Abstract
Lung cancer is one of the most common malignancies worldwide. Since the global outbreak of the coronavirus disease 2019 (COVID-19) pandemic in 2020, the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on lung cancer has been extensively studied. Despite reports about SARS-CoV-2 infection inducing a significant increase in the number of medical visits for patients with cancer, the virus has also been reported to produce some unknown benefits. The present study reports the case of a patient with lung cancer whose tumor lesion was reduced in size after SARS-CoV-2 infection even though the therapeutic regimen remained unchanged. Although the mechanism involved is not yet understood, this case supports the novel idea of applying SARS-CoV-2 in oncolytic virotherapy.
Keywords: lung cancer, severe acute respiratory syndrome coronavirus 2, tumor reduction, oncolytic virotherapy, drug resistance
Introduction
Lung cancer is one of the most common malignancies worldwide and the leading cause of cancer-associated death. As shown in the cancer data of 2020 from the International Agency for Research on Cancer, 19.3 million new cancer cases were estimated to occur globally. Of these, 2,206,771 new cases (11.1%) in all ages and sexes were attributed to lung cancer, which accounted for 18% (n=1,796,144) of all cancer-related mortalities (1). Over the past few decades, lung cancer treatment has undergone tremendous changes, with immunotherapy and targeted therapies experiencing rapid advancement and becoming the dominant treatments. Tyrosine kinase inhibitors (TKIs), checkpoint inhibitors and chimeric antigen receptor T-cell therapy, among others, have significantly improved the survival rate and quality of life of patients with lung cancer beyond the abilities of standard chemotherapeutic and radiotherapy regimens (2). However, limited by gene mutation, individual differences, unclear mechanisms of tumor development and other factors, the long-term efficacy of these therapies remains unsatisfactory, as the survival time of patients with stage IV lung cancer rarely exceeds 12 months (3). Therefore, the exploration of new treatments for lung cancer remains necessary.
Since the global outbreak of the coronavirus disease 2019 (COVID-19) pandemic in 2020, the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on lung cancer has been extensively studied. Similar to other respiratory viruses, SARS-CoV-2 primarily spreads through droplets, thus infecting respiratory mucosal epithelial cells, and causes a series of inflammatory reactions, which include recruitment of macrophages, and increased local secretion of inflammatory cytokines [e.g., interleukin (IL)-6 and IL-1β] and chemokines [e.g., interferon (INF)-α, IFN-γ, monocyte chemoattractant protein-1 and IFNγ inducible protein 10] into the peripheral blood. The cytokines and chemokines induce more T helper 1 cells to enter into the lungs, mediating immune responses that attack and kill virus-infected cells (4). This reaction undoubtedly aggravates the lung cancer burden, leading to more complications. SARS-CoV-2 infection, based on the severity of illness, is grouped into the following categories of asymptomatic infection, viral pneumonia, acute respiratory distress syndrome and death (5). However, a recent report indicated that SARS-CoV-2 is not entirely harmful, and that it can also bring some benefits with unknown mechanisms (6). A number of patients with malignancies experienced tumor reduction or significant remission during SARS-CoV-2 infection, even though their therapeutic regimens were unchanged (7).
The present study reviewed the case of a patient with advanced lung cancer who was initially prescribed TKI agents but received poor efficacy. After the patient had tested negative for SARS-CoV-2 for ~3 weeks, imaging showed significant tumor reduction although the therapeutic schedule remained unchanged. To the best of our knowledge, this is the first description of lung cancer reduction after SARS-CoV-2 infection.
Case report
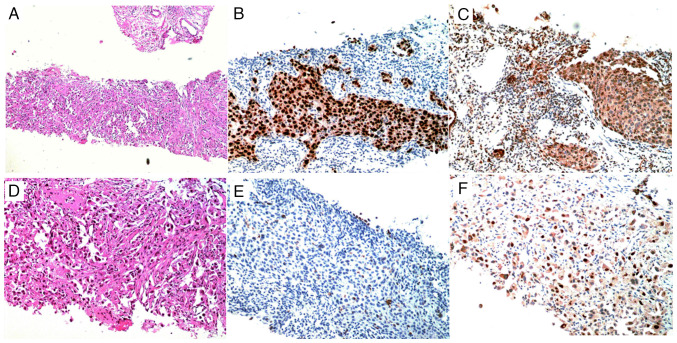
A 60-year-old Chinese male, with no significant previous history or family history of illness, who had smoked ~10 cigarettes per day for 25 years, presented to the Department of Respiratory and Critical Care Medicine, Eighth Medical Center of the Chinese People's Liberation Army General Hospital (Beijing, China) complaining of right chest pain and discomfort, fatigue, anorexia and weight loss for 3 weeks in March 2022. Although a physical examination suggested no significant abnormalities, serum tumor marker levels [carcinoembryonic antigen (CEA), 1,352 ng/ml; carbohydrate antigen 125 (CA125), 83.45 U/l; CA15-3, 66.98 U/l; CA724, 33.45 U/l; and serum keratin 19 fragment (CA21-1), 42.91 ng/l) were obviously beyond the normal ranges (CEA, 0–5 ng/ml; CA125, 0–35 U/ml; CA15-3, 0–25 U/l; CA724, 0–6 U/l; and CA21-1, 0–3 ng/l). Moreover, a chest computed tomography (CT) scan showed a 17.0×14.5-mm space-occupying lesion in the center of the inferior lobes of the right lung, with mixed ground-glass nodules and irregular margins. A core needle biopsy was performed on the lung lesion using B-ultrasound guidance. The pathological diagnosis based on hematoxylin-eosin staining (Data S1) and immunohistochemistry (Data S1) demonstrated staining characteristics of lung adenocarcinoma, with positivity for thyroid transcription factor-1 and napsin A, and negativity for cytokeratin 5/6 (Fig. 1).
Figure 1.
Pathological results after core needle biopsy. (A and D) H&E staining [(A), ×40 magnification and (D), ×100 magnification] showed the pathological type of lung adenocarcinoma, with a tumor mass that appeared to be of mixed histological pattern, including acinar, papillary, bronchioloalveolar and solid patterns. IHC (×100 magnification) showed (B) positive thyroid transcription factor-1 expression, (C) positive Napsin A expression, (E) negative cytokeratin 5/6 expression and (F) sporadic cells positive for tumor protein 63 expression.
In order to determine the mutation that could be treated with targeted therapy, a next-generation sequencing (NGS) assay (GeneseeqPrime™; Geneseeq Technology, Inc.; Data S1) was performed to detect 437 cancer-related genes, including a total of 1.53 MB bases involving exons, fusion-related introns and microsatellite regions. The result revealed that six genes (EGFR, TP53, WAS, DOT1L, EPHA2 and SMAD4) had tumor-specific mutations. Immunotherapy-associated gene mutations of lung cancer only occurred in EGFR, with the exception of TP53, rather than other genes [such as BRAF V600, Kirsten rat sarcoma viral oncogene homolog, mesenchymal epithelial transition factor (MET), human epidermal growth factor receptor 2, anaplastic lymphoma kinase, ret proto-oncogene and ROS proto-oncogene 1].
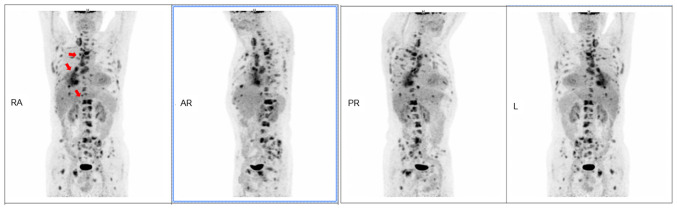
Subsequently, to further evaluate the general condition of the patient, a fluorine-18-fluorodeoxyglucose positron emission tomography-CT scan was performed, which revealed multiple metastases, including those in the pleura, frontal lobe of the brain and liver (maximum diameter, 1.1 cm) (Fig. 2). Given the overall condition of the patient and the sequencing results, in order that there was the opportunity of surgery after neoadjuvant chemotherapy, the patient decided to accept targeted therapy instead of a conventional regimen. The patient initially underwent treatment with orally administered gefitinib (250 mg once daily, for 5 months). However, follow-up CT showed a continually deteriorating condition, with an enlarged primary lesion (18.6×17.2 mm) and multiple metastatic lesions after 5 months. Given the possibility of gefitinib resistance, a tumor individualized treatment molecular assay (Anzekong®; Anhui Anlong Gene Technology, Inc.), which covered 550 cancer-related gene sites, revealed EGFR T790M resistance but EGFR L858R sensitivity, and the patient was recommended to receive an orally administration of osimertinib (80 mg once daily) instead of gefitinib. A distinctive curative effect was not present, nor was a partial response, after 1 month of medication. A follow-up CT scan showed that the size of the primary lesion had not significantly decreased (17.4×15 mm at the inferior lobe of the right lung).
Figure 2.
Whole-body positron emission tomography/computed tomography scan. Images revealed an FDG-avid mass with a maximum standardized uptake value of 8.8 in the lower right lung. Additionally, increased accumulation of FDG in the right paratracheal lymph nodes, liver and bone marrow was also noted (red arrows). FDG, fluorine-18-fluorodeoxyglucose; R, right; AR, anterior right; PR, posterior right; L, left.
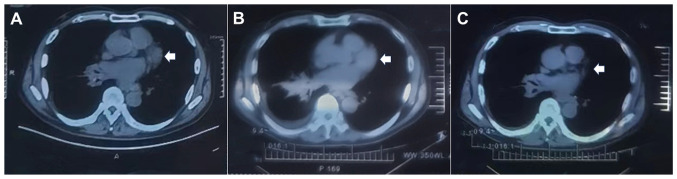
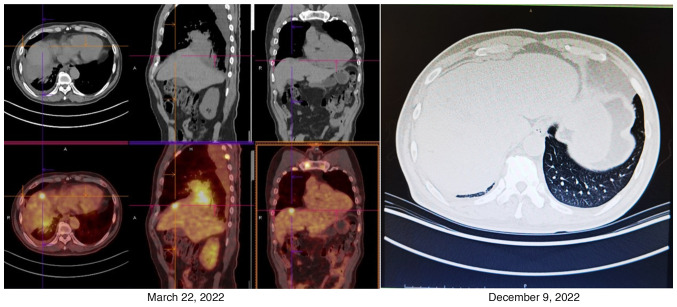
In October 2022 (~45 days from the start of osmertinib administration), the patient was diagnosed with COVID-19 [by serum reverse transcription (RT)-PCR test; Data S1] and was isolated in a hotel. During this period, the patient took ibuprofen (0.4 g, twice per day, for 2 weeks), dextromethorphan (20 mg, twice per day, for 3 weeks) and some Chinese patent medicines [lianhua qingwen granules (1 bag, three times per day, for 2 weeks) and compound glycyrrhiza oral solution (5 ml, as needed, for 3 weeks)] to treat a fever and cough, but the administration of osimertinib was not changed. By the middle of November 2022 (~65 days from the start of osmertinib administration), blood samples tested negative for SARS-CoV-2 (RT-PCR). Unexpectedly, the patient experienced chest pain relief and a continuous remission of the other symptoms, such as fatigue and poor appetite, leading to an improved quality of life. The values of several lung carcinoma-related tumor markers (CA125, 64.61 U/ml; CA15-3, 31.45 U/ml; CA724, 3.81 U/ml; and CA21-1, 9.281 ng/ml) in December 2022, compared with those in March 2022, were markedly decreased, and a CT scan revealed that the primary and metastatic lesions were also significantly reduced in size, especially the lung lesion (0.8×0.7 cm), and metastatic lesions were significantly reduced in size or had even locally disappeared (Figs. 3 and 4). The patient was still alive and being followed up every 2 weeks at the time of publication of the present study.
Figure 3.
Sequential chest computed tomography scans. There was substantial regression of the lung lesions over time (white arrows). (A) April 2022, (B) September 2022 and (C) December 2022.
Figure 4.
Changes to the metastatic lesion in the liver. Positron emission tomography/CT showed that there were multiple scattered metastatic lesions (maximum diameter, 1.1 cm) in the right lobe of the liver in March 2022. However, the follow-up CT scan performed in December 2022 showed that the metastatic lesions were significantly reduced in size or had even locally disappeared.
Discussion
The current patient presented with a reduction in tumor size on imaging, decreased serum tumor marker levels, decreased pain and relief from fatigue after the SARS-CoV-2 infection, without a change to the therapeutic schedule. Combining data on the clinical manifestations, serological test results and the patient's symptoms, it was concluded that the tumor in this patient with advanced lung cancer had spontaneously reduced, and the patient's medical condition had improved.
Spontaneous tumor reduction after SARS-CoV-2 infection is rare. Generally, patients with cancer, as a immunocompromised group, showed a higher mortality rate when infected (7). However, recent reports have demonstrated that the imaging and serological symptoms of certain cancer patients were relieved after SARS-CoV-2 infection (8). This effect of SARS-CoV-2 infection occurs not only in hematological malignancies, such as Epstein-Barr virus-positive classical Hodgkin lymphoma (9), follicular lymphoma (10), acute myeloid leukemia (11) and chronic lymphocytic leukemia (12), but also in solid tumors such as colon cancer (13,14) and renal cell carcinoma (15). Although the underlying mechanism has not been entirely understood, researchers have speculated that SARS-CoV-2 can activate the body's innate immunity. By binding to the angiotensin-converting enzyme 2/neuropilin-1 complex on the surface of infected cell membranes through the surface spike proteins, it changes the natural tolerance of innate immunity through non-major histocompatibility complex-restricted pathways, enhances the recognition of macrophages and dendritic cells, thus inducing excessive inflammatory responses, and activates more cytotoxic T cells to destroy infected cells (16). In other words, SARS-CoV-2 infection makes tumor cells more susceptible to attack by immune cells and destruction. Furthermore, some researchers believe that cross-reactivity may occur between SARS-CoV-2 antigens and specific tumor antigens, thus activating the tumor-specific anti-tumor response of T cells to mediate the oncolytic effect (17). Additionally, activating natural killer cells is also reported to be an essential factor involved in oncolysis (18). We hypothesized (despite it not being confirmed) that SARS-CoV-2 infection plays an indispensable role in superior drug sensitivity and lower resistance to antineoplastic agents. Therefore, SARS-CoV-2 maybe exert the effect of an oncolytic virus (OV) on mediating the enhancement of the sensitivity of targeted anticancer agents, improving their efficacy, and promoting tumor dissolution and regression.
Oncolytic virotherapy is a promising avenue of immunotherapy that exerts its oncolytic effect by actively identifying and infecting tumor cells, replicating and eventually killing tumor cells with different regulatory mechanisms, such as inducing an innate immune response, but with immune exemption in normal cells (19). Although few viruses have currently been successfully developed into Food and Drug Administration-approved treatments for use in clinical regimens, the discovery of OVs can be traced back as far as the mid to late 19th century, when Dock (20) described a patient with myeloid leukemia who has suffered a spontaneous reduction of abnormal leukocyte counts after an influenza epidemic. Following this, similar reports became more common, mainly finding patients with hematological tumors who experienced transient remission after coincidental viral infection. With more and more researchers working to modify the natural wild-type lytic virus, further modifications have been able to provide a more powerful anticancer effect (21). Currently, various viruses, including RNA viruses such as rhinovirus, poliovirus, measles virus and vesicular stomatitis virus, and DNA viruses, such as adenovirus and herpes simplex virus, have been used in cancer treatment (22). Due to their single-stranded structure, RNA viruses have some natural disadvantages in use as preclinical virus models of anticancer therapy compared with DNA viruses. The mutation rate of RNA viruses is 1×10−6−10−4 per base per cell, while that of DNA viruses is 1×10−8−10−6. Therefore, if RNA viruses are used as vectors and injected into patients, the patients may face more unpredictable risks (23). Compared with DNA viruses, RNA viruses are more easily integrated into the host genome, which also brings unpredictable risks to patients. Thus, if SARS-CoV-2 is to be used as an oncolytic therapy vector, a series of genetically engineered modifications need to be conducted. Similar to the case of combining poliovirus and rhinovirus to form a recombinant poliovirus chimera (24), it is necessary to further attenuate pathogenicity and cytotoxicity, and enhance specificity and security if SARS-CoV-2 is to play a role in antitumor immunomodulation.
In conclusion, tumor reduction after SARS-CoV-2 infection is a rare condition. Although the exact cause and mechanism are unknown, the clinical symptoms and diagnostic tests in the present report suggest that SARS-CoV-2 infection may alter the immunomodulatory response or drug resistance, leading to improved antitumor effects. This case also provides a rationale and inspiration for exploring SARS-CoV-2 as a viral vector in oncolytic virotherapy, although further evidential studies are required. Due to the present study reporting only a single case, there was no way for a controlled trial to be conducted. Therefore, this case is shared with other researchers and physicians to aid in further elucidation of the mechanism involved.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the Key Research and Development Project of Hainan (grant no. ZDYF2023SHFZ117) and the Military Medical Science and Technology Youth Cultivation Program (grant no. 2019NPY11)
Availability of data and materials
The NGS data generated in the present study are not publicly available to preserve patient anonymity. All other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XMZ, JYC and JS drafted the manuscript and conceived the study. SYG and FYZ participated in the analysis, collection and interpretation of data, and NSQ obtained PET/CT images and analyzed patient data from the NGS assay. NSQ and XMZ confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for the case study to be published.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Petty WJ, Paz-Ares L. Emerging strategies for the treatment of small cell lung cancer: A review. JAMA Oncol. 2023;9:419–429. doi: 10.1001/jamaoncol.2022.5631. [DOI] [PubMed] [Google Scholar]
- 3.Vokes NI, Pan K, Le X. Efficacy of immunotherapy in oncogene-driven non-small-cell lung cancer. Ther Adv Med Oncol. 2023;15:17588359231161409. doi: 10.1177/17588359231161409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: Signaling pathways and treatment implications. Mol Cancer. 2021;20:76–82. doi: 10.1186/s12943-021-01363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsamakis K, Gavriatopoulou M, Schizas D, Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E, Sioulas AD, Tsamakis C, et al. Oncology during the COVID-19 pandemic: Challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20:441–447. doi: 10.3892/ol.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bounassar-Filho JP, Boeckler-Troncoso L, Cajigas-Gonzalez J, Zavala-Cerna MG. SARS-CoV-2 as an oncolytic virus following reactivation of the immune system: A review. Int J Mol Sci. 2023;24:243–249. doi: 10.3390/ijms24032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meo C, Palma G, Bruzzese F, Budillon A, Napoli C, de Nigris F. Spontaneous cancer remission after COVID-19: Insights from the pandemic and their relevance for cancer treatment. J Transl Med. 2023;21:273–278. doi: 10.1186/s12967-023-04110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahimmanesh I, Shariati L, Dana N, Esmaeili Y, Vaseghi G, Haghjooy Javanmard S. Cancer occurrence as the upcoming complications of COVID-19. Front Mol Biosci. 2021;8:813175. doi: 10.3389/fmolb.2021.813175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurlapski M, Romanowicz G, Taszner M, Zaucha JM. SARS-CoV-2-induced remission of advanced classical Hodgkin lymphoma. Pol Arch Intern Med. 2022;13:132–139. doi: 10.20452/pamw.16266. [DOI] [PubMed] [Google Scholar]
- 10.Sollini M, Gelardi F, Carlo-Stella C, Chiti A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the ‘flare phenomenon’ to the ‘abscopal effect’. Eur J Nucl Med Mol Imaging. 2021;48:2652–2654. doi: 10.1007/s00259-021-05275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barkhordar M, Rostami FT, Yaghmaie M, Abbaszadeh M, Chahardouli B, Mousavi SA. Spontaneous complete remission of acute myeloid leukemia in the absence of disease-modifying therapy following severe pulmonary involvement by coronavirus infectious disease-19. Case Rep Hematol. 2022;2022:2603607. doi: 10.1155/2022/2603607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulbul H, Nazli HE, Olgun A, Togay A, Kahraman DS. Spontaneous remission of chronic lymphocytic leukemia in a patient with SARS-CoV2. Leuk Res Rep. 2022;18:103–106. doi: 10.1016/j.lrr.2022.100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottaiano A, Scala S, D'Alterio C, Trotta A, Bello A, Rea G, Picone C, Santorsola M, Petrillo A, Nasti G. Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection. Ther Adv Med Oncol. 2021;13:17588359211011455. doi: 10.1177/17588359211011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottaiano A, Santorsola M, Circelli L, Cascella M, Petrillo N, Perri F, Casillo M, Granata V, Ianniello M, Izzo F, et al. Genetic landscape of colorectal cancer patients manifesting tumor shrinkage during SARS-Cov-2 infection. Ther Adv Med Oncol. 2022;14:17588359221138388. doi: 10.1177/17588359221138388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchler T, Fiser L, Benesova J, Jirickova H, Votrubova J. Spontaneous regression of metastatic renal cell carcinoma after SARS-CoV-2 infection: A report of two cases. Curr Oncol. 2021;28:3403–3407. doi: 10.3390/curroncol28050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Anton-Plagaro C, Shoemark DK, Simon-Gracia L, Bauer M, Hollandi R, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192:415–421. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 18.Pasin F, Mascalchi Calveri M, Calabrese A, Pizzarelli G, Bongiovanni I, Andreoli M, Cattaneo C, Rignanese G. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed. 2020 Aug 10;91 doi: 10.23750/abm.v91i3.10141. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin D, Shen Y, Liang T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct Target Ther. 2023;8:156–173. doi: 10.1038/s41392-023-01407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dock G. The influence of complicating diseases upon leukemia. Am J Med Sci. 1904;127:563–592. doi: 10.1097/00000441-190412740-00001. [DOI] [Google Scholar]
- 21.Kristin DP, Greg MD. Integrating innate and adaptive immunity in oncolytic virus therapy. Trends Cancer. 2023;23:190–196. doi: 10.1016/j.trecan.2023.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun CO, Hong J, Yoon AR. Current clinical landscape of oncolytic viruses as novel cancer immunotherapeutic and recent preclinical advancements. Front Immunol. 2022;13:95–104. doi: 10.3389/fimmu.2022.953410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscolini M, Tassone E, Hiscott J. Oncolytic immunotherapy: Can't start a fire without a spark. Cytokine Growth Factor Rev. 2020;56:94–101. doi: 10.1016/j.cytogfr.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Hamad A, Yusubalieva GM, Baklaushev VP, Chumakov PM, Lipatova AV. Recent developments in glioblastoma therapy: Oncolytic viruses and emerging future strategies. Viruses. 2023;15:106–119. doi: 10.3390/v15020547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NGS data generated in the present study are not publicly available to preserve patient anonymity. All other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.