Abstract
Malignant struma ovarii (MSO) with synchronous primary thyroid cancer in the neck is extremely rare and lacks a treatment consensus. A 44-year-old woman presenting with a left ovarian cyst was admitted to Peking Union Medical College Hospital (Beijing, China) Ultrasonography showed a 6 cm solid-cystic left ovarian mass with plentiful blood signals. Other notable findings were an elevated CA125 level and a suspected malignant thyroid nodule. A unilateral salpingo-oophorectomy (USO) was conducted, and the surgical pathology was papillary thyroid cancer (PTC) arising in a struma ovarii. The patient underwent a total thyroidectomy and cervical lymph node dissection, and the pathology of the right lobe nodule was follicular-variant PTC without capsule invasion or lymph node metastasis (5 mm; pT1aN0M0). No further adjuvant therapy was administered. The serum thyroglobulin value was normal before surgery and was undetectable after thyroidectomy. During regular follow-up examinations over 4 years, the patient remained well with no evidence of disease (NED). In a literature review, another 13 cases of MSO coexisting with cervical thyroid cancer that had reported outcomes were found. The MSO was confined to the ovary in all cases. A total of nine patients received radioiodine therapy (RAI) treatment after total thyroidectomy. Two patients relapsed and were successfully cured with RAI after the initial surgery Only one patient died due to another disease, while 11 patients showed NED and the remaining patient was alive with the disease after a median follow-up time of 2 years. This data suggests that USO with personalized RAI may be a preferred option for MSO confined to the ovary plus synchronous primary thyroid cancer due to the conferred satisfactory prognosis.
Keywords: MSO, thyroid cancer, surgery, adjuvant therapy, prognosis
Introduction
Struma ovarii (SO) is a unique monodermal teratoma that is defined as having thyroid tissue accounting for >50% of teratoma components (1). It is estimated that 2–5% of ovarian teratomas and <1% of all ovarian tumors can be classified as SO (2). Malignant transformation of SO, namely malignant struma ovarii (MSO), is an extremely rare entity that is observed in ~5% of the cases (3). Management of MSO remains controversial due to its rarity (4). Conservative surgery with personalized radioiodine therapy (RAI), aggressive treatment combined with comprehensive staging surgery with total thyroidectomy (TT), and RAI regardless of the presence of metastatic diseases, have been proposed in previous studies (5–7). The feasibility of fertility preservation and the survival outcomes have also been evaluated, but the risk factors are inconsistent (8).
Although patients with MSO suffer a significantly increased risk of primary thyroid cancer in the neck, to the best of our knowledge, there are currently only a small number of cases documented in the literature (9). The current study presents a case of MSO coexisting with primary cervical papillary thyroid cancer (PTC) to further investigate the clinical characteristics, treatment options and survival outcomes of this particularly rare entity.
Case report
A 44-year-old woman was admitted to Peking Union Medical College Hospital (Beijing, China) in September 2019 due to an ovarian mass first identified ~3 years ago. An ultrasound examination revealed a 7.1×4.7-cm solid-cystic mass in the left adnexal area, with plentiful blood signals identified by color Doppler imaging (Fig. 1A). A 3-cm anterior uterine myoma was also noted. Routine thyroid ultrasonography showed a 0.5-cm solid nodule in the right lobe near the isthmus, which was potentially malignant (Thyroid Imaging Reporting and Data System grade 4c) (10). Multiple cervical lymph nodes measuring 1.1–1.3 cm, located at right region VI, were detected. Another notable finding was an elevated serum CA125 level (67 U/ml; normal range, 0–35 U/ml). The patient was euthyroid and the serum thyroglobulin (TG) level was also within the normal range (23.1 ng/ml; normal range, 1.4–78 ng/ml).
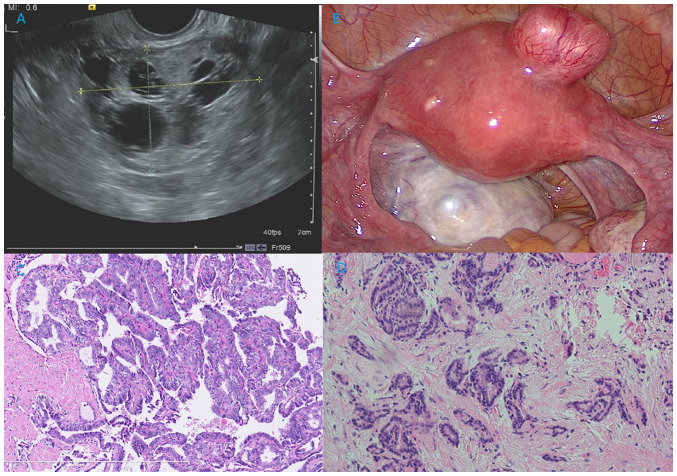
Figure 1.
Ultrasound and pathologic image of this patient. (A) Ultrasound of the ovarian mass revealed a round, multilocular, solid-cystic shape. Several round, echoless regions could be noted, which was similar to a palette. (B) Intraoperative exploration showed a 6-cm solid-cystic left ovarian mass with plentiful vascularization, and a 3-cm myoma was also noted in the anterior uterine wall. (C) Pathological analysis of MSO showed PTC arising in SO. Papillary structures lined by one or more layers of tumor cells were observed. The tumor cells were crowded, in round or oval nuclei shapes, and the nuclei of some neoplastic cells were enlarged, clear, ‘ground glass opacity (unclear nuclei) and overlapping (H&E staining; ×100 magnification). (D) Pathological analysis of primary thyroid cancer in the neck demonstrating non-typical papillary structures (H&E staining; ×100 magnification). H&E, hematoxylin and eosin.
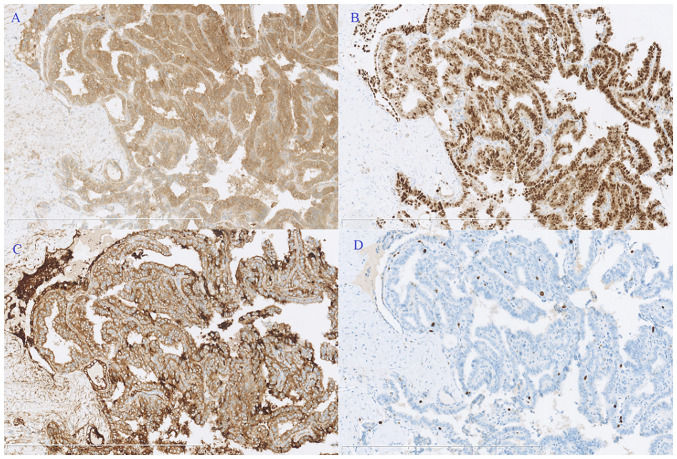
An exploratory laparoscopy was conducted, showing a 6-cm solid-cystic left ovarian mass with plentiful vascularization, and a 3-cm myoma was also noted in the anterior uterine wall (Fig. 1B). No positive findings were detected on the right ovary or in the abdominopelvic cavity. An ovarian cystectomy and myomectomy were conducted. The pathology (10% formalin solution, 20°C for 30 min; 10 µm; HE stain, 20°C for 30 min; light microscope) of the ovarian mass showed struma ovarii with focal PTC (3 mm; Fig. 1C). Papillary structures lined by one or more layers of tumor cells were observed, and the nuclei of some neoplastic cells were enlarged, clear, ‘ground glass opacity (unclear nuclei)’ and overlapping. Immunohistochemical staining demonstrated positive expression of calretinin thyroxin and thyroid transcription factor-1, with a Ki-67 index of 5% (Fig. 2). A unilateral salpingo-oophorectomy (USO) was performed 2 weeks later based on this result. No tumor cells were found upon peritoneal washing and no residual tumor could be identified in the second surgical pathological analysis. Approximately 2 months later, the patient underwent a TT, and the frozen section of the right lobe nodule revealed papillary growth with enlarged, ground glass opacity features of the tumor cells, confirming the diagnosis of a PTC. Therefore, a right zone VI cervical lymph node resection (ipsilateral central node dissection) was performed, but the pathological results showed chronic inflammation. The final pathology showed follicular-variant PTC (5 mm) without capsular involvement or vascular invasion, and no extrathyroidal extension (Fig. 1D). Postoperative contrast enhanced computed tomography scans of the neck, thorax, abdomen and pelvic cavity, and whole-body iodine scans showed no residual lesions. Therefore, the stage of thyroid cancer in the neck was pT1aN0M0 (AJCC TNM staging system for thyroid cancer) (11).
Figure 2.
Immunohistochemical staining. Positive expression of (A) calretinin, (B) thyroid transcription factor-1 and (C) thyroxin, and (D) the Ki-67 index was 5%. Magnification, ×100.
The patient recovered uneventfully and the serum TG level rapidly decreased to an undetectable level (<1.4 ng/ml) postoperatively. No further adjuvant therapy was conducted, and a combination of TG and CA125 assessment, and imaging examinations (cervical and pelvic ultrasonography), were performed at 3–6 month intervals for follow-up. To date, no evidence of MSO or cervical thyroid cancer has been detected for >4 years.
Literature review
A comprehensive literature review was performed in PubMed (pubmed.ncbi.nlm.nih.gov/), Web of Science (https://www.webofscience.com/wos/), Scopus (https://www.scopus.com/) database using the following keywords: ‘malignant struma ovarii’; ‘metastatic malignant struma ovarii’; ‘malignant ovarian teratoma’; ‘thyroid carcinoma arising in struma ovarii’; ‘struma ovarii’. The present study also evaluated references cited by these articles. Only articles in English published between 1960 and 2023 were examined. The other 13 known cases of MSO coexisting with primary thyroid cancer in the neck that had detailed clinical characteristics, treatment information and results of follow-up were identified and summarized (Table SI) (12–24). Several cases of MSO with thyroid cancer in the neck without follow-up outcomes were excluded (25,26). Benign struma ovarii, or MSO without coexisted with thyroid cancer in the neck, or patients without documented clear clinical characteristics, treatment, and outcomes were also excluded.
The median age of the patients was 44.0 years (range, 30–78 years) and the condition mostly occurred in those individuals aged in their 40s. PTC was the most common pathological subtype in both MSO and thyroid cancer. Only two cases of FTC in MSO were noted, but none had FTC in the neck in this group. The pelvic surgery at initial treatment included USO, bilateral salpingo-oophorectomy (BSO), hysterectomy with BSO and comprehensive staging surgery without fertility-sparing. A total of 9 patients also received RAI at varied doses (28.3–179 mCi). Although two patients relapsed in the group who received surgery alone during follow-up, they were both cured by RAI at recurrence (12,22). One of these patients also received repeated surgery.
The median follow-up time was 2 years, 11 patients remain alive with NED (12–14,16–22,24) and one was alive with the disease (23). Only 1 patient died of another disease (15).
Discussion
The risk of the coexistence of thyroid cancer in the neck is significantly increased in patients with MSO, compared with common population (4). In several large cohort studies, the incidence of cervical thyroid cancer in patients with MSO was 2.6–8.8% (4–6), while the highest incidence of thyroid cancer in the neck was ~10 cases per 10,0000 person-years (27). Researchers have proposed that this may be attributed to more rigorous thyroid screening and ‘field cancerization’ in patients with MSO (6,17). Leong et al (17) found that the two malignancies may be independent in existence, but the early genomic instability may explain these multifocal carcinogeneses. Poli et al (28) identified similar histomorphological and molecular features in thyroid components of MSO (such as BRAF, RAS and KIT mutations) and primary thyroid cancer in a series of 6 patients, and a review of 48 cases reported molecular profiles. In primary thyroid cancer, some specific gene mutations, including BRAFV600E, and telomerase reverse transcriptase promoter mutations are significantly associated with the prognosis (29). Hence, routine thyroid imaging examinations should be recommended in patients with MSO, and gene detection methods for thyroid cancer are also encouraged.
Patients with MSO usually have satisfactory survival outcomes (9). In 2015, Goffredo et al (4) found the 5-, 10- and 20-year overall survival (OS) rate in these patients was 96.7, 94.3 and 84.9%, respectively (4). Even in patients with metastatic MSO, the outcomes remained promising with 5-, 10- and 15-year OS rates of 89.3, 82.4 and 65.9%, respectively, in a large cohort of 79 patients (6). Furthermore, patients with MSO confined to the ovary were found to have better outcomes, with a 5- and 20-year disease-specific survival (DSS) rate of 95.3 and 88.7%, respectively (5). These findings are also consistent with other similar case reports and systematic reviews that revealed inexact 5-year OS rate >90% (30,31). Therefore, it has always been controversial whether conservative or aggressive treatment should be administered in patients with MSO.
Most of these patients underwent aggressive management with pelvic and thyroid surgery, and TT followed by RAI, while 4 patients received surgery alone. However, according to the European Society for Medical Oncology guidelines for thyroid cancer (32), only 3 patients needed RAI after TT, if MSO confined to the ovary was not considered as an indication for RAI. Marti et al (14) suggested that pelvic surgery alone may be enough for MSO confined to the ovary, as the 25-year cumulative recurrence rate was only 7.5%. Our previous study found that the benefit of RAI was unclear in terms of lowering the recurrence (94.4 and 60.1% with and without RAI, respectively) and DSS (95.3%) rates in a cohort of 125 patients with MSO confined to the ovary (5). Furthermore, although 2 patients had a relapse in the present literature review, including 1 patient who should have received initial RAI due to metastatic disease but did not, they were both successfully cured by RAI with or without repeat surgery. This suggested that some patients might not need RAI at initial treatment but that they were being given it, possibly leading to overtreatment.
At present, most patients with MSO are administered RAI based on the risk stratifications of primary thyroid cancer (9). For example, Yassa et al (33) suggested that patients with tumors >2 cm in diameter, disease outside the ovaries or aggressive histological features should be considered for postoperative RAI therapy. However, directly applying the risk stratifications of primary thyroid cancer to MSO may not be suitable and lacks evidence from large cohorts, which would inevitably result in most patients being given RAI (13). The size of the ovarian mass can easily be much larger than the thyroid nodules, and the mass size does not equal the carcinoma size in MSO (6). The cancer components are mostly very small focal struma components, and measuring tumor size in a situation where cancer and teratomatous components blend would be extremely difficult. Furthermore, studies found that no potential risk factors were identified in MSO confined to the ovary, while age >55 years and International Federation of Gynecology and Obstetrics stage (34) IV were prognostic factors in metastatic MSO in large cohorts, but the mass size, presence of ascites, surgical options and pathological subtypes were not (5,6,9).
In the literature review cohort of patients with MSO coexisting with primary thyroid cancer, no patients died of the disease, confirming the excellent survival outcomes in this subgroup and that coexistence with primary thyroid cancer does not seem to be a risk factor. Therefore, the risk stratifications of these two synchronous malignancies may be better determined independently. Reserving TT for patients with MSO with suspected primary thyroid cancer or for those planning to receive RAI may be more reasonable. Whether the patients receive RAI therapy should be determined by the risk stratification of both the cervical thyroid cancer and the MSO (5,6,9,14). Namely, personalized RAI may be preferred in MSO confined to the ovary while RAI is recommended in patients with metastatic MSO (5,6).
Previously, several studies with large sample sizes have fully discussed the significance of conservative surgery (5,6,9). Given the fact that MSO is greatly different from common epithelial ovarian carcinoma in terms of biological behavior and survival outcome, less aggressive surgery should also be advocated. In patients with MSO confined to the ovary or metastatic MSO, more aggressive surgery did not significantly improve the survival outcomes (5,6). The current case received USO without adjuvant therapy but showed NED for >4 years, again supporting the conservative surgical option.
Due to the extremely rare nature of MSO, more evidence of practical risk stratifications and application of RAI should be gathered to determine in which circumstance and doses RAI should be applied. Survival outcomes from long-term close follow-up should also be further investigated.
In conclusion, the prognosis of patients with MSO confined to the ovary with synchronous primary thyroid cancer in the neck is satisfactory, and USO with personalized RAI therapy may be a preferred treatment option balancing the quality of life and therapeutic effect. Whether RAI is administered should be based on the risk group of cervical thyroid cancer in this population.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MSO
malignant struma ovarii
- PTC
papillary thyroid cancer
- RAI
radioiodine therapy
- USO
unilateral salpingo-oophorectomy
Funding Statement
The present study was supported by the National High-Level Hospital Clinical Research Funding (grant no. 2022-PUMCH-B-083) and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant no. 2022-I2M-C&T-B-023).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
SL wrote the manuscript and participated in study conceptualization. RH completed the pathological analysis and participated in the literature review. JY conceived the study design and modified the manuscript. All authors have read and approved the manuscript. SL, RP and JY confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval no. S-K1198). Written informed consent to participate in the study was obtained from the patient.
Patient consent for publication
Written informed consent for publication of clinical details and/or clinical images was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Devaney K, Snyder R, Norris HJ, Tavassoli FA. Proliferative and histologically malignant struma ovarii: A clinicopathologic study of 54 cases. Int J Gynecol Pathol. 1993;12:333–343. doi: 10.1097/00004347-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: Tumor types and imaging characteristics. Radiographics. 2001;21:475–490. doi: 10.1148/radiographics.21.2.g01mr09475. [DOI] [PubMed] [Google Scholar]
- 3.Hanby A, Walker C. Pathology and genetics: Tumours of the breast and female genital organs. WHO classification of tumours series-volume IV. Lyon, France: IARC Press. Breast Cancer Res. 2004;6:133. doi: 10.1186/bcr788. [DOI] [Google Scholar]
- 4.Goffredo P, Sawka AM, Pura J, Adam MA, Roman SA, Sosa JA. Malignant struma ovarii: A population-level analysis of a large series of 68 patients. Thyroid. 2015;25:211–215. doi: 10.1089/thy.2014.0328. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Yang T, Xiang Y, Li X, Zhang L, Deng S. Clinical characteristics and survival outcomes of malignant struma ovarii confined to the ovary. BMC Cancer. 2021;21:383. doi: 10.1186/s12885-021-08118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Yang T, Li X, Zhang L, Shi H, Cheng N, Lang J. FIGO stage IV and age over 55 years as prognostic predicators in patients with metastatic malignant struma ovarii. Front Oncol. 2020;10:584917. doi: 10.3389/fonc.2020.584917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrimali RK, Shaikh G, Reed NS. Malignant struma ovarii: The west of Scotland experience and review of literature with focus on postoperative management. J Med Imaging Radiat Oncol. 2012;56:478–482. doi: 10.1111/j.1754-9485.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaco-Levy R, Peng RY, Snyder MJ, Osmond GW, Veras E, Bean SM, Bentley RC, Robboy SJ. Malignant struma ovarii: A blinded study of 86 cases assessing which histologic features correlate with aggressive clinical behavior. Arch Pathol Lab Med. 2012;136:172–178. doi: 10.5858/arpa.2011-0092-OA. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Kong S, Wang X, Zhang X, Yin M, Yang J. Survival outcomes and prognostic predictors in patients with malignant struma ovarii. Front Med (Lausanne) 2021;8:774691. doi: 10.3389/fmed.2021.774691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 12.Dardik RB, Dardik M, Westra W, Montz FJ. Malignant struma ovarii: Two case reports and a review of the literature. Gynecol Oncol. 1999;73:447–451. doi: 10.1006/gyno.1999.5355. [DOI] [PubMed] [Google Scholar]
- 13.Janszen EW, van Doorn HC, Ewing PC, de Krijger RR, de Wilt JH, Kam BL, de Herder WW. Malignant struma ovarii: Good response after thyroidectomy and I ablation therapy. Clin Med Oncol. 2008;2:147–152. doi: 10.4137/cmo.s410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marti JL, Clark VE, Harper H, Chhieng DC, Sosa JA, Roman SA. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: A series of 4 patients and a review of 53 reported cases. Thyroid. 2012;22:400–406. doi: 10.1089/thy.2011.0162. [DOI] [PubMed] [Google Scholar]
- 15.Leite I, Cunha TM, Figueiredo JP, Félix A. Papillary carcinoma arising in struma ovarii versus ovarian metastasis from primary thyroid carcinoma: A case report and review of the literature. J Radiol Case Rep. 2013;7:24–33. doi: 10.3941/jrcr.v7i10.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnamurthy A, Ramshankar V, Vaidyalingam V, Majhi U. Synchronous papillary carcinoma thyroid with malignant struma ovarii: A management dilemma. Indian J Nucl Med. 2013;28:243–245. doi: 10.4103/0972-3919.121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong A, Roche PJ, Paliouras M, Rochon L, Trifiro M, Tamilia M. Coexistence of malignant struma ovarii and cervical papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:4599–4605. doi: 10.1210/jc.2013-1782. [DOI] [PubMed] [Google Scholar]
- 18.Brusca N, Del Duca SC, Salvatori R, D'Agostini A, Cannas P, Santaguida MG, Virili C, Bianchi L, Gargano L, Centanni M. A case report of thyroid carcinoma confined to ovary and concurrently occult in the thyroid: Is conservative treatment always advised? Int J Endocrinol Metab. 2015;13:e18220. doi: 10.5812/ijem.18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma D, Guseva NV, Dahmoush L, Robinson RA. Struma ovarii with malignant transformation and germline KIT mutation: A case report with review of the literature. Int J Gynecol Pathol. 2016;35:442–447. doi: 10.1097/PGP.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 20.Middelbeek RJW, O'Neill BT, Nishino M, Pallotta JA. Concurrent intrathyroidal thyroid cancer and thyroid cancer in struma ovarii: A case report and literature review. J Endocr Soc. 2017;1:396–400. doi: 10.1210/js.2017-00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes-Lima CJ, Nikiforov YE, Lee W, Burman KD. Synchronous independent papillary thyroid carcinomas in struma ovarii and the thyroid gland with different RAS mutations. J Endocr Soc. 2018;2:944–948. doi: 10.1210/js.2018-00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzelepis EG, Barengolts E, Garzon S, Shulan J, Eisenberg Y. Unusual case of malignant struma ovarii and cervical thyroid cancer preceded by ovarian teratoma: Case report and review of the literature. Case Rep Endocrinol. 2019;2019:7964126. doi: 10.1155/2019/7964126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donato S, Simões H, Leite V. Malignant struma ovarii with concurrent thyroid cancer: Outcomes during and after pregnancy. Eur Thyroid J. 2021;10:523–527. doi: 10.1159/000512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo GT, Minkowitz J, Kapustin DA, Fan J, Minkowitz G, Minkowitz M, Dowling E, Matloob A, Asti D, Dhar M, et al. Synchronous thyroid cancer and malignant struma ovarii: Concordant mutations and microRNA profile, discordant loss of heterozygosity loci. Diagn Pathol. 2023;18:47. doi: 10.1186/s13000-023-01336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd JC, Williams BA, Rigby MH, Kieser K, Offman S, Shirsat H, Trites JRB, Taylor SM, Hart RD. Malignant Struma ovarii in a 30-year old nulliparous patient. Thyroid Res. 2017;10:3. doi: 10.1186/s13044-017-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim ST, Jeong HJ, Chung MJ, Yim CY, Sohn MH. Malignant struma ovarii demonstrated on post-therapy radioiodine scan after total thyroidectomy for papillary thyroid cancer. Clin Nucl Med. 2008;33:429–431. doi: 10.1097/RLU.0b013e3181708297. [DOI] [PubMed] [Google Scholar]
- 27.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poli R, Scatolini M, Grosso E, Maletta F, Gallo M, Liscia D, Nelva A, Cesario F, Forte G, Metovic J, et al. Malignant struma ovarii: Next-generation sequencing of six cases revealed Nras, Braf, and Jak3 mutations. Endocrine. 2021;71:216–224. doi: 10.1007/s12020-020-02438-7. [DOI] [PubMed] [Google Scholar]
- 29.Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, Hahn S, Park DJ, Park YJ. Effects of coexistent BRAF V600E and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: A meta-analysis. Thyroid. 2017;27:651–660. doi: 10.1089/thy.2016.0350. [DOI] [PubMed] [Google Scholar]
- 30.Siegel MR, Wolsky RJ, Alvarez EA, Mengesha BM. Struma ovarii with atypical features and synchronous primary thyroid cancer: A case report and review of the literature. Arch Gynecol Obstet. 2019;300:1693–1707. doi: 10.1007/s00404-019-05329-z. [DOI] [PubMed] [Google Scholar]
- 31.Ayhan S, Kilic F, Ersak B, Aytekin O, Akar S, Turkmen O, Akgul G, Toyran A, Turan T, Kimyon Comert G. Malignant struma ovarii: From case to analysis. J Obstet Gynaecol Res. 2021;47:3339–3351. doi: 10.1111/jog.14902. [DOI] [PubMed] [Google Scholar]
- 32.Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, Papotti MG, Berruti A, ESMO Guidelines Committee, Electronic address. clinicalguidelines@esmo.org: Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856–1883. doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 33.Yassa L, Sadow P, Marqusee E. Malignant struma ovarii. Nat Clin Pract Endocrinol Metab. 2008;4:469–472. doi: 10.1038/ncpendmet0887. [DOI] [PubMed] [Google Scholar]
- 34.Prat J. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26:87–89. doi: 10.3802/jgo.2015.26.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study are included in the figures and/or tables of this article.