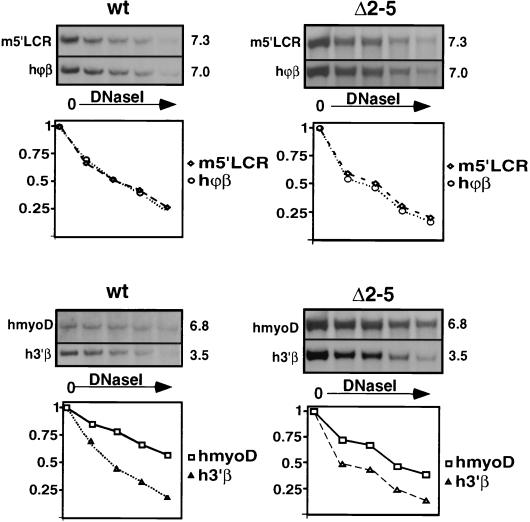
The formation of HSs in the β-globin and other loci is usually indicative of an open chromatin structure (22, 54), but it has been observed that HSs can form in a locus which is in a DNase I-resistant closed conformation (17). We therefore analyzed the general DNase I sensitivity of the human β-globin locus by examining the relative sensitivities of restriction fragments outside of transcribed regions, which did not contain HSs (Fig. 6). The unexpressed human myoD gene on the transferred chromosome 11 served as a resistant control (hmyoD), and a fragment from the DNase I-sensitive endogenous mouse β-globin locus (m5′LCR) served as a control for an open chromatin conformation. The analysis shows that the similarly sized fragment from the human ϕβ pseudogene region (hϕβ) and the murine m5′LCR fragment show almost identical digestion kinetics and that the human fragment from the region 3′ of the adult β-globin gene (h3′β) is more sensitive to DNase I than is the much larger hmyoD fragment. We conclude that like the wild-type β-globin locus, the Δ2–5 locus is sensitive to DNase I. Since the lines we analyzed were kept in culture for several months prior to analysis, we conclude that the sensitivity of the β-globin locus is maintained at least several hundred cell divisions after deletion of 5′HS2 to 5′HS5. These results demonstrate that 5′HS2 to 5′HS5 are not required for maintenance of the open chromatin structure of the human β-globin locus.
FIG. 6.
General DNase I sensitivity of the human β-globin locus. Nuclei were digested with increasing amounts of DNase I, and DNA was purified, cut with EcoRI, and analyzed by Southern blotting. Hybridization against the probes indicated on the left of the panel was performed. (The sizes of the resulting bands in kilobases are given on the right.) Quantitation, with the undigested band set at 1, is shown below. Probes: ϕβ and 3′β, in the human ϕβ-globin pseudogene and 3′ of the human β-globin gene; m5′LCR, sensitive region upstream of the murine β-globin LCR; hmyoD, the human myoD gene region on chromosome 11 as a resistant control.
DISCUSSION
The many experiments that have been performed to unravel the complexities of the β-globin regulation have led to a model that the β-globin LCR contains three activities: an erythroid cell-specific enhancer, a chromatin-opening activity (both of which are located in 5′HS1 to 5′HS4), and an insulating element (5′HS5) (23). The data presented here show that the postulated chromatin-opening and -insulating activities are dispensable in the erythroid background whereas the enhancer activity is crucial for transcription of any of the β-globin genes in every cell, even when the deletion of the LCR is performed in the background of an erythroid cell able to transcribe β-globin.
Role of the LCR in transcription.
Although DNase I hypersensitivity is observed at 5′HS1 and 5′HS5 after deletion of 5′HS2 to 5′HS4, the presence of these elements is not sufficient for any measurable transcription of the β-globin genes in the MEL and GM979 cell backgrounds, correlating with their lack of activity in transfections and transgenic-mouse analyses (9, 21, 32). The continuous requirement for the LCR for transcription of the genes in the β-globin cluster is compatible with a number of current models of LCR function. For example, activators bound by the LCR may interact directly with components of the β-globin gene promoters to facilitate the assembly of transcription complexes. It has been suggested that such an interaction is mediated through the LCR binding protein NF-E2 and the TATA binding protein TAFII130 (2). The LCR may also influence transcription by changing the subnuclear localization of the β-globin locus, for example, through interactions with the nuclear matrix. Furthermore, transcription of the LCR itself (3, 62) may play a role in regulating the expression of the β-globin genes. Clearly, all of these potential activities of the LCR could be affected by our LCR deletions.
In the chicken β-globin locus, the DNase I-sensitive domain coincides with the region of increased acetylation of histone H4 (28). It is possible that the LCR serves as an anchor for proteins involved in acetylation of histones within the β-globin locus, which in turn could trigger transcription (reviewed in reference 59). However, as mentioned above, sodium butyrate, an inhibitor of histone deacetylase, did not activate transcription from LCR-deleted β-globin loci, suggesting that maintenance of global histone acetylation is not the sole pathway of LCR action. Nonetheless, these experiments do not eliminate the possibility that the LCR is required to maintain specific patterns of histone acetylation (50) that may not be reproduced by inhibition of histone deacetylation.
The requirement for the continuous presence of the LCR to maintain β-globin transcription is reminiscent of the few cases in which enhancers were deleted from their natural chromosomal position. Deletion of the upstream enhancer of the human β-globin locus (HS-40) in MEL cells led to a severe reduction of α- and θ-globin expression. Formation of constitutive and erythroid cell-specific HSs in the locus was tested only in the presence of the selectable marker replacing the HS-40 enhancer and was found to be unaffected (4). The α-globin enhancer, however, does not possess LCR activity and lies in a constitutively open chromatin environment; therefore, it is not likely to play a role in chromatin opening (10). Deletion of the immunoglobulin heavy-chain enhancer region after the establishment of transcriptional competence in stable lines resulted in loss of expression of the μ heavy-chain gene (25). This observation and more recent homologous recombination studies (48) imply that the heavy-chain enhancer is required for both initiation and maintenance of transcription. However the effect of the deletion on chromatin structure was not analyzed in either study. It has also been shown that continuous binding of activated glucocorticoid receptor to the glucocorticoid response element of the TAT gene and HS formation is required for the maintenance of TAT transcription, although DNase I hypersensitivity of the promoter is independent of hormone induction (53).
The mutations we studied in this investigation eliminated all detectable β-globin gene expression in populations of cells and individual cells. Thus, our studies have not been informative about whether the LCR predominantly controls the level of expression within each cell or whether it controls the probability that expression is activated in a given cell (see the discussion in reference 42). The creation of less severe mutations in the LCR, resulting in reduction rather than elimination of expression, is required before this issue can be expressed.
Role of the LCR in maintenance of an open chromatin structure.
In some transgenic-mouse analyses, deletion of LCR HSs has been reported to result in position-dependent variegated expression patterns, heterochromatin formation, and/or lack of formation of the remaining HSs (14, 43, 54, 66). We cannot exclude the possibility that the deletion of the LCR results in small local changes in the chromatin structure of the β-globin locus which are not detected by our assays and which influence the transcription or other properties of the locus. However, we clearly did not observe the global changes in chromatin structure and the heterochromatinization of the locus which have been described in some of the cases when the β-globin locus was inactivated by deletions of the LCR. Our experiments show that in erythroid cells, maintenance of the β-globin locus in an open chromatin structure does not require the LCR. Since these LCR deletions were made in the erythrocyte environment, our experiments did not address whether the human β-globin LCR plays a role in opening the chromatin of the locus or in keeping the locus open in cells of earlier erythroid stages. We reported previously that the degree of inactivation of a reporter gene after recombinase-driven deletion of an enhancer varies strongly between integration sites (66). In addition, transgenic-mouse analyses have revealed that constructs containing incomplete human β-globin or CD2 LCRs demonstrate heterochromatinization and loss of HS formation predominantly when the transgenes were integrated near the centromere (14, 43). The failure of our LCR deletions to close the β-globin locus suggests that either the locus is not located in a region subjected to spreading heterochromatin or there may be as yet undetected elements in the β-globin locus that prevent heterochromatinization (see below). Our finding of an open chromatin structure of the β-globin locus in the absence of transcription reinforces the notion that transcription of the β-globin genes is not required for the maintenance of the open conformation of the β-globin chromatin domain. In particular, our experiments demonstrate that the maintenance of the open conformation of the β-globin locus is not the consequence of postulated LCR-promoter interactions.
Comparison of our LCR deletions to the Hispanic thalassemia phenotype.
The deletion of 5′HS2 to 5′HS4 and of 5′HS2 to 5′HS5 recapitulates the transcriptional phenotype of the Hispanic thalassemia deletion. Our analysis of transcription in the GM979 cells revealed that in addition to the adult β-globin genes, the embryonic and fetal human β-globin genes are inactivated in the endogenous locus by deletion of 5′HS2 to 5′HS5. In contrast, the chromatin phenotype of Hispanic thalassemia is not reproduced by the deletion of 5′HS2 to 5′HS4 or 5′HS2 to 5′HS5: the remaining HSs are formed, and the chromatin remains in an open conformation. This discrepancy may be due to the different history of the respective chromosomes and the cellular backgrounds in which the deletions were performed. Alternatively, there may be as yet uncharacterized regulatory elements either in the region upstream of 5′HS5, which is deleted in Hispanic thalassemia, or in the region upstream of the 5′ Hispanic breakpoint, which is moved closer to the β-globin genes by the deletion. It is also possible that multiple elements spread throughout the locus contribute to maintaining the open chromatin structure of the locus.
Role of 5′HS5.
Our results raise the possibility that there are regulatory elements located upstream of 5′HS5. If 5′HS5 functioned as an insulator, as has been suggested (8, 39), the β-globin locus would be shielded from the effects of these elements. We found that in the erythroid background, the deletion of 5′HS5 had only a small effect on transcription of the β-globin genes and no effect on the formation of neighboring HSs. These results argue that in the endogenous locus in erythroid cells, 5′HS5 is not required to shield the locus from proposed neighboring heterochromatin. It is possible, however, that 5′HS5 plays a role earlier in development or in restricting β-globin expression to erythroid cells, since loss of tissue-specific expression has been observed after the deletion of components of other LCRs (6, 51).
Phenotype of the 5-neo-4 insertion.
We reported previously that insertion of a Friend enhancer/promoter-driven hygromycin B or neomycin resistance gene between 5′HS1 and 5′HS2 abolished β-globin transcription completely (15, 34). In comparison, the insertion of a pgk promoter neomycin resistance gene cassette between 5′HS4 and 5′HS5 described here reduces the transcription of the β-globin genes significantly but does not abolish it. The mechanism by which integration of a transcribed gene into the LCR negatively affects transcription is not known, but our result shows that a gene does not have to be located between the LCR 5′HSs with strong enhancer activity (5′HS2 to 5′HS4) and the β-globin genes to interfere with transcription. In addition, our results show that the repression observed in the 5-neo-4 insertion cannot be due to blocking the influence of 5′HS5 on expression, since removal of 5′HS5 had a much smaller effect on β-globin expression. For both insertions, LCR activity was restored by the subsequent excision of the selection marker, showing that the effects of the insertion on the locus are reversible.
Comparison to the mouse β-globin LCR mutations.
It is interesting to compare the phenotypes of the deletions in the human β-globin LCR described here to those observed in mice when deletions were created in the murine β-globin LCR by homologous recombination (references 16 and 31 and unpublished data). Deletions of individual HSs have only small negative effects on transcription of the β-globin genes and no effect on the formation of neighboring HSs. This includes deletion of the region homologous to human 5′HS5, which further argues against an essential role of that region in β-globin regulation (3b).
We have also analyzed the effect of deletion of murine 5′HS1 to 5′HS5 on the transcription and chromatin structure of the murine β-globin locus in tissue culture cells (13b). Similar to the results reported here for the human locus, we observed that deletion of the murine LCR in erythroid cells has no effect on the chromatin structure of the locus: it remains in a DNase I-sensitive conformation in erythroid cells. However, the mouse LCR deletion results in partial, rather than complete, reduction in β-globin gene transcription. We are currently examining if the residual transcription in the mouse locus is due to the elimination of 5′HS1 (which we did not delete in our analysis of the human locus reported here), the different cellular backgrounds in which these assays were performed, the different histories of the human and murine chromosomes used in the analyses, or a difference in the regulation of the two loci. Whatever the basis of this difference, it is clear that components of the LCR, as defined by 5′HS1 to 5′HS5, are required for high-level transcription of the genes in the human and mouse β-globin loci, even in the context of an open chromatin conformation in erythroid cells. Both systems offer the opportunity to insert specific sequences into chromosomes with the LCR deletion and determine the requirements for high-level transcription in the endogenous β-globin locus.
ACKNOWLEDGMENTS
We thank Michael Bender, Mike Bulger, and Steve Fiering for critical reading of the manuscript, and we thank the Hutchinson Cancer Center Image Analysis Laboratory for assistance in PhosphorImager analysis.
This work was supported by fellowships from the Deutsche Forschungsgemeinschaft and the Leukemia Research Foundation to A.R., a fellowship from the American Cancer Society to D.C., and National Cancer Institute grant CA54337 and NIH grants DK52854 and DK 44746 to M.G.
REFERENCES
- 1.Aladjem M I, Groudine M, Brody L L, Dieken E S, Fournier R E K, Wahl G M, Epner E M. Participation of the human β-globin locus control region in initiation of DNA replication. Science. 1995;270:815–819. doi: 10.1126/science.270.5237.815. [DOI] [PubMed] [Google Scholar]
- 2.Amrolia P J, Ramamurthy L, Saluja D, Tanese N, Jane S M, Cunningham J M. The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the α- and β-globin gene loci in an erythroid cell line. Proc Natl Acad Sci USA. 1997;94:10051–10056. doi: 10.1073/pnas.94.19.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashe H L, Monks J, Wijgerde M, Fraser P, Proudfoot N J. Intergenic transcription and transinduction of the human β-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Bender, M. Unpublished data.
- 3b.Bender, M., A. Reik, J. Close, A. Telling, E. Epner, S. Fiering, R. Hardison, and M. Groudine. Description and targeted deletion of 5′HS 5 and 6 of the mouse β-globin locus control region. Blood, in press. [PubMed]
- 4.Bernet A, Sabatier S, Picketts D J, Ouzana R, Morlé F, Higgs D R, Godet J. Targeted inactivation of the major positive regulatory element (HS-40) of the human α-globin gene locus. Blood. 1995;86:1202–1211. [PubMed] [Google Scholar]
- 5.Blom van Assendelft G, Hanscombe O, Grosveld F, Greaves D R. The β-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989;56:969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- 6.Bonifer C, Yannoutsos N, Krüger G, Grosveld F, Sippel A E. Dissection of the locus control function located on the chicken lysozyme gene domain in transgenic mice. Nucleic Acids Res. 1994;22:4202–4210. doi: 10.1093/nar/22.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown P A, Padgett R W, Hardies S C, Hutchison III C A, Edgell M H. Beta-globin transcript found in induced erythroleukemia cells is homologous to the beta h0 and beta h1 genes. Proc Natl Acad Sci USA. 1982;79:2753–2757. doi: 10.1073/pnas.79.9.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Buchholz F, Ringrose L, Angrand P O, Rossi F, Stewart A F. Different thermostabilities of FLP and Cre recombinases: implications for applied site-specific recombination. Nucleic Acids Res. 1996;24:4256–4262. doi: 10.1093/nar/24.21.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung J H, Whiteley M, Felsenfeld G. A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 9.Collis P, Antoniou M, Grosveld F. Definition of the minimal requirements within the human β-globin gene and the dominant control region for high level expression. EMBO J. 1990;9:233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craddock C F, Vyas P, Sharpe J A, Ayyub H, Wood W G, Higgs D R. Contrasting effects of α and β globin regulatory elements on chromatin structure may be related to their different chromosomal environments. EMBO J. 1995;14:1718–1726. doi: 10.1002/j.1460-2075.1995.tb07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin P T, Liu D, Liu W, Chang J C, Kan Y W. Human β-globin gene expression in transgenic mice is enhanced by a distant DNaseI hypersensitive site. Proc Natl Acad Sci USA. 1989;86:7082–7086. doi: 10.1073/pnas.86.18.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhar V, Nandi A, Schildkraut C L, Skoultchi A I. Erythroid-specific nuclease-hypersensitive sites flanking the human β-globin domain. Mol Cell Biol. 1990;10:4324–4333. doi: 10.1128/mcb.10.8.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieken E S, Epner E M, Fiering S, Fournier R E K, Groudine M. Efficient modification of human chromosomal alleles using recombination-proficient chicken/human microcell hybrids. Nat Genet. 1996;12:174–182. doi: 10.1038/ng0296-174. [DOI] [PubMed] [Google Scholar]
- 13a.Epner, E. Unpublished data.
- 13b.Epner, E., A. Reik, D. Cimbora, A. Telling, M. Bender, S. Fiering, T. Enver, D. Martin, M. Kennedy, G. Keller, and M. Groudine. The β-globin LCR is not necessary for an open chromatin structure or transcription of the mouse β-globin locus. Mol. Cell, in press.. [DOI] [PubMed]
- 14.Festenstein R, Tolaini M, Corbella P, Mamalaki C, Parrington J, Fox M, Miliou A, Jones M, Kioussis D. Locus control region function and heterochromatin-induced position effect variegation. Science. 1996;271:1123–1125. doi: 10.1126/science.271.5252.1123. [DOI] [PubMed] [Google Scholar]
- 15.Fiering S, Kim C G, Epner E M, Groudine M. An “in-out” strategy using gene targeting and FLP recombinase for the functional dissection of complex DNA regulatory elements: analysis of the β-globin locus control region. Proc Natl Acad Sci USA. 1993;90:8469–8473. doi: 10.1073/pnas.90.18.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiering S, Epner E, Robinson K, Zhuang Y, Telling A, Hu M, Martin D I K, Enver T, Ley T J, Groudine M. Targeted deletion of 5′HS2 of the murine β-globin LCR reveals that it is not essential for proper regulation of the β-globin locus. Genes Dev. 1995;9:2203–2213. doi: 10.1101/gad.9.18.2203. [DOI] [PubMed] [Google Scholar]
- 17.Forrester W C, Takegawa S, Papayannopoulou T, Stamatoyannopoulos G, Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987;15:10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester W C, Novak U, Gelinas R, Groudine M. Molecular analysis of the human β-globin locus activation region. Proc Natl Acad Sci USA. 1989;86:5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrester W C, Epner E, Driscoll M C, Enver T, Brice M, Papayannopoulou T, Groudine M. A deletion of the human β-globin locus activation region causes a major alteration in chromatin structure and replication across the entire β-globin locus. Genes Dev. 1990;4:1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- 20.Fraser P, Hurst J, Collis P, Grosveld F. DNaseI hypersensitive sites 1, 2, and 3 of the human β-globin dominant control region direct position-independent expression. Nucleic Acids Res. 1990;18:3503–3508. doi: 10.1093/nar/18.12.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser P, Pruzina S, Antoniou M, Grosveld F. Each hypersensitive site of the human β-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev. 1993;7:106–113. doi: 10.1101/gad.7.1.106. [DOI] [PubMed] [Google Scholar]
- 22.Garrick D, Sutherland H, Robertson G, Whitelaw E. Variegated expression of a globin transgene correlates with chromatin accessibility but not methylation status. Nucleic Acids Res. 1996;24:4902–4909. doi: 10.1093/nar/24.24.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geyer P K. The role of insulator elements in defining domains of gene expression. Curr Opin Genet Dev. 1997;7:242–248. doi: 10.1016/s0959-437x(97)80134-7. [DOI] [PubMed] [Google Scholar]
- 24.Gronostajski R M, Sadowski R D. Determination of DNA sequences essential for FLP-mediated recombination by a novel method. J Biol Chem. 1985;260:12320–12327. [PubMed] [Google Scholar]
- 25.Grosschedl R, Marx M. Stable propagation of the active transcriptional state of an immunoglobulin μ gene requires continuous enhancer function. Cell. 1988;55:645–654. doi: 10.1016/0092-8674(88)90223-1. [DOI] [PubMed] [Google Scholar]
- 26.Grosveld F, Blom van Assendelft G, Greaves D R, Kollias G. Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 27.Gu H, Zou Y R, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 28.Hebbes T R, Clayton A L, Thorne A W, Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNaseI sensitivity in the chicken β-globin chromosomal domain. EMBO J. 1994;13:1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins M J, Smilinch N J, Sait S, Koenig A, Pongratz J, Gessler M, Richard III C W, James M R, Sanford J P, Kim B-W, Cattelane J, Nowak N J, Winterpacht A, Zabel B U, Munroe D J, Bric E, Housman D E, Jones C, Nakamura Y, Gerhard D S, Shows T B. An ordered NotI fragment map of human chromosome band 11p15. Genomics. 1994;23:211–222. doi: 10.1006/geno.1994.1479. [DOI] [PubMed] [Google Scholar]
- 30.Hug B A, Moon A M, Ley T J. Structure and function of the murine β-globin locus control region 5′ HS-3. Nucleic Acids Res. 1992;20:5771–5778. doi: 10.1093/nar/20.21.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hug B A, Wesselschmidt R L, Fiering S, Bender M A, Epner E, Groudine M, Ley T J. Analysis of mice containing a targeted deletion of beta-globin locus control region 5′ hypersensitive site 3. Mol Cell Biol. 1996;16:2906–2912. doi: 10.1128/mcb.16.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson J D, Petrykowska H, Philipsen S, Miller W, Hardison R. Role of DNA sequences outside the cores of DNase hypersensitive sites (HSs) in functions of the β-globin locus control region. J Biol Chem. 1996;271:11871–11878. doi: 10.1074/jbc.271.20.11871. [DOI] [PubMed] [Google Scholar]
- 33.Jarman A P, Higgs D R. Nuclear scaffold attachment sites in the human globin gene complexes. EMBO J. 1988;7:3337–3344. doi: 10.1002/j.1460-2075.1988.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C G, Epner E M, Forrester W C, Groudine M. Inactivation of the human β-globin gene by targeted insertion into the β-globin locus control region. Genes Dev. 1992;6:928–938. doi: 10.1101/gad.6.6.928. [DOI] [PubMed] [Google Scholar]
- 35.Kioussis D, Festenstein R. Locus control regions: overcoming heterochromatin-induced gene inactivation in mammals. Curr Opin Genet Dev. 1997;7:614–619. doi: 10.1016/s0959-437x(97)80008-1. [DOI] [PubMed] [Google Scholar]
- 36.Kitsberg D, Selig S, Keshet I, Cedar H. Replication structure of the human beta-globin gene domain. Nature. 1993;366:588–590. doi: 10.1038/366588a0. [DOI] [PubMed] [Google Scholar]
- 37.Kulozik A E, Bail S, Bellan-Koch A, Bartrann C R, Kohne E, Kleihauer E. The proximal element of the β-globin locus control region is not functionally required in vivo. J Clin Invest. 1991;87:2142–2146. doi: 10.1172/JCI115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leach R J, Thayer M J, Schafer A J, Fournier R E K. Physical mapping of human chromosome 17 using fragment-containing microcell hybrids. Genomics. 1989;5:167–176. doi: 10.1016/0888-7543(89)90043-8. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Stamatoyannopoulos G. Hypersensitive site 5 of the human β locus control region functions as a chromatin insulator. Blood. 1994;84:1399–1401. [PubMed] [Google Scholar]
- 40.Lichter P, Tang C-J C, Call K, Hermanson G, Evans G A, Housman D, Ward D C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990;247:64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Chang J C, Moi P, Liu W, Kan Y W, Curtin P T. Dissection of the enhancer activity of β-globin 5′ DNaseI-hypersensitive site 2 in transgenic mice. Proc Natl Acad Sci USA. 1992;89:3899–3903. doi: 10.1073/pnas.89.9.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin D, Fiering S, Groudine M. Regulation of beta-globin gene expression. Straightening out the locus. Curr Opin Genet Dev. 1996;6:488–495. doi: 10.1016/s0959-437x(96)80072-4. [DOI] [PubMed] [Google Scholar]
- 43.Milot E, Strouboulis J, Trimborn T, Wijgerde M, de Boer E, Langeveld A, Tan-Un K, Vergeer W, Yannoutsos N, Grosveld F, Fraser P. Heterochromatin effects on the frequency and duration of LCR-mediated gene transcription. Cell. 1996;87:105–114. doi: 10.1016/s0092-8674(00)81327-6. [DOI] [PubMed] [Google Scholar]
- 44.Moi P, Kan Y W. Synergistic enhancement of globin gene expression by activator protein-1-like proteins. Proc Natl Acad Sci USA. 1990;87:9000–9004. doi: 10.1073/pnas.87.22.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon A M, Ley T J. Conservation of the primary structure, organization, and function of the human and mouse β-globin locus activation regions. Proc Natl Acad Sci USA. 1990;87:7693–7697. doi: 10.1073/pnas.87.19.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon A M, Ley T J. Functional properties of the β-globin locus control region in K562 erythroleukemia cells. Blood. 1991;77:2272–2284. [PubMed] [Google Scholar]
- 47.Ney P A, Sorrentino B P, McDonagh K T, Nienhuis A W. Tandem AP-1-binding sites within the human β-globin dominant control region functions as an inducible enhancer in erythroid cells. Genes Dev. 1990;4:993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- 48.Oancea A E, Berru M, Shulman M J. Expression of the (recombinant) endogenous immunoglobulin heavy-chain locus requires the intronic matrix attachment regions. Mol Cell Biol. 1997;17:2658–2668. doi: 10.1128/mcb.17.5.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Gorman S, Fox D T, Wahl G M. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science. 1991;251:1351–1355. doi: 10.1126/science.1900642. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill L P, Turner B M. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J. 1995;14:3946–3957. doi: 10.1002/j.1460-2075.1995.tb00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz B J, Cado D, Chen V, Diaz P W, Winoto A. Adjacent DNA elements dominantly restrict the ubiquitous activity of a novel chromatin-opening region to specific tissues. EMBO J. 1997;16:5037–5045. doi: 10.1093/emboj/16.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pruzina S, Hanscombe O, Whyatt D, Grosveld F, Philipsen S. Hypersensitive site 4 of the human β-globin locus control region. Nucleic Acids Res. 1991;19:1413–1419. doi: 10.1093/nar/19.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reik A, Schütz G, Stewart A F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancer. EMBO J. 1991;10:2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reitman M, Lee E, Westphal H, Felsenfeld G. An enhancer/locus control region is not sufficient to open chromatin. Mol Cell Biol. 1993;13:3990–3998. doi: 10.1128/mcb.13.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryan T M, Behringer R R, Martin N C, Townes T M, Palmiter R D, Brinster R L. A single erythroid-specific DNaseI super-hypersensitive site activates high levels of human β-globin gene expression in transgenic mice. Genes Dev. 1989;3:314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- 56.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by CRE recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senecoff J F, Bruckner R C, Cox M M. The FLP recombinase of the yeast 2-micron plasmid: characterization of its recombination site. Proc Natl Acad Sci USA. 1985;82:7270–7274. doi: 10.1073/pnas.82.21.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorrentino B, Ney P, Bodine D, Nienhuis A W. A 46 base pair enhancer sequence within the locus activating region is required for induced expression of the γ-globin gene during erythroid development. Nucleic Acids Res. 1990;18:2721–2731. doi: 10.1093/nar/18.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 60.Talbot D, Collis P, Anotiou M, Vidal M, Brosveld F, Greaves D R. A dominant control region from the human β-globin locus conferring integrations site independent gene expression. Nature. 1989;338:352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- 61.Tuan D, Solomon W, Li Q, London I M. The ’beta-like globin’ gene domain in human erythroid cells. Proc Natl Acad Sci USA. 1985;82:6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuan D, Kong S, Hu K. Transcription of the hypersensitive site HS2 enhancer in erythroid cells. Proc Natl Acad Sci USA. 1992;89:11219–11223. doi: 10.1073/pnas.89.23.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuan D Y, Solomon W B, London I M, Lee D P. An erythroid-specific, developmental-stage-independent enhancer far upstream of the human “β-like globin” genes. Proc Natl Acad Sci USA. 1989;86:2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Gijlswijk R P, Zijlmans H J, Wiegant J, Bobrow M N, Erickson T J, Adler K E, Tanke H J, Raap A K. Fluorochrome-labeled tyramides: use in immunocytochemistry and fluorescence in situ hybridization. J Histochem Cytochem. 1997;45:375–382. doi: 10.1177/002215549704500305. [DOI] [PubMed] [Google Scholar]
- 65.Walters M C, Fiering S, Eidemiller J, Magis W, Groudine M, Martin D I K. Enhancers increase the probability but not the level of gene expression. Proc Natl Acad Sci USA. 1995;92:7125–7129. doi: 10.1073/pnas.92.15.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walters M C, Magis W, Fiering S, Eidemiller J, Scalzo D, Groudine M, Martin D I K. Transcriptional enhancers act in cis to suppress position-effect variegation. Genes Dev. 1996;10:185–195. doi: 10.1101/gad.10.2.185. [DOI] [PubMed] [Google Scholar]
- 67.Wiegant J. Nonradioactive in situ hybridization application manual. Indianapolis, Ind: Boehringer Mannheim Biochemicals; 1996. In situ hybridization to human metaphase chromosomes using DIG-, biotin, or fluorochrome-labeled DNA probes and detection with fluorochrome conjugates; pp. 62–71. [Google Scholar]
- 68.Yu J, Bock J H, Slightom J L, Villeponteau B. A 5′ β-globin matrix-attachment region and the polyoma enhancer together confer position-independent transcription. Gene. 1994;139:139–145. doi: 10.1016/0378-1119(94)90747-1. [DOI] [PubMed] [Google Scholar]
- 69.Zitnik G, Hines P, Stamatoyannopoulos G, Papapyannopoulou T. Murine erythroleukemia cell line GM979 contains factors that can activate silent chromosomal human γ-globin genes. Proc Natl Acad Sci USA. 1991;88:2530–2534. doi: 10.1073/pnas.88.6.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]