Abstract
Introduction
In the placebo-controlled, phase 3 PACIFIC trial, durvalumab significantly prolonged progression-free survival (PFS) (p < 0.0001) and overall survival (OS) (p = 0.00251) in patients with unresectable stage III NSCLC and no progression after platinum-based concurrent chemoradiotherapy (cCRT). Pneumonitis or radiation pneumonitis (PRP) was common in both arms. We report exploratory analyses evaluating the association of symptomatic (grade ≥2) PRP (G2+PRP) with baseline factors and clinical outcomes.
Methods
Patients with WHO performance status of 0 or 1 were randomized (2:1) to 12 months of durvalumab or placebo, 1 to 42 days after cCRT. Associations between baseline factors and on-study G2+PRP in durvalumab-treated patients were investigated using univariate and multivariate logistic regression. PFS and OS were analyzed using Cox proportional hazards models adjusted for time-dependent G2+PRP plus covariates for randomization stratification factors without and with additional baseline factors.
Results
On-study G2+PRP occurred in 94 of 475 (19.8%) and 33 of 234 patients (14.1%) on durvalumab and placebo, respectively (median follow-up, 25.2 mo); grade greater than or equal to 3 PRP was uncommon (4.6% and 4.7%, respectively). Time to onset and resolution of G2+PRP was similar with durvalumab and placebo. Univariate and multivariate analyses identified patients treated in Asia, those with stage IIIA disease, those with performance status of 1, and those who had not received induction chemotherapy as having a higher risk of G2+PRP. PFS and OS benefit favoring durvalumab versus placebo was maintained regardless of time-dependent G2+PRP.
Conclusions
Factors associated with higher risk of G2+PRP with durvalumab after cCRT were identified. Clinical benefit was maintained regardless of on-study G2+PRP, suggesting the risk of this event should not deter the use of durvalumab in eligible patients with unresectable stage III NSCLC.
Keywords: Immunotherapy, Radiotherapy, Durvalumab, Pulmonary toxicity, Locally advanced NSCLC
Introduction
Historically, the standard of care for patients with unresectable stage III NSCLC and adequate performance status (PS) was platinum-based chemotherapy and radiotherapy (RT) administered concurrently (or sequentially in patients considered unsuitable for concurrent treatment), leading to 2-year overall survival (OS) rates between 50% and 60%.1, 2, 3
Immune checkpoint inhibition (ICI) of the programmed cell death-(ligand) 1 (PD-[L]1) pathway has revolutionized the management of advanced NSCLC. PACIFIC (NCT02125461) was the first phase 3 trial to reveal the benefits of ICI in nonmetastatic NSCLC; patients with unresectable stage III NSCLC and no tumor progression after platinum-based, concurrent chemoradiotherapy (cCRT) were randomized to receive durvalumab or placebo for up to 12 months. Durvalumab significantly prolonged progression-free survival (PFS) (stratified hazard ratio [HR] = 0.52, 95% confidence interval [CI]: 0.42–0.65, p < 0.0001) and OS (stratified HR = 0.68, 95% CI: 0.53–0.87, p = 0.00251), with manageable safety and no detrimental impact on quality of life versus placebo.4, 5, 6, 7 Moreover, a 5-year update revealed sustained OS and durable PFS benefit with durvalumab.8 The PACIFIC regimen (consolidation durvalumab after platinum-based CRT) received approvals in the United States of America, Japan, Europe, and elsewhere6,9,10 and has become a standard of care in this setting.
Although ICI is generally well tolerated compared with chemotherapy, ICI-related toxicities may occur.11,12 Of these, pneumonitis can be the most serious13 and is of particular concern in the post-CRT setting, as it is also a common complication of radiation (RT-pneumonitis). When ICI-related pneumonitis is suspected, clinical vigilance is required to effectively diagnose and manage this adverse event (AE).14 Interventions range from observation (for grade 1 [asymptomatic] pneumonitis; graded per Common Terminology Criteria for Adverse Events [CTCAE] and typically diagnosed based on radiological findings) to temporary or permanent ICI cessation and immunosuppressive therapy (for grade ≥2 [symptomatic] pneumonitis).12,15
Challenges associated with the differential diagnosis of pneumonitis with ICI delivered soon after CRT, and differences in diagnostic procedures across centers (as allowed by the PACIFIC protocol, which did not mandate specific tests), meant the attribution of pneumonitis to ICI could not be established reliably in PACIFIC. Therefore, all-cause pneumonitis (pneumonitis or RT-pneumonitis) was reported. Any-grade pneumonitis or RT-pneumonitis occurred in 33.9% and 24.8% of patients with durvalumab and placebo, respectively; grade 3 or 4 events were reported in 3.4% and 3.0% of patients, respectively.6 With broad application of the PACIFIC regimen, the diagnosis and management of pneumonitis or RT-pneumonitis in this setting, particularly clinically relevant grade greater than or equal to 2 events, has become a subject of considerable interest. To give further insight to clinicians, we report detailed, post hoc analyses from PACIFIC that characterize grade greater than or equal to 2 pneumonitis or RT-pneumonitis and evaluate its potential association with baseline factors and clinical outcomes.
Materials and Methods
Patients, Study Design, and Treatment
PACIFIC is a phase 3, randomized, double-blind trial of adult patients with unresectable stage III NSCLC, WHO PS 0 or 1, any tumor PD-L1 status, and no tumor progression after platinum-based cCRT (≥2 cycles). Patients with unresolved grade greater than 2 toxicities (CTCAE version 4.03), or grade greater than or equal to 2 pneumonitis or RT-pneumonitis, from prior cCRT were excluded. Participants were randomized (2:1), 1 to 42 days after completing cCRT, to durvalumab (10 mg/kg intravenously) or placebo, administered every 2 weeks for 12 months or until confirmed progression, initiation of alternative anticancer therapy, unacceptable toxicity, or consent withdrawal. Randomization was stratified by age, sex, and smoking history. All patients provided written informed consent for participation in the trial. Further details of the trial design are published elsewhere.4
The primary end points were PFS (according to Response Evaluation Criteria in Solid Tumors version 1.1; assessed by blinded independent central review) and OS. Secondary end points included time to death or distant metastasis (TTDM; blinded independent central review) and safety.
Assessment of Pneumonitis or RT-Pneumonitis
Pneumonitis or RT-pneumonitis was investigator assessed (CTCAE version 4.03) and defined as a focal or diffuse inflammation of the lung parenchyma; this included diagnoses of acute interstitial pneumonitis, interstitial lung disease, pneumonitis, pulmonary fibrosis, alveolitis, diffuse alveolar damage, and RT-pneumonitis. In all cases, institutional standards in serologic, immunologic, and histologic testing were recommended in the protocol to rule out neoplastic, infectious, or other possible causes. On-study pneumonitis or RT-pneumonitis was defined as a de novo event that occurred during study treatment or a preexisting grade 1 event that worsened during the study and within 90 days of last dose of study medication or before the initiation of subsequent anticancer therapy (whichever occurred earlier). We characterized time to onset and resolution of grade greater than or equal to 2 pneumonitis or RT-pneumonitis and investigated the association of grade greater than or equal to 2 pneumonitis or RT-pneumonitis with (1) baseline characteristics and (2) study treatment exposure. Moreover, we investigated the association of grade greater than or equal to 2 pneumonitis or RT-pneumonitis with efficacy and safety outcomes and summarized the clinical management and outcomes of grade greater than or equal to 2 pneumonitis or RT-pneumonitis cases. Guidelines for managing ICI-related toxicities, including pneumonitis (see the Supplementary Methods), were provided to the investigators; these were considered recommendations, and, ultimately, management decisions were left to the investigator’s clinical judgment.
Statistical Analyses
All analyses were based on the data cutoff for the primary OS analysis (March 22, 2018).5 The following were summarized with descriptive statistics using the as-treated population: times to onset and resolution of grade greater than or equal to 2 pneumonitis or RT-pneumonitis; study treatment exposure by the presence or absence of grade greater than or equal to 2 pneumonitis or RT-pneumonitis; treatment-emergent, all-causality AEs by the presence or absence of grade greater than or equal to 2 pneumonitis or RT-pneumonitis; and clinical management and outcomes of grade greater than or equal to 2 pneumonitis or RT-pneumonitis cases.
We investigated associations between baseline clinical characteristics and grade greater than or equal to 2 pneumonitis or RT-pneumonitis with descriptive statistics and regression analyses using the intent-to-treat population. Univariate logistic regression models were used to assess the relative risk by odds ratios (ORs) and 95% CIs for occurrence of grade greater than or equal to 2 pneumonitis or RT-pneumonitis for individual baseline characteristics. Multivariate logistic regression models were used to investigate the relative importance of these characteristics and interactions between them, through backward selection. Regression analyses were performed in the durvalumab arm using the as-treated population; similar analyses were not performed for placebo because the sample size was insufficient.
To investigate the possible impact of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis on clinical benefit with durvalumab versus placebo, we performed analyses to assess whether there was any change in observed treatment effects (HRs) for PFS, OS, and TTDM when adjusting for the occurrence of this event. Aligned with the methodology used for prespecified time-to-event analyses from PACIFIC, Cox proportional hazards models were used to estimate HRs and 95% CIs (based on the intent-to-treat population) for durvalumab versus placebo stratified by age, sex, and smoking history (the randomization stratification factors). Two models were fitted to the efficacy data to assess the impact of grade greater than or equal to 2 pneumonitis or RT-pneumonitis. Occurrence of on-study pneumonitis is a post-baseline event and as such has an implicit association with duration of therapy and thus the efficacy measures; therefore, model 1 added a covariate for time-dependent grade greater than or equal to 2 pneumonitis or RT-pneumonitis. Acknowledging the risk of (time-dependent) pneumonitis is also affected by baseline characteristics; model 2 incorporated further covariates for additional baseline characteristics (disease stage, histology [squamous vs nonsquamous], best response to prior therapy, WHO PS, region, and race).
Results
Patients and Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis
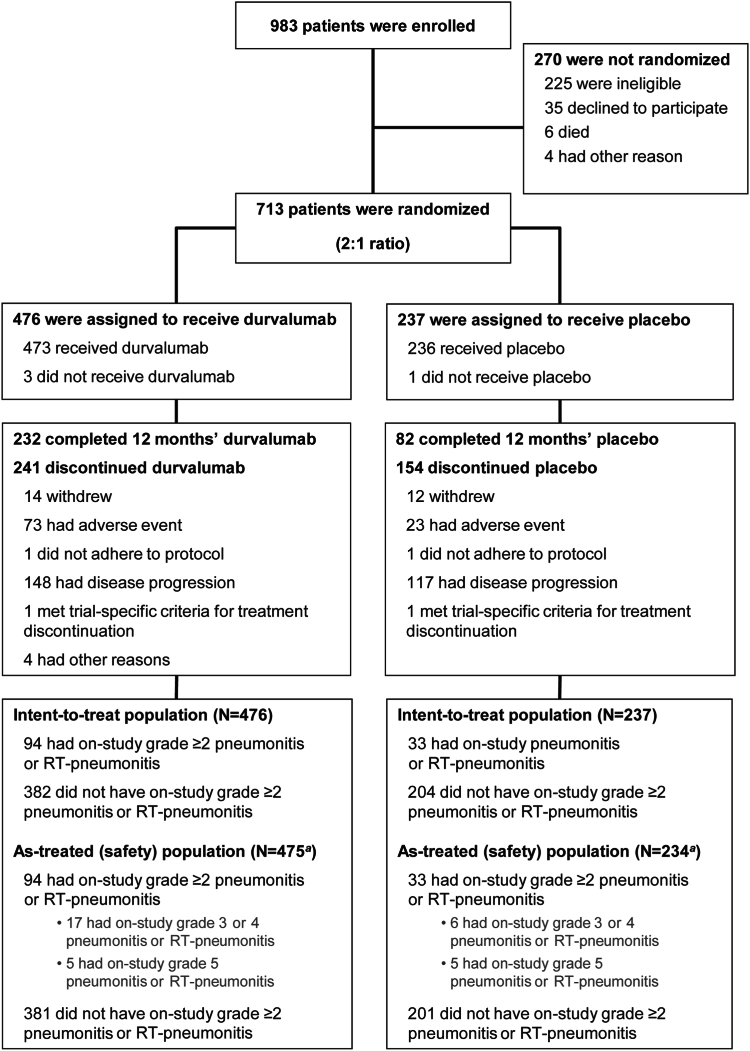
In the PACIFIC trial, 709 of 713 patients who were randomized to durvalumab (n/N = 473 of 476) or placebo (n/N = 236 of 237) received a study treatment (Fig. 1); two patients assigned to placebo erroneously received one dose of durvalumab and were included in the durvalumab as-treated population. As of March 22, 2018 (median follow-up, 25.2 mo [range, 0.2–43.1]), on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis had occurred in 94 of 475 (19.8%) and 33 of 234 patients (14.1%) who received durvalumab and placebo, respectively (as-treated population); 22 of 475 (4.6%) and 11 of 234 patients (4.7%) experienced grade greater than or equal to 3 events and five of 475 (1.1%) and five of 234 (2.1%) experienced grade 5 (fatal) events, respectively. One patient (durvalumab) with preexisting grade 1 pneumonitis or RT-pneumonitis at baseline experienced on-study worsening of their event (to grade 2). Recurrence of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis was uncommon (durvalumab, three of 475 [0.6%]; placebo, two of 234 [0.9%]), and no patients experienced more than 2 events. A breakdown of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis events by the investigators’ preferred attribution term is provided (Supplementary Table 1).
Figure 1.
CONSORT Diagram. Shown are data collected up to March 22, 2018, the last data cutoff for which safety data (including pneumonitis or RT-pneumonitis) was analyzed. Patients who completed (protocol-defined) 12 months’ durvalumab or placebo are those for whom the electronic case report form revealed that they had received the maximum number of cycles of study treatment. aTwo patients who were randomized to placebo erroneously received one dose of durvalumab and were included in the durvalumab as-treated population. RT, radiotherapy.
Although the PACIFIC protocol originally mandated randomization to study treatment within 14 days of completing CRT, this criterion was amended to 42 days to allow enrollment of patients recovering from CRT-related toxicities.4 Among patients who received durvalumab and were randomized less than 14 days post-CRT, 20 of 120 (16.7%) experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis (five of 120 [4.2%] had grade greater than or equal to 3 events; none were fatal); meanwhile, 74 of 355 (20.8%) who were randomized more than or equal to 14 days post-CRT experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis (17 of 355 [4.8%] had grade greater than or equal to 3 events; five of 355 had fatal events [1.4%]). Among patients who received placebo and were randomized less than 14 days post-CRT, five of 60 (8.3%) experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis (three of 60 [5.0%] had grade greater than or equal to 3 events; two of 60 had fatal events [3.3%]); meanwhile, 28 of 174 (16.1%) patients who were randomized more than or equal to 14 days post-CRT experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis (eight of 174 [4.6%] had grade greater than or equal to 3 events; three of 174 had fatal events [1.7%]).
Times to Onset and Resolution of Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis
Median time to onset of grade greater than or equal to 2 pneumonitis or RT-pneumonitis from initiation of durvalumab (53.5 d [range: 2–406]; n = 94) and placebo (55.0 d [14–253]; n = 33) was similar; onset occurred more than 90 days after the first dose in 18 of 94 (19.1%) and seven of 33 patients (21.2%) with durvalumab and placebo, respectively. Median time to onset from the completion of RT was also similar with durvalumab (70.0 d [range: 21–433]; n = 94) and placebo (79.0 d [34–271]; n = 33). Excluding events ongoing at the data cutoff, median time to resolution of grade greater than or equal to 2 pneumonitis or RT-pneumonitis (or death) was similar with durvalumab (57.5 d [2–588]; n = 64) and placebo (52.0 d [4–186]; n = 19). Times to onset and resolution of grade greater than or equal to 2 pneumonitis or RT-pneumonitis according to the time elapsed between RT completion and randomization to durvalumab or placebo are provided (Supplementary Table 2).
Association Between Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis and Baseline Characteristics
Baseline characteristics were generally well balanced irrespective of the occurrence of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis (Supplementary Tables 3–5). Nevertheless, in both the durvalumab and placebo arms, a higher proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis were of Asian ethnicity (durvalumab, 35 of 94 [37.2%]; placebo, 16 of 33 [48.5%]) versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (durvalumab, 85 of 382 [22.3%]; placebo, 56 of 204 [27.5%]); a higher proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis were treated in Asia (durvalumab, 31 of 94 [33.0%]; placebo, 15 of 33 [45.5%]) versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (durvalumab, 78 of 382 [20.4%]; placebo, 53 of 204 [26.0%]); a higher proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis were nonsmokers (durvalumab, 18 of 94 [19.1%]; placebo, six of 33 [18.2%]) versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (durvalumab, 25 of 382 [6.5%]; placebo, 15 of 204 [7.4%]); and a lower proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis had prior chronic obstructive pulmonary disease (COPD) (durvalumab, 18 of 94 [19.1%]; placebo, four of 33 [12.1%]) versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (durvalumab, 101 of 382 [26.4%]; placebo, 54 of 204 [26.5%]). Moreover, in the durvalumab arm only, a higher proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis had PS 1 (60 of 94 [63.8%]) versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (180 of 382 [47.1%]), and a higher proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis had stage IIIA disease (61 of 94 [64.9%]) versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (191 of 382 [50.0%]).
Most patients received a RT dose between 54 and 66 gray (Gy) (Supplementary Table 6). The median total RT dose among patients with on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis (durvalumab, 60.0 Gy [range: 55.0–70.2]; placebo, 60.6 Gy [60.0–70.2]) was numerically lower versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (durvalumab, 61.5 Gy [45.0–70.2]; placebo, 63.0 Gy [54.0–70.2]), regardless of treatment arm. Carboplatin–paclitaxel was the most used chemotherapy regimen during platinum-based CRT, followed by cisplatin–etoposide; similar proportions of patients received these regimens regardless of the occurrence of grade greater than or equal to 2 pneumonitis or RT-pneumonitis in both the durvalumab and placebo arms (Supplementary Table 6). A small proportion of patients received induction chemotherapy before CRT4; in the durvalumab arm only, a lower proportion of patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis (13 of 94 [13.8%]) received induction chemotherapy versus those without grade greater than or equal to 2 pneumonitis or RT-pneumonitis (110 of 382 [28.8%]) (Supplementary Table 6).
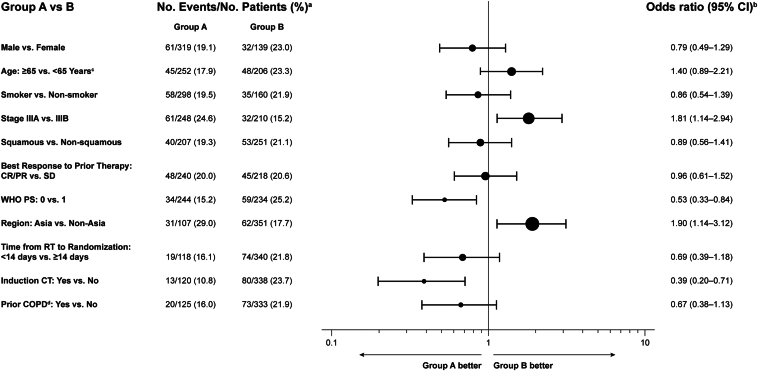
Univariate analyses in durvalumab-treated patients identified a higher risk of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis among patients treated in Asia (OR, 1.90; 95% CI: 1.14–3.12), patients with stage IIIA disease (OR, 1.81; 95% CI: 1.14–2.94), patients with PS 1 (OR, 0.53; 95% CI: 0.33–0.84), and patients who had not received induction chemotherapy (OR, 0.39; 95% CI: 0.20–0.71) (Fig. 2). These factors were also associated with significantly higher risk in multivariate backward-selection analyses (nominal p < 0.05) (Supplementary Table 7). The multivariate analyses also identified patients without prior COPD as having a higher risk of grade greater than or equal to 2 pneumonitis or RT-pneumonitis, and multivariate interaction models further identified significant interactions (nominal p < 0.1) between (1) PS and prior COPD and (2) geographic region and induction chemotherapy (Supplementary Table 7).
Figure 2.
Univariate analyses of baseline factors potentially associated with grade greater than or equal to 2 pneumonitis or RT-pneumonitis in durvalumab-treated patients. aSeventeen patients were excluded from the analyses due to missing data for stage and/or best response to prior therapy. bThe OR of grade ≥2 pneumonitis or RT-pneumonitis occurrence was derived using logistic regression; an OR for group A versus group B of <1 implies lower risk of grade ≥2 pneumonitis or RT-pneumonitis for group A relative to group B. cAge at randomization. dCOPD was analyzed as a grouped term comprising diagnoses of COPD, bronchitis chronic, emphysema, and obstructive airways disorder. CI, confidence interval; COPD, chronic obstructive pulmonary disease; CR, complete response; CT, chemotherapy; OR, odds ratio; PR, partial response; RT, radiotherapy; SD, stable disease; PS, performance status.
Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis and Efficacy Outcomes
In the Cox analyses adjusting for time-dependent occurrence of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis, OS, PFS, and TTDM benefit of durvalumab versus placebo was maintained in both model 1 (which used the trial stratification factors and time-dependent grade ≥2 pneumonitis or RT-pneumonitis as covariates) and model 2 (which further incorporated additional baseline factors); results from both models were consistent with the intent-to-treat analyses (Table 1). The time-dependent covariate for grade greater than or equal to 2 pneumonitis or RT-pneumonitis was not a significant factor for PFS and TTDM in either model (nominal p > 0.1) but was nominally significant for OS in both models (patients with grade ≥2 pneumonitis or RT-pneumonitis had an increased risk of death at any given time versus those without the event); however, the benefit of durvalumab versus placebo was unchanged when the time-dependent covariate was incorporated into the OS models. Results from Cox models for OS, PFS, and TTDM in subsets of patients with and without on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis (with treatment as the only covariate) are provided (Supplementary Table 8); these subset analyses were limited to patients who received more than or equal to 24 weeks of treatment to address potential bias from misclassification of patients who were not on study treatment long enough to experience pneumonitis.
Table 1.
OS, PFS, and TTDM Adjusted for the Time-Dependent Occurrence of Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis and for the Intent-to-Treat Population
| Outcome | No. of Events/No. of Patients (%) |
HR (95% CI) for Durvalumab vs. Placebo |
||
|---|---|---|---|---|
| Durvalumab | Placebo | Adjusted for Time-Dependent Grade ≥2 Pneumonitis or RT-Pneumonitis | ITT Analysisa | |
| OS | 183/476 (38.4) | 116/237 (48.9) | ||
| Model 1b | 0.67 (0.53–0.85) | 0.68 (0.53–0.87)5,6 | ||
| Model 2c | 0.63 (0.50–0.80) | − | ||
| PFS (BICR) | 243/476 (51.1) | 173/237 (73.0) | ||
| Model 1b | 0.54 (0.44–0.66) | 0.51 (0.41–0.63)5 | ||
| Model 2c | 0.52 (0.42–0.64) | − | ||
| TTDM (BICR) | 182/476 (38.2) | 126/237 (53.2) | ||
| Model 1b | 0.55 (0.44–0.70) | 0.53 (0.41–0.68)5 | ||
| Model 2c | 0.51 (0.41–0.65) | − | ||
BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; TTDM, time to death or distant metastasis.
For the ITT analyses, PFS, OS, and TTDM were assessed using a stratified Cox proportional hazards model (to estimate HRs and 95% CIs), adjusted for trial stratification factors: age at randomization (<65 vs. ≥65 y), sex (male vs. female), and smoking history (smoker vs. never smoked).4, 5, 6 HR less than 1 favors durvalumab over placebo.
Model 1 (the base model) accounts for stratification factors at randomization (aligned with the ITT analyses) and the time-dependent occurrence of grade greater than or equal to 2 pneumonitis or RT-pneumonitis.
Model 2 is the base model plus additional baseline factors: stage of disease (IIIA vs. IIIB), histology (squamous vs. nonsquamous), best response to prior anticancer therapy (complete response vs. partial response vs. stable disease), WHO performance status (0 vs. 1), region (Asia vs. Europe vs. North America and South America), and race (White vs. Black or African American vs. Asian vs. Other).
Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis and Safety Outcomes
Among patients who experienced on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis, the incidence of serious AEs was proportionally higher with durvalumab (53.2%) versus placebo (36.4%); meanwhile, the incidence of grade 3 or 4 AEs and AEs leading to treatment discontinuation was similar between the treatment arms (Table 2).
Table 2.
Treatment-Emergent, All-Causality Adverse Events by Presence or Absence of Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis
|
AE Category |
Present |
Absent |
||
|---|---|---|---|---|
| Durvalumab (n = 94) | Placebo (n = 33) | Durvalumab (n = 381) | Placebo (n = 201) | |
| Any-grade, n (%) | 94 (100) | 33 (100) | 366 (96.1) | 189 (94.0) |
| Grade 3 or 4, n (%) | 32 (34.0) | 9 (27.3) | 123 (32.3) | 57 (28.4) |
| Grade 5 (fatal), n (%) | 7 (7.4) | 6 (18.2) | 14 (3.7) | 9 (4.5) |
| Leading to discontinuation, n (%) | 34 (36.2) | 11 (33.3) | 39 (10.2) | 12 (6.0) |
| Serious, n (%) | 50 (53.2) | 12 (36.4) | 88 (23.1) | 42 (20.9) |
AE, adverse event; RT, radiotherapy.
When comparing patients with on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis and patients without this event, the incidence of grade 3 or 4 AEs was similar, both for durvalumab and placebo (Table 2). With durvalumab, the incidence of AEs leading to treatment discontinuation (36.2% versus 10.2%), serious AEs (53.2% versus 23.1%), and fatal AEs (7.4% versus 3.7%) was higher among patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis. Likewise, with placebo, the incidence of AEs leading to treatment discontinuation (33.3% versus 6.0%), serious AEs (36.4% versus 20.9%), and fatal AEs (18.2% versus 4.5%) was higher among patients with grade greater than or equal to 2 pneumonitis or RT-pneumonitis.
Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis and Study Treatment Exposure
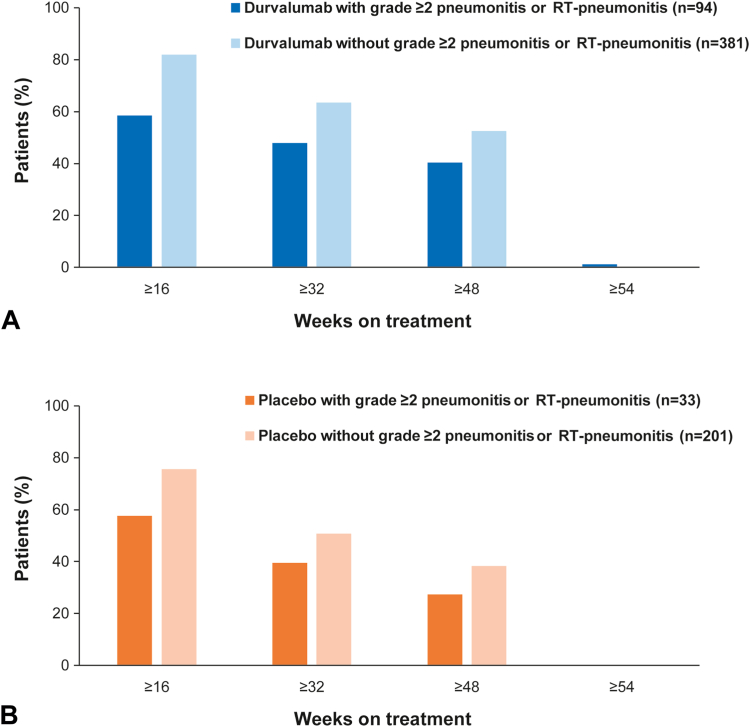
Cumulative exposure to durvalumab (Fig. 3A) and placebo (Fig. 3B) was proportionally lower among patients with on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis versus those who did not experience this event. Correspondingly, a lower proportion of patients who experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis completed the (protocol-defined) 12 months of durvalumab therapy (37 of 94 [39.4%] with and 195 of 382 [51.5%] without the event); the trend was similar for placebo (nine of 33 [27.3%] and 73 of 204 [36.0%], respectively) (Supplementary Table 9). With durvalumab, 76 of 94 (80.9%) patients who experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis stopped treatment to manage this AE; 54 of 76 (71.1%) of these patients were rechallenged with durvalumab, and five of 54 (9.3%) of the rechallenged patients went on to permanently discontinue treatment due to worsening or recurrence of pneumonitis or RT-pneumonitis.
Figure 3.
Study treatment exposure by presence or absence of grade greater than or equal to 2 pneumonitis or RT-pneumonitis in patients who received (A) durvalumab and (B) placebo. RT, radiotherapy.
Clinical Management and Outcomes of Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis
With durvalumab, the most common interventions for grade greater than or equal to 2 pneumonitis or RT-pneumonitis were administration of any-dose (84 of 94 [89.4%]) and high-dose systemic corticosteroids (53 of 94 [56.4%]; defined as a dose equating to ≥40 mg prednisone daily) and temporary interruption of treatment (49 of 94 [52.1%]) (Table 3); intervention rates were generally similar with placebo. Corticosteroid administration for a continuous period of more than or equal to 12 weeks to manage grade greater than or equal to 2 pneumonitis or RT-pneumonitis was required by 25 of 94 (26.6%) and five of 33 (15.2%) patients who experienced this event with durvalumab and placebo, respectively. Additional immunosuppression (beyond corticosteroids) to manage grade greater than or equal to 2 pneumonitis or RT-pneumonitis was uncommon with durvalumab (two of 94 [2.1%]; both patients received infliximab) and placebo (one of 33 [3.0%]; the patient received cyclophosphamide and tacrolimus); all three patients who received additional immunosuppression subsequently had fatal pneumonitis or RT-pneumonitis events. Overall, 28 of 94 (29.8%) and 10 of 33 patients (30.3%) permanently discontinued durvalumab and placebo, respectively, to manage grade greater than or equal to 2 pneumonitis or RT-pneumonitis. For durvalumab, this included 12 of 72 (16.7%) and 11 of 17 patients (64.7%) with events of maximum grade 2 and 3, respectively (no grade 4 events were reported); similar proportions of patients discontinued placebo at each toxicity grade.
Table 3.
Management and Outcomes of Grade Greater Than or Equal to 2 Pneumonitis or RT-Pneumonitis
| No. of Patients (Durvalumab – n = 94) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade ≥2 Pneumonitis or RT-Pneumonitis |
Management or Intervention |
Clinical Outcome |
|||||||
| Highest CTCAE Grade | Any AE | Temporary Treatment Interruption | Permanent Treatment Discontinuation | Any-Dose Corticosteroid | High-Dose Corticosteroida | Other Treatmentb | Resolvedc | Not Resolved | Fatal |
| 2 | 72 | 45d | 12d | 63 | 37 | 0 | 47 | 25 | – |
| 3 | 17 | 4 | 11e | 16 | 11 | 0 | 12 | 5 | – |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 5 | 5 | 0 | 5 | 5 | 5 | 2 | 0 | 0 | 5 |
| Total, n (%) | 94 (100) | 49 (52.1) | 28 (29.8) | 84 (89.4) | 53 (56.4) | 2 (2.1) | 59 (62.8) | 30 (31.9) | 5 (5.3) |
| No. of Patients (Placebo – n = 33) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade ≥2 Pneumonitis or RT-Pneumonitis |
Management or Intervention |
Clinical Outcome |
|||||||
| Highest CTCAE Grade | Any AE | Temporary Treatment Interruption | Permanent Treatment Discontinuation | Any-Dose Corticosteroid | High-Dose Corticosteroida | Other Treatmentb | Resolvedc | Not resolved | Fatal |
| 2 | 22 | 15d | 3d | 19 | 14 | 0 | 15 | 7 | – |
| 3 | 6 | 3 | 4e | 6 | 6 | 0 | 2 | 4 | – |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 5 | 5 | 0 | 3f | 5 | 4 | 1 | 0 | 0 | 5 |
| Total, n (%) | 33 (100) | 18 (54.5) | 10 (30.3) | 30 (90.9) | 24 (72.7) | 1 (3.0) | 17 (51.5) | 11 (33.3) | 5 (15.2) |
Note: Data are tabulated according to responses in the AE eCRF. In the course of the study, patients could receive several interventions for pneumonitis or RT-pneumonitis (e.g., a patient could have both “interrupted” and “discontinued” study treatment).
AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; eCRF, electronic case report form; RT, radiotherapy.
A dose that equates to at least 40 mg prednisone daily.
Two durvalumab-treated patients received infliximab and one placebo-treated patient received cyclophosphamide and tacrolimus.
Resolution of pneumonitis or RT-pneumonitis was determined by the investigator in accordance with local practice.
Among patients with an event of maximum (max.) grade 2, 21 (durvalumab, 17; placebo, 4) had grade 2 events for which a treatment dose action of interruption or discontinuation was not recorded on the AE eCRF: six (durvalumab, 3; placebo, 3) had an exposure record indicating “treatment cycle delay” for reason of AE during the event; three (durvalumab, 2; placebo, 1) had a record indicating “treatment cycle delay” for reason of AE at one of the next two doses after the event ended; four (all durvalumab) had an event occur after completing the max. number of cycles of study treatment, so no dose action could be taken; three (all durvalumab) had treatment end shortly after the start of the event; and five (all durvalumab) had no dose action taken either during or soon after the event.
Among patients with an event of max. grade 3, nine (durvalumab, 6; placebo, 3) had grade 3 events for which treatment discontinuation was not recorded on the AE eCRF: four (durvalumab, 2; placebo, 2) had an event occur after completing the max. number of cycles of study treatment, so no dose action could be taken (one additional patient discontinued placebo for grade 3 pneumonitis or RT-pneumonitis [and is tabulated as discontinuing at this toxicity grade] but subsequently experienced a separate on-study grade 5 event, for which no dose action could be taken); and five (durvalumab, 4; placebo, 1) interrupted, but did not permanently discontinue, study treatment.
Among patients with an event of max. grade 5, two (both placebo) had grade 5 events for which a treatment discontinuation was not recorded on the AE eCRF: one had already discontinued treatment due to a previous separate grade 3 pneumonitis or RT-pneumonitis event (as mentioned previously) and one had an event occur after completing the max. number of cycles of study treatment, so no dose action could be taken in either case.
In PACIFIC, resolution of pneumonitis or RT-pneumonitis was determined by the investigator in accordance with local practice. At the data cutoff, 59 of 94 (62.8%) and 17 of 33 patients (51.5%) who experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis with durvalumab and placebo, respectively, had an event resolve. Among patients with events of maximum grade 2, 47 of 72 (65.3%) and 15 of 22 (68.2%) who received durvalumab and placebo, respectively, achieved resolution. Among patients who experienced events of maximum grade 3, 12 of 17 (70.6%) and two of six (33.3%) who received durvalumab and placebo, respectively, achieved resolution. Overall, pneumonitis or RT-pneumonitis events had a fatal outcome in five of 94 (5.3%) and five of 33 patients (15.2%) with durvalumab and placebo, respectively.
Discussion
In the PACIFIC trial, on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis occurred in 19.8% and 14.1% of patients who received durvalumab and placebo, respectively; grade greater than or equal to 3 pneumonitis or RT-pneumonitis was uncommon (<5%). Median time to onset of grade greater than or equal to 2 pneumonitis or RT-pneumonitis from initiation of durvalumab and placebo was similar, and most cases occurred within 3 months (approximately 80%). In patients who received the PACIFIC regimen, univariate and multivariate analyses identified patients treated in Asia (versus non-Asia), those with stage IIIA disease (versus IIIB), those with PS 1 (versus 0), and those who had not received induction chemotherapy (versus those who had) as having a higher risk of experiencing grade greater than or equal to 2 pneumonitis or RT-pneumonitis. Clinical benefit with durvalumab was maintained regardless of on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis. Moreover, durvalumab exhibited a manageable safety profile broadly irrespective of this event. Reassuringly, fatal pneumonitis or RT-pneumonitis events were uncommon, and the incidence was comparable with durvalumab (1.1%) and placebo (2.1%). These findings are generally consistent with previously reported analyses of on-study, any-grade pneumonitis or RT-pneumonitis from PACIFIC.16,17
Pneumonitis is of concern to patients with NSCLC as pulmonary function can already be compromised by local tumor burden and co-existent pulmonary and cardiac comorbidities. Moreover, pneumonitis is a common complication of RT, and prior chest RT correlates with pneumonitis occurrence on ICI therapy, suggesting possible synergy between these treatments with regard to the development of pneumonitis.18, 19, 20, 21 Therefore, exploring the association of pneumonitis occurring on ICI therapy with clinically relevant factors, and assessing its impact on the efficacy of ICI (as reported here), is vital to informing patient management and potential risk assessment for the development of pneumonitis.
Patients who experienced on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis in PACIFIC had a worse OS prognosis compared with patients who did not experience this event. This is consistent with findings from other studies of patients who received ICI, which suggest that (unlike other ICI-related AEs), pneumonitis correlates with poorer survival.22, 23, 24 Nevertheless, it is not possible to conclude specifically on the prognostic impact of ICI-related pneumonitis based on data from PACIFIC as RT-pneumonitis is also associated with poorer OS,25 and the causes of pneumonitis could not be established reliably in the post-CRT treatment setting.
The incidence of any-grade and grade greater than or equal to 2 pneumonitis or RT-pneumonitis in PACIFIC was higher compared with trials of PD-1 and PD-L1 ICI in stage IIIB or IV NSCLC.4,6,26, 27, 28, 29, 30, 31 This is likely owing to curative-intent RT received before enrollment, a hypothesis supported by the higher incidence of pneumonitis or RT-pneumonitis in the placebo arm of PACIFIC relative to the control arms of the aforementioned studies (where, unlike PACIFIC, the treatment paradigm did not mandate receipt of RT 1–42 d before starting ICI).4,6,26, 27, 28, 29, 30, 31 Although pneumonitis or RT-pneumonitis was common in PACIFIC, most events were grade 1 or 2 (asymptomatic or presenting with mild symptoms not requiring oxygen supplementation), consistent with other studies of ICI administered after CRT.4,6,32, 33, 34, 35
There did not seem to be a clinically meaningful association between the incidence or severity of grade greater than or equal to 2 pneumonitis or RT-pneumonitis and whether patients were randomized within or beyond 14 days of completing CRT, suggesting that durvalumab initiation should not be delayed for the sole purpose of reducing the risk of pneumonitis. Nevertheless, the timing of durvalumab initiation after CRT in PACIFIC was not random and may have been biased by associations with potentially prognostic baseline characteristics; for example, patients with smaller disease volumes and a lower lung RT dose may have recovered from CRT more rapidly, possibly allowing them to start durvalumab earlier.
The finding that patients treated in Asia were at higher risk of grade greater than or equal to 2 pneumonitis or RT-pneumonitis with the PACIFIC regimen (found in both univariate and multivariate analyses) aligns with the high incidence of this event observed in real-world Asian cohorts.33, 34, 35, 36 Grade greater than or equal to 2 pneumonitis or RT-pneumonitis incidence was also higher among patients from Asia in the placebo arm of PACIFIC. These results align with the finding that pneumonitis events were more common among Asian patients who receive other anticancer agents (e.g., EGFR tyrosine kinase inhibitors37). Thus, Asian patients may have a higher risk of being diagnosed with having pneumonitis related to anticancer therapy in general.
The higher risk of grade greater than or equal to 2 pneumonitis or RT-pneumonitis among patients with stage IIIA (versus IIIB) NSCLC (found in both univariate and multivariate analyses) was unexpected as, typically, larger lung volumes are irradiated (a known risk factor for RT-pneumonitis18) in patients with stage IIIB disease. We are uncertain of the factors underpinning this finding; however, the risk factor analyses are limited by small patient numbers and their post hoc nature and may be biased by unobserved imbalances in baseline factors that correlate with the incidence or severity of pneumonitis or RT-pneumonitis. For instance, RT-planning parameters were not collected in PACIFIC, and several correlate with the severity of pneumonitis (e.g., intensity-modulated RT is associated with lower rates of grade ≥3 pneumonitis versus three-dimensional conformal RT,38 and larger tumor volume is associated with a larger area of normal lung being irradiated, leading to a higher risk of RT-pneumonitis).39
The higher risk of grade greater than or equal to 2 pneumonitis or RT-pneumonitis among patients who did not receive (pre-CRT) induction chemotherapy (found in both univariate and multivariate analyses) was also unexpected. Theoretically, induction chemotherapy may have reduced the patients’ tumor volume, leading to reduced RT treatment volumes and less pulmonary toxicity during and after CRT; there is some evidence to support this.18,40 Nevertheless, this finding should be interpreted cautiously given the aforementioned limitations of the risk factor analyses and because information on disease volume is not available. Moreover, this particular analysis is likely biased as few Asian patients (who are more susceptible to developing pneumonitis) received induction chemotherapy in PACIFIC41; indeed, a nominally significant (p < 0.1) interaction between geographic region and the use of induction chemotherapy was identified.
Grade greater than or equal to 2 pneumonitis or RT-pneumonitis did not affect clinical benefit with durvalumab in the adjusted Cox analyses: the treatment effect estimates were consistent with the intent-to-treat results. Therefore, benefit was observed even though patients who experienced grade greater than or equal to 2 pneumonitis or RT-pneumonitis were less likely to complete the 12-month treatment course. Our finding that pneumonitis or RT-pneumonitis did not affect benefit with durvalumab aligns with findings from a small, real-world cohort of patients who received durvalumab after CRT.42 Nevertheless, as on-study pneumonitis or RT-pneumonitis may occur as a late side effect of prior CRT or because of study treatment, interpretation of the exploratory models we report (which adjusted for grade ≥2 pneumonitis or RT-pneumonitis as a time-dependent factor) is subject to potential bias. In addition, the exploratory models used did not account for the time elapsed from CRT to randomization to study treatment as a potentially prognostic baseline factor; future studies should seek to address this.
Aligned with management guidelines,12,15 interruption of durvalumab and administration of corticosteroids were common interventions for grade greater than or equal to 2 pneumonitis or RT-pneumonitis. Most patients with events of maximum grade 2 did not permanently discontinue durvalumab; treatment rechallenge in this scenario may be appropriate as most had their events resolve. Grade greater than or equal to 2 pneumonitis or RT-pneumonitis lasted approximately 8 weeks (median), indicating that standard steroid tapers of 4 to 6 weeks may be insufficient. Moreover, the wide range of time to resolution for grade greater than or equal to 2 pneumonitis or RT-pneumonitis indicates that some patients require longer management. Indeed, some patients required corticosteroids for a continuous period of greater than or equal to 12 weeks (as observed in other studies43), and a higher proportion required this to manage grade greater than or equal to 2 pneumonitis or RT-pneumonitis in the durvalumab arm versus the placebo arm. As expected, permanent discontinuation of durvalumab was common among patients with grade greater than or equal to 3 pneumonitis or RT-pneumonitis.
In PACIFIC, attribution of pneumonitis to ICI or RT was determined according to local practice44; lack of central review meant that attribution could not be established reliably, so analyses of pneumonitis were performed regardless of attribution for the purposes of the current report. Although clinical presentation and radiographic evaluation do not always allow for discrimination between RT- and ICI-related pneumonitis, future studies may benefit from the use of radiomics to distinguish between these causes.45 Further research is also required to determine whether the features and outcomes of pneumonitis occurring post-CRT differ in their natural history when occurring on, or in the absence of, ICI. Moreover, there is evidence that RT dose-volume factors, including RT techniques, correlate with the occurrence or severity of pneumonitis in patients with stage III NSCLC who undergo CRT.38,46,47 The association of these parameters with pneumonitis should be investigated in the context of the PACIFIC regimen. Initial efforts have begun with retrospective, real-world analyses identifying elevated lung V20 (the percentage of lung volume receiving a RT dose >20 Gy) as a risk factor for grade greater than or equal to 2 pneumonitis or RT-pneumonitis.35,48, 49, 50 RT dose-volume parameters are being collected in the observational PACIFIC-R study (NCT02125461), presenting a possible avenue for further investigation of their relationship with pneumonitis.
In conclusion, factors associated with higher risk of grade greater than or equal to 2 pneumonitis or RT-pneumonitis with the PACIFIC regimen were identified; however, the post hoc analyses of risk factors are limited in scope and other factors, including RT parameters, which were not collected in PACIFIC, could have contributed to these findings. These results therefore warrant further study and validation in other populations. Nevertheless, clinical benefit with durvalumab was maintained in patients who experienced on-study, grade greater than or equal to 2 pneumonitis or RT-pneumonitis, and most achieved resolution of this event, suggesting the risk of grade greater than or equal to 2 pneumonitis or RT-pneumonitis should not deter use of the PACIFIC regimen in eligible patients with unresectable stage III NSCLC.
CRediT Authorship Contribution Statement
Johan F. Vansteenkiste: Resources, Investigation, Conceptualization, Writing—review and editing.
Jarushka Naidoo: Resources, Investigation, Conceptualization, Writing—review and editing.
Corinne Faivre-Finn: Resources, Investigation, Conceptualization, Writing—review and editing.
Mustafa Özgüroğlu: Resources, Investigation, Conceptualization, Writing—review and editing.
Augusto Villegas: Resources, Investigation, Writing—review and editing.
Davey Daniel: Resources, Investigation, Writing—review and editing.
Shuji Murakami: Resources, Investigation, Writing—review and editing.
Rina Hui: Resources, Conceptualization, Investigation, Writing—review and editing.
Ki Hyeong Lee: Resources, Investigation, Writing—review and editing.
Byoung Chul Cho: Resources, Investigation, Writing—review and editing.
Kaoru Kubota: Resources, Investigation, Writing—review and editing.
Helen Broadhurst: Formal Analysis, Methodology, Writing—review and editing.
Catherine Wadsworth: Conceptualization, Writing—review and editing.
Michael Newton: Conceptualization, Writing—review and editing.
Piruntha Thiyagarajah: Conceptualization, Writing—review and editing.
Scott J. Antonia: Resources, Investigation, Writing—review and editing.
Disclosure
Dr. Vansteenkiste reports receiving institutional research funding from Merck Sharp & Dohme; receiving advisory, consulting fees from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, and Roche; receiving payment or honoraria from Merck, Merck Sharp & Dohme, and Sanofi; having participation on a data safety monitoring or advisory board from PDCline, Daiichi Sankyo, and Boehringer Ingelheim; and having a leadership role in the Lung Education Group for European Society for Medical Oncology (ESMO). Prof. Naidoo reports receiving institutional research funding from AstraZeneca, Bristol-Myers Squibb, Roche/Genentech, Amgen, Mirati, Pfizer, Takeda, and Novartis; advisory and/or consulting fees from AstraZeneca, Bristol-Myers Squibb, Takeda, Pfizer, Daiichi Sankyo, Roche/Genentech, Kaleido Biosciences, Amgen, Novartis, NGM Pharmaceuticals, Elevation Oncology, and Mirati; honoraria from AstraZeneca, Bristol-Myers Squibb, Takeda, Pfizer, Daiichi Sankyo, Roche/Genentech, Kaleido Biosciences, Amgen, Novartis, NGM Pharmaceuticals, Elevation Oncology, and Mirati; support for attending meetings from AstraZeneca and Roche/Genentech; and having participation on a data safety monitoring or advisory board for Bristol-Myers Squibb, AstraZeneca, and Daiichi Sankyo. Prof. Faivre-Finn reports receiving institutional research funding from AstraZeneca, Merck Sharp & Dohme, and Elekta; institutional consulting fees, honoraria, and support for attending meetings and/or travel from AstraZeneca and Merck Sharp & Dohme; and having participation on a data safety monitoring or advisory board from AstraZeneca and Merck Sharp & Dohme/Merck. Dr. Villegas reports receiving honoraria and support for attending meetings and/or travel from AstraZeneca. Dr. Daniel reports receiving institutional research funding from Genentech, Roche, Celgene, Guardant, Janssen, Bristol-Myers Squibb, Merck, Novartis, AbbVie, ARMO Biosciences, Lilly Merus, Daiichi Sankyo, EQRX, and G1 Therapeutics; is a member of the Board of Directors for the Common Spirit Memorial Hospital Board, and the Board of Governors for Tennessee Oncology. Dr. Murakami reports receiving institutional research funding from AstraZeneca, Takeda, Hugai Pharma, Sanofi, Merck Sharp & Dohme, Daiichi Sankyo, Ono Pharmaceutical, and Janssen Pharma; honoraria from AstraZeneca, Chugai Pharma, Takeda, Eli Lilly, Merck Sharp & Dohme, Pfizer, Novartis, and Taiho Pharmaceutical. Dr. Hui reports receiving institutional research funding from AstraZeneca, Eisai, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, OncoSec, Roche, and Seagen; payment or honoraria from AstraZeneca, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, and Roche; having participation on an advisory board for Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, Eli Lilly, Merck, Merck Sharp & Dohme, Novartis, Oncosec, Pfizer, Roche, Seagen, Takeda, and Zai Lab; and having a leadership or fiduciary role in the ESMO Congress 2021 Scientific Committee, the ESMO Congress 2022 Scientific Committee, and the International Association for the Study of Lung Cancer Education Committee. Dr. Lee reports having participation on an advisory board for Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, AstraZeneca, and Eli Lilly; and receipt of study drugs from Merck. Dr. Cho reports receiving institutional research funding from MOGAM Institute, LG Chem, Oscotec, Interpark Bio Convergence Corp., GIInnovation, GI-Cell, Abion, AbbVie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Oncology, CJ Bioscience, CJ Blossom Park, Cyrus, Dizal Pharma, Genexine, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Nuvalent, Oncternal, Ono, Regeneron, Dong-A ST, Bridgebio Therapeutics, Yuhan, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, JINTSbio, Hanmi, and CHA Bundang Medical Center; royalties from Champions Oncology, Crown Bioscience, and Imagen; consulting fees from Abion, BeiGene, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, CJ, CureLogen, Cyrus Therapeutics, Ono, Onegene Biotechnology, Yuhan, Pfizer, Eli Lilly, GI-Cell, Guardant, HK Inno-N, Imnewrun Biosciences Inc., Janssen, Takeda, Merck Sharp & Dohme, Janssen, Medpacto, Blueprint Medicines, RandBio, and Hanmi; payment or honoraria from American Society of Clinical Oncology, AstraZeneca, Guardant, Roche, ESMO, International Association for the Study of Lung Cancer, Korean Cancer Association, Korean Society of Medical Oncology, Korean Society of Thyroid-Head and Neck Surgery, Korean Cancer Study Group, Novartis, Merck Sharp & Dohme, The Chinese Thoracic Oncology Society, and Pfizer; membership on a Scientific Advisory Board for Kanaph Therapeutic Inc., Bridgebio Therapeutics, Cyrus Therapeutics, Guardant Health, and Oscotec Inc.; membership on the Board of Directors for Interpark Bio Convergence Corp. and J INTS BIO; stock ownership in TheraCanVac, Inc., Gencurix Inc., Bridgebio Therapeutics, Kanaph Therapeutic Inc., Cyrus Therapeutics, Interpark Bio Convergence Corp., and J INTS BIO; employment by the Yonsei University Health System; and is a founder of DAAN Biotherapeutics. Dr. Kubota reports receiving research funding from AstraZeneca; payment or honoraria from AstraZeneca, Merck Sharp & Dohme, Merck, Ono, Chugai, Taiho, Kyowa-Kirin, Bristol-Myers Squibb, Novartis, Eli Lilly, Pfizer, Nihon-Kayaku, and BIH; and a leadership or fiduciary role for the Japan Multi-National Trial Organization, Japan Clinical Oncology Group, Thoracic Oncology Research Group, North East Japan Study Group, and Japan Association of Medical Translation for Cancer. Ms. Broadhurst reports receiving consulting fees from AstraZeneca, employment with AstraZeneca (under contract from Plus-Project Ltd.), and stock ownership in AstraZeneca. Ms. Wadsworth, Dr. Newton, and Dr. Thiyagarajah report employment and stock ownership in AstraZeneca. Dr. Antonia reports research funding from AstraZeneca and Regeneron; consulting and/or scientific advisory board fees from Memgen, RAPT Biotherapeutics, Achilles Therapeutics, Xilis, Taurus Therapeutics, Shoreline Therapeutics, Immutep, and Leap Therapeutics; royalties or licenses from H. Lee Moffitt Cancer Center; stock ownership in Shoreline Therapeutics, Leap Therapeutics, RAPT Biotherapeutics, and Achilles Therapeutics; receipt of drug supply for a clinical trial from Amgen; and support for attending meetings and/or travel from Achilles Therapeutics and RAPT Biotherapeutics. Dr. Özgüroğlu reports no disclosures.
Acknowledgments
Prof. Corinne Faivre-Finn is supported by a grant from the National Institute for Health Research Manchester Biomedical Research Centre. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Aaron Korpal, PhD, of Ashfield MedComms (Manchester, United Kingdom), an Inizio Company, and was funded by AstraZeneca. The PACIFIC trial (NCT02125461) was funded by AstraZeneca.
Data Sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Data for studies directly listed on Vivli can be requested through Vivli at: www.vivli.org.
Data for studies not listed on Vivli could be requested through Vivli at: https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/.
The AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Footnotes
Cite this article as: Vansteenkiste JF, Naidoo J, Faivre-Finn C, et al. Symptomatic pneumonitis with durvalumab after concurrent chemoradiotherapy in unresectable stage III NSCLC. JTO Clin Res Rep. 2024;5:100638.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100638.
Supplementary Data
References
- 1.Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):IV1–IV21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 2.Bradley J.D., Paulus R., Komaki R., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senan S., Brade A., Wang L., et al. PROCLAIM: a randomized, phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced non-squamous non-small cell lung cancer. J Clin Oncol. 2016;34:953–962. doi: 10.1200/JCO.2015.64.8824. [DOI] [PubMed] [Google Scholar]
- 4.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Eng J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 5.Antonia S.J., Villegas A., Daniel D., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Eng J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency (EMA) Imfinzi (durvalumab) product information. https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf
- 7.Hui R., Özgüroğlu M., Villegas A., et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1670–1680. doi: 10.1016/S1470-2045(19)30519-4. [DOI] [PubMed] [Google Scholar]
- 8.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration Durvalumab prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761069s035lbl.pdf
- 10.Pharmaceuticals and Medical Devices Agency List of approved products: financial year 2018. https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html
- 11.Naidoo J., Page D.B., Li B.T., et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haanen J., Obeid M., Spain L., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217–1238. doi: 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishino M., Sholl L.M., Hodi F.S., Hatabu H., Ramaiya N.H. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Eng J Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 16.Vansteenkiste J.F., Naidoo J., Faivre-Finn C., et al. MA05.02 PACIFIC subgroup analysis: pneumonitis in stage III, unresectable NSCLC patients treated with durvalumab vs. placebo after CRT. J Thorac Oncol. 2018;13(suppl):S370–S371. [Google Scholar]
- 17.Vansteenkiste J.F., Naidoo J., Faivre-Finn C., et al. Efficacy of durvalumab in patients with stage III NSCLC who experience pneumonitis (PACIFIC) Ann Oncol. 2019;30(suppl 5):V592–V593. [Google Scholar]
- 18.Giuranno L., Ient J., De Ruysscher D., Vooijs M.A. Radiation-induced lung injury (RILI) Front Oncol. 2019;9:877. doi: 10.3389/fonc.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voong K.R., Hazell S.Z., Fu W., et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clin Lung Canc. 2019;20:E470–E479. doi: 10.1016/j.cllc.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaverdian N., Lisberg A.E., Bornazyan K., et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suresh K., Voong K.R., Shankar B., et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 22.Fukihara J., Sakamoto K., Koyama J., et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20:442–450. doi: 10.1016/j.cllc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Suresh K., Naidoo J. Lower survival in patients who develop pneumonitis following immunotherapy for lung cancer. Clin Lung Cancer. 2020;21:e169–e170. doi: 10.1016/j.cllc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Suresh K., Psoter K.J., Voong K.R., et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14:494–502. doi: 10.1016/j.jtho.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Bütof R., Kirchner K., Appold S., et al. Potential clinical predictors of outcome after postoperative radiotherapy of non-small cell lung cancer. Strahlenther Onkol. 2014;190:263–269. doi: 10.1007/s00066-013-0501-4. [DOI] [PubMed] [Google Scholar]
- 26.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 29.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 30.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 32.Durm G.A., Althouse S.K., Sadiq A.A., et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14–179. J Clin Oncol. 2018;36:8500. [Google Scholar]
- 33.Inoue H., Ono A., Kawabata T., et al. Clinical and radiation dose-volume factors related to pneumonitis after treatment with radiation and durvalumab in locally advanced non-small cell lung cancer. Invest New Drugs. 2020;38:1612–1617. doi: 10.1007/s10637-020-00917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura Y., Mouri A., Kaira K., et al. Chemoradiotherapy followed by durvalumab in patients with unresectable advanced non-small cell lung cancer: management of adverse events. Thorac Cancer. 2020;11:1280–1287. doi: 10.1111/1759-7714.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito G., Oya Y., Taniguchi Y., et al. Real-world survey of pneumonitis/radiation pneumonitis among patients with locally advanced non-small cell lung cancer treated with chemoradiotherapy after durvalumab approval: a multicenter retrospective cohort study (HOPE-005/CRIMSON) J Clin Oncol. 2020;38:9039. [Google Scholar]
- 36.Saito S., Abe T., Kobayashi N., et al. Incidence and dose-volume relationship of radiation pneumonitis after concurrent chemoradiotherapy followed by durvalumab for locally advanced non-small cell lung cancer. Clin Transl Radiat Oncol. 2020;23:85–88. doi: 10.1016/j.ctro.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh C.H., Park H.S., Kim K.W., Pyo J., Hatabu H., Nishino M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: meta-analysis of 153 cohorts with 15,713 patients: meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer. 2018;123:60–69. doi: 10.1016/j.lungcan.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Chun S.G., Hu C., Choy H., et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marks L.B., Bentzen S.M., Deasy J.O., et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amin N.P., Miften M., Thornton D., Ryan N., Kavanagh B., Gaspar L.E. Effect of induction chemotherapy on estimated risk of radiation pneumonitis in bulky non-small cell lung cancer. Med Dosim. 2013;38:320–326. doi: 10.1016/j.meddos.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Faivre-Finn C., Spigel D.R., Senan S., et al. Impact of prior chemoradiotherapy-related variables on outcomes with durvalumab in unresectable stage III NSCLC (PACIFIC) Lung Cancer. 2021;151:30–38. doi: 10.1016/j.lungcan.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Hassanzadeh C., Sita T., Savoor R., et al. Implications of pneumonitis after chemoradiation and durvalumab for locally advanced non-small cell lung cancer. J Thorac Dis. 2020;12:6690–6700. doi: 10.21037/jtd-20-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naidoo J., Cottrell T.R., Lipson E.J., et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Canc. 2020;8:E000840. doi: 10.1136/jitc-2020-000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naidoo J., Vansteenkiste J.F., Faivre-Finn C., et al. Characterizing immune-mediated adverse events with durvalumab in patients with unresectable stage III NSCLC: a post-hoc analysis of the PACIFIC trial. Lung Cancer. 2022;166:84–93. doi: 10.1016/j.lungcan.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Traverso A., Tohidinezhad F., Bontempi D., Dekker A., Hendriks L., De Ruysscher D. P1.15-01 Differential diagnosis of pneumonitis in metastatic NSCLC (non-small cell lung cancer) patients receiving immunotherapy with radiomics. J Thorac Oncol. 2022;17:S118–S119. [Google Scholar]
- 46.Jain V., Berman A.T. Radiation pneumonitis: old problem, new tricks. Cancers (Basel) 2018;10:222. doi: 10.3390/cancers10070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsui K., Ogata T., Watanabe K., et al. Radiation pneumonitis after definitive concurrent chemoradiotherapy with cisplatin/docetaxel for non-small cell lung cancer: analysis of dose-volume parameters. Cancer Med. 2020;9:4540–4549. doi: 10.1002/cam4.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shintani T., Kishi N., Matsuo Y., et al. Incidence and risk factors of symptomatic radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent chemoradiotherapy and consolidation durvalumab. Clin Lung Cancer. 2021;22:401–410. doi: 10.1016/j.cllc.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Diamond B.H., Belani N., Masel R., et al. Predictors of pneumonitis in patients with locally advanced non-small cell lung cancer treated with definitive chemoradiation followed by consolidative durvalumab. Adv Radiat Oncol. 2022;8 doi: 10.1016/j.adro.2022.101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao R.W., Day C.N., Yu N.Y., et al. Dosimetric predictors of pneumonitis in locally advanced non-small cell lung cancer patients treated with chemoradiation followed by durvalumab. Lung Cancer. 2022;170:58–64. doi: 10.1016/j.lungcan.2022.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.