Abstract
This study aimed to compare and evaluate the efficacy of the blood pressure (BP) control and cholesterol‐lowering effects and safety of combination therapy with telmisartan, rosuvastatin, and ezetimibe versus rosuvastatin and ezetimibe double therapy or telmisartan single therapy in dyslipidemia patients with hypertension. After a wash‐out/therapeutic lifestyle change period of ≥4 weeks, a total of 100 eligible patients were randomized and received one of three treatments for 8 weeks: (1) telmisartan 80 mg/rosuvastatin 20 mg/ezetimibe 10 mg (TRE), (2) rosuvastatin 20 mg/ezetimibe 10 mg (RE), or (3) telmisartan 80 mg (T). The primary endpoint was the efficacy evaluation of TRE by comparing changes in mean sitting systolic blood pressure (msSBP) and mean percentage change in low‐density lipoprotein‐C (LDL‐C) from baseline after 8 weeks of treatment.
The least square (LS) mean (SE) changes in msSBP at 8 weeks compared with baseline were −23.02 (3.04) versus −7.18 (3.09) mmHg in the TRE and RE groups, respectively (p < .0001), and −25.80 (2.74) versus −14.92 (2.65) mmHg in the TRE and T groups, respectively (p = .0005). The percentage changes in the mean (SD) LDL‐C at 8 weeks compared with baseline were −54.97% (3.49%) versus −0.17% (3.23%) in the TRE and T groups, respectively (p < .0001). No serious adverse events occurred, and no statistically significant differences in the incidence of overall AEs and adverse drug reactions occurred among the three groups.
TRE therapy significantly decreased msSBP and LDL‐C compared to RE or T therapy with comparable safety and tolerability profiles.
Keywords: combination therapy, dyslipidemia, ezetimibe, hypertension, rosuvastatin, telmisartan
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide, representing 32% of all global deaths, according to the World Health Organization report in 2021. 1 The two most significant risk factors for CVD morbidity and mortality, hypertension and dyslipidemia, are frequently accompanied. 2 , 3 In Korea, 59.9% of people with hypertension have dyslipidemia, 4 and 34.6% of people received combination treatments for hypertension and dyslipidemia in 2018. 5 Managing hypertension and dyslipidemia is essential for reducing the overall risk of CVD. This often requires taking multiple medications to control both chronic conditions. 6 Additionally, fixed‐dose combinations (FDC) of antihypertensive and lipid‐lowering medication could increase adherence by easing the pill burden. 7 , 8
Triple combination therapy of telmisartan, amlodipine, and rosuvastatin has become an effective method for treating high‐risk hypertension and dyslipidemia. Triple therapy can successfully target various pathways involved in BP regulation and lipid control by using three drugs with various mechanisms of action, leading to greater control. 9 , 10 , 11 Additionally, the FDC treatment of aspirin, ramipril, and atorvastatin had higher adherence and was more effective for secondary cardiovascular prevention than usual care. 12
Angiotensin II receptor blockers (e.g., Telmisartan) are one of the preferred first‐line treatments for hypertension. 13 Hydroxymethylglutaryl‐coenzyme A (HMG‐CoA) reductase inhibitor (hereafter statin), the first‐line treatment for dyslipidemia, prevents adverse cardiovascular events by reducing mainly low‐density lipoprotein cholesterol (LDL‐C) and its pleiotropic effects. 14 , 15 , 16 Ezetimibe reduces cholesterol transport from the small intestine to the liver by preventing cholesterol absorption in the small intestine. Ezetimibe reduces blood cholesterol in a complementary way to statin. 17
Recent evidence indicated that rosuvastatin/ezetimibe (RE) combination therapy effectively lowered LDL‐C levels compared to statin monotherapy, and more patients with combination medication achieved their goal LDL‐C levels. 18
The aim of this study was to compare and evaluate the efficacy of the BP control and cholesterol‐lowering effect and safety of combination therapy with telmisartan, rosuvastatin, and ezetimibe (TRE) versus RE double or telmisartan (T) single therapy in dyslipidemia patients with hypertension.
2. PATIENTS AND METHODS
2.1. Study patients
Persons who met the inclusion/exclusion criteria were randomly assigned to either the TRE, RE, or T groups and were treated for 8 weeks.
Men or women aged over 19 years with dyslipidemia accompanied by essential hypertension and requiring medical treatment were included. After a ≥4‐week wash‐out/therapeutic lifestyle change (TLC) period, patients with mean sitting systolic blood pressure (msSBP) ≥ 140 mmHg and mean sitting diastolic blood pressure (msDBP) < 110 mmHg were eligible for the trial. Additionally, patients who met the LDL‐C criteria for CVD risk as defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) 19 were included in the study.
Patients with severe hypertension (msSBP ≥ 180 mmHg or msDBP ≥ 110 mmHg), a BP difference between both arms of msSBP ≥ 20 mmHg or msDBP ≥ 10 mmHg, fasting LDL‐C > 250 mg/dL or triglycerides (TG) ≥ 500 mg/dL were excluded.
2.2. Study design
This study was a randomized, double‐blind, multicenter, therapeutic confirmatory, phase III clinical trial. The enrollment of persons was conducted at 18 nationwide sites in the Republic of Korea from September 2019 to June 2021. This study was conducted by the International Conference on Harmonisation–Good Clinical Practice (ICH‐GCP) guidelines and the Declaration of Helsinki. Additionally, the Institutional Review Boards (IRBs) of each participating center approved the study protocol.
The study design is presented in Figure 1. Eligible persons were instructed to follow at least 4 weeks of washout/TLC before randomization. All lipid‐modifying and antihypertension medications were prohibited for at least 4 weeks (6 weeks for fibrates). Persons were instructed on patient education for the lifestyle change and underwent diet and exercise therapy for at least 4 weeks (screening period). They maintained TLC during the treatment period. The cardiovascular risk group criteria of the NCEP ATP III 19 were used as a stratification factor; Group1: no other risk factors and LDL‐C ≥160 mg/dL, Group2: ≥1 major risk factors and a 10‐year CVD risk indicated by Framingham risk score < 10% (LDL‐C ≥160 mg/dL), Group3: ≥1 major risk factors and a 10‐year risk score 10%−20% (LDL‐C ≥130 mg/dL), Group4: coronary artery disease (CAD) or CAD equivalents or ≥1 major risk factors and a 10‐year risk score > 20% (LDL‐C ≥100 mg/dL).
FIGURE 1.
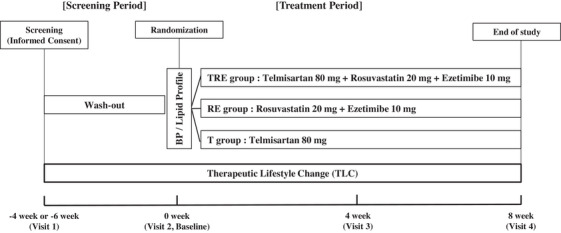
Study design. BP, blood pressure; RE, rosuvastatin/ezetimibe; T, telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe.
At randomization, persons were re‐evaluated for eligibility criteria, and if satisfactory, they were randomly assigned to one of three groups in a 1:1:1 ratio within strata. All persons received three investigational drugs, including a placebo to maintain double‐blinding. The TRE group received three active tablets, telmisartan 80 mg (Micardis, Boehringer Ingelheim Pharma Co, Seoul, Korea), rosuvastatin 20 mg (Crestor, AstraZeneca Pharma Co, Seoul, Korea), and ezetimibe 10 mg (Ezetrol, MSD Technology Singapore Pte Ltd.). The RE group received two active tablets, rosuvastatin 20 mg (Crestor, AstraZeneca Pharma Co, Seoul, Korea) and ezetimibe 10 mg (Ezetrol, MSD Technology Singapore Pte Ltd.) and a placebo tablet for telmisartan (ChongKunDang Pharm. Co, Seoul, Korea). The T group received one active tablet, telmisartan 80 mg (Micardis, Boehringer Ingelheim Pharma Co, Seoul, Korea) and two placebo tablets for rosuvastatin and ezetimibe (ChongKunDang Pharm. Co, Seoul, Korea). The placebo tablets had the same appearance as each active tablet.
During the clinical trial period, all persons were recommended to take the prescribed investigational drugs once a day, at a fixed time every day, if possible. No dose adjustment was performed during the entire clinical trial period.
2.3. Outcomes
The primary endpoints were the mean changes in msSBP compared between the TRE and RE groups and the mean percentage change in LDL‐C compared between the TRE and T groups at week 8 compared to the baseline for each.
The secondary endpoints were the mean changes in msSBP and msDBP and the achievement rate of target BP (msSBP/msDBP < 140/90 mmHg) from baseline to 4 and 8 weeks of treatment compared between the TRE and RE groups; the mean percentage change and the mean change in LDL‐C, total cholesterol (TC), triglyceride (TG), and high‐density lipoprotein cholesterol (HDL‐C), and the LDL‐C treatment goal achievement rate according to the NCEP ATP III Guideline 19 (Group 1: < 160 mg/dL, Group 2, 3: < 130 mg/dL, Group 4: < 100 mg/dL) from baseline to 4 and 8 weeks of treatment compared between the TRE and T groups.
In addition, the exploratory endpoints were BP evaluation compared between the TRE and T groups and lipid profile evaluation compared between the TRE and RE groups.
BP was evaluated after the person relaxed for at least 5 min, using the same arm and sphygmomanometer (HEM‐7080IC; Omron Health Care, Tokyo, Japan). BP was measured twice after screening, and mean SBP and DBP were calculated from the average of the two measurements. The central laboratory analyzed the lipid profiles.
Adverse events (AEs), laboratory tests, concomitant drugs, vital signs, and physical examination were evaluated for safety endpoints.
2.4. Statistical analysis
This study was designed under assumptions that TRE therapy is superior to RE combination in reducing BP and superior to T alone in lowering LDL‐C levels. The expected difference in mean change (SD) in BP from baseline between the TRE and RE groups was −15.4 mmHg (16.9 mmHg), and the expected difference in mean percent change (SD) in LDL‐C from baseline between the TRE and T groups was −62.7% (22%). 20 Sample sizes were calculated for each estimate with 90% power and a two‐sided level set at 5%; the larger number was selected, which was the size to assess the change in BP. A sample size of 99 patients was produced considering a 20% drop‐off rate and the randomization ratio of 1:1:1 (33 patients in each group).
The major analysis set for evaluation of efficacy was full analysis sets (FAS). Changes in BP and percent changes in LDL‐C from baseline were compared between groups and analyzed using an analysis of covariance (ANCOVA) model, considering the baseline and stratification factors (risk group). The achievement rate of the BP target and the target LDL‐C level were compared between groups using the Cochran–Mantel–Haensqel test, in which the stratification factor (risk group) was corrected as a covariate. Comparison within each group for changes compared to the baseline was performed through the paired‐samples t‐test. Wilcoxon signed‐rank test was performed for non‐normal data. The mean msSBP and LDL‐C from baseline to 4 and 8 weeks of treatment were compared between groups and analyzed using the Independent t‐test. All analyses were two‐sided, and p values < .05 were considered statistically significant. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Inc, Cary, North Carolina, USA).
3. RESULTS
3.1. Participant disposition and baseline characteristics
One hundred participants were randomly assigned to receive TRE (n = 33), RE (n = 33), or T (n = 34) therapy (Figure 2). Furthermore, 11 participants dropped out of the study, and 89 completed treatments. Among the enrolled 100 persons, one person had not administered study drugs, and 99 were analyzed for safety evaluation. For efficacy, 96 persons were analyzed as the FAS, excluding four persons whose BP or lipid profile had not been evaluated during the clinical trial. The demographic and baseline clinical characteristics, including baseline BP and lipid profiles, were similar among all groups (Table 1). No significant differences were observed.
FIGURE 2.
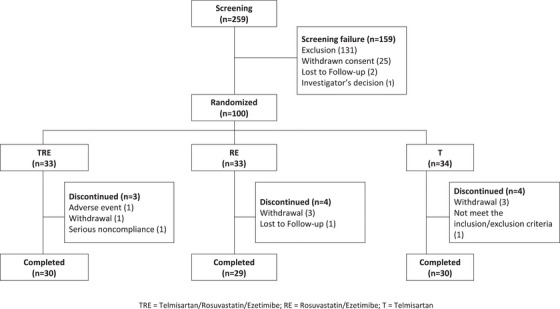
Flowchart describing person disposition.
TABLE 1.
Baseline demographic and clinical characteristics of the study participants.
| Characteristic | TRE a (No. = 33) | RE a (No. = 31) | T a (No. = 32) | p b |
|---|---|---|---|---|
| Age, mean (SD), y | 63.64 (10.65) | 62.58 (10.76) | 60.75 (9.73) | .4169K |
| Male | 26 (78.79) | 24 (77.42) | 22 (68.75) | .6017C |
| BMI, mean (SD), kg/m2 | 26.19 (2.76) | 26.50 (3.50) | 25.73 (2.51) | .5830A |
| NCEP ATP III risk category | ||||
| ≥1 risk factor | 29 (87.88) | 29 (93.55) | 24 (75.00) | .1076F |
| CHD and CHD risk equivalents | 15 (45.45) | 17 (54.84) | 15 (46.88) | .7237C |
| Group category c | ||||
| Group1 | 3 (9.09) | 2 (6.45) | 3 (9.38) | .9876F |
| Group2 | 2 (6.06) | 3 (9.68) | 4 (12.50) | |
| Group3 | 8 (24.24) | 8 (25.81) | 7 (21.88) | |
| Group4 | 20 (60.61) | 18 (58.06) | 18 (56.25) | |
| Drug therapy before enrollment | ||||
| Antihypertensive | 28 (84.85) | 24 (77.42) | 27 (84.38) | .6879C |
| Lipid‐lowering | 25 (75.76) | 21 (67.74) | 25 (78.13) | .6170C |
| Lipid and BP baseline, mean (SD) | ||||
| LDL‐C, mg/dL | 162.79 (35.17) | 155.68 (28.11) | 156.56 (34.48) | .5862K |
| Total cholesterol, mg/dL | 227.94 (37.86) | 219.61 (33.09) | 225.72 (38.39) | .6451A |
| Triglyceride, mg/dL | 183.58 (82.67) | 180.35 (83.65) | 175.03 (70.84) | .9898K |
| HDL‐C, mg/dL | 45.42 (9.73) | 44.61 (7.36) | 48.84 (12.39) | .3810K |
| SiSBP, mmHg | 154.68 (10.79) | 152.77 (9.84) | 153.53 (9.30) | .7855K |
| SiDBP, mmHg | 91.18 (9.22) | 89.61 (9.10) | 94.03 (7.21) | .1208A |
Abbreviations: BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; SiDBP, sitting diastolic blood pressure; SiSBP, sitting systolic blood pressure; RE, rosuvastatin/ezetimibe; T, telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe.
Data are presented as the number (percentage) of patients unless otherwise noted.
p values between telmisartan/rosuvastatin/ezetimibe and rosuvastatin/ezetimibe and telmisartan group. [ANOVA (A) or Kruskal–Wallis Test (K) or Chi‐square test (C) or Fisher's exact test (F)].
Group1:no other risk factors and LDL‐C ≥160 mg/dL, Group2: ≥1 major risk factors and a 10‐year cardiovascular disease (CVD) risk indicated by Framingham risk score < 10% (LDL‐C ≥160 mg/dL), Group3: ≥1 major risk factors and a 10‐year risk score 10%−20% (LDL‐C ≥130 mg/dL), Group4: coronary artery disease (CAD) or CAD equivalents or a 10‐year risk score > 20% (LDL‐C ≥100 mg/dL).
3.2. Efficacy
The LS mean (SE) changes in msSBP from baseline after 8 weeks of treatment were −23.02 (3.04) and −7.18 (3.09) mmHg in the TRE and RE groups, respectively (Table 2). Treatment with TRE resulted in a greater reduction in BP than treatment with RE (differences, −15.85 mmHg [95% CI, −23.00 to −8.69 mmHg], p < .0001). The LS mean (SE) changes in msSBP from baseline to after 8 weeks of treatment were −25.80 (2.74) and −14.92 (2.65) mmHg in the TRE and T groups, respectively. The differences between the TRE and the T groups were also statistically significant (differences, −10.88 mmHg [95% CI, −17.40 to −4.36 mmHg], p = .0015) (Table 2). The LS mean (SE) change in msDBP from baseline to 8 weeks was −10.89 (1.49) and −1.15 (1.50) mmHg in the TRE and the RE groups, respectively. The differences between the TRE and the RE groups were −9.74 mmHg [95% CI, −13.24 to −6.24 mmHg] and it was statistically significant (p < .0001) (Table 2). A significantly higher number of persons achieved target BP at week 8 in the TRE group (69.70%, 23 persons) compared to the RE group (25.81%, eight persons, p = .0005) (Figure 3A). The mean (SD) msSBP from baseline to 4 and 8 weeks of treatment was from 154.68 (10.79) mmHg to 134.44 (13.02) mmHg and 130.88 (12.76) mmHg in the TRE group, which was a more significant reduction than in the RE group, from 152.77 (9.84) mmHg to 149.72 (16.69) mmHg and 145.60 (17.09) mmHg (p = .0001 at 4 weeks and p < .0001 at 8 weeks) (Figure 3B). The effects of lowered msSBP were comparable at 4 and 8‐week follow‐ups.
TABLE 2.
Changes in blood pressure from baseline to week 8.
| Variable | TRE (No. = 33) | RE (No. = 31) | T (No. = 32) |
|---|---|---|---|
| MSSBP | |||
| Mean (SD) | −23.80 (12.82) | −7.18 (15.94) | −12.97 (15.28) |
| Treatment difference | |||
| LS mean (SE) | −23.02 (3.04) | −7.18 (3.09) | – |
| LS mean (SE) | −25.80 (2.74) | – | −14.92 (2.65) |
| LS mean difference [95% CI] | – | −15.85 [−23.00, −8.69] | −10.88 [−17.40, −4.36] |
| p † | – | <.0001 | .0015 |
| MSDBP | |||
| Mean (SD) | −11.00 (7.87) | −0.50 (8.63) | −6.56 (7.22) |
| Treatment difference | |||
| LS mean (SE) | −10.89 (1.49) | −1.15 (1.50) | – |
| LS mean (SE) | −12.27 (1.50) | – | −6.58 (1.46) |
| LS mean difference [95% CI] | – | −9.74 [−13.24, −6.24] | −5.68 [−9.31, −2.06] |
| p † | – | <.0001 | .0026 |
Treatment difference was calculated as telmisartan/rosuvastatin/ezetimibe group minus rosuvastatin/ezetimibe group or telmisartan group.
Abbreviations: CI, Confidence Interval; LS mean, Least Square Mean; MSSBP, mean sitting systolic blood pressure; MSDBP, mean sitting diastolic blood pressure; SD, Standard Deviation; SE, Standard Error; RE, rosuvastatin/ezetimibe; T, telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe.
p value for ANCOVA, with Group(stratification variable) as a covariate.
FIGURE 3.
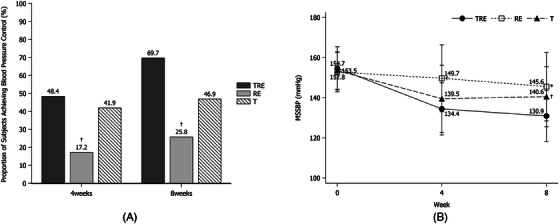
(A) Achievement rate of the BP target (msSBP/msDBP < 140/90 mmHg) at 4 and 8 weeks of treatment († p < .05 vs. TRE by Cochran‐Mantel‐ Haensqel test). (B) The mean msSBP from baseline to 4 and 8 weeks (* p < .0001 and † p < .05 vs. TRE by independent t‐test). msDBP, mean sitting diastolic blood pressure; msSBP, mean sitting systolic blood pressure; RE, rosuvastatin/ezetimibe; T, telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe.
The LS mean (SE) percentage changes in mean LDL‐C at 8 weeks compared with baseline values were −54.97% (3.49%) and −0.17% (3.23%) in the TRE and T groups, respectively (Table 3). Treatment with TRE had a more effect on the lipid control than T alone (differences, −54.80% [95% CI, −62.76% to −46.83%], p < .0001). The LS mean (SE) percentage changes in TC after the 8‐week treatment were −40.31% (2.53%) and 1.40% (2.40%) in the TRE and T groups, respectively. The difference between the TRE and the T groups was −41.72% [95% CI, −47.56% to −35.87%], and it was statistically significant (p < .0001) (Table 3). TG levels were significantly decreased in the TRE group than the T group (differences, −45.00% [95% CI, −63.96% to −26.05%], p < .0001) (Table 3). The percentage changes in mean HDL‐C were 1.69% (2.79%) and −3.20% (2.77%) in the TRE and T groups, respectively. There was no statistically significant difference in HDL‐C level (differences, 4.89% [95% CI, −1.82% to 11.61%], p = .1500), but it showed a tendency to increase after administration of the investigational drug.
TABLE 3.
Percent changes in lipid variables from baseline to week 8.
| Variable | TRE (No. = 33) | RE (No. = 31) | T (No. = 32) |
|---|---|---|---|
| LDL‐C | |||
| Mean (SD) | −61.38 (14.25) | −58.18 (22.17) | −4.49 (19.09) |
| Treatment difference | |||
| LS mean (SE) | −62.56 (4.16) | −59.93 (4.06) | – |
| LS mean (SE) | −54.97 (3.49) | – | −0.17 (3.23) |
| LS mean difference [95% CI] | – | −2.63 [−12.08, 6.82] | −54.80 [−62.76, −46.83] |
| p † | – | .5790 | <.0001 |
| Total cholesterol | |||
| Mean (SD) | −45.24 (12.41) | −41.75 (16.73) | −2.32 (13.60) |
| Treatment difference | |||
| LS mean (SE) | −45.33 (3.27) | −42.71 (3.18) | – |
| LS mean (SE) | −40.31 (2.53) | – | 1.40 (2.40) |
| LS mean difference [95% CI] | – | −2.62 [−9.97, 4.73] | −41.72 [−47.56, −35.87] |
| p † | – | .4784 | <.0001 |
| Triglyceride | |||
| Mean (SD) | −27.37 (30.11) | −29.21 (30.11) | 18.38 (45.94) |
| Treatment difference | |||
| LS mean (SE) | −24.29 (5.55) | −26.42 (5.60) | – |
| LS mean (SE) | −27.13 (7.97) | – | 17.87 (7.70) |
| LS mean difference [95% CI] | – | 2.13 [−10.85, 15.11] | −45.00 [−63.96, −26.05] |
| p† | – | .7436 | <.0001 |
| HDL‐C | |||
| Mean (SD) | 3.04 (14.93) | 8.54 (14.55) | −3.12 (12.75) |
| Treatment difference | |||
| LS mean (SE) | 3.09 (3.08) | 7.64 (3.05) | – |
| LS mean (SE) | 1.69 (2.79) | – | −3.20 (2.77) |
| LS mean difference [95% CI] | – | −4.55 [−11.33, 2.22] | 4.89 [−1.82, 11.61] |
| p† | – | .1836 | .1500 |
Treatment difference was calculated as telmisartan/rosuvastatin/ezetimibe group minus rosuvastatin/ezetimibe group or telmisartan group.
Abbreviations: CI, Confidence Interval; LS mean, Least Square Mean; RE, rosuvastatin/ezetimibe; SD, Standard Deviation; SE, Standard Error; T, telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe.
p value for ANCOVA, with Group(stratification variable) as a covariate.
The percentage of persons who achieved the target LDL‐C after 8 weeks of treatment was 96.97% and 12.50% in the TRE and T groups, respectively (p < .0001) (Figure 4A). The mean (SD) LDL‐C from baseline to 4 and 8 weeks of treatment were from 162.79 (35.17) mg/dL to 58.94 (22.47) mg/dL and 61.48 (21.35) mg/dL in the TRE group, which was more significant than in the T group, from 156.56 (34.48) mg/dL to 151.71 (42.49) mg/dL and 149.38 (43.59) mg/dL (p < .0001) (Figure 4B).
FIGURE 4.
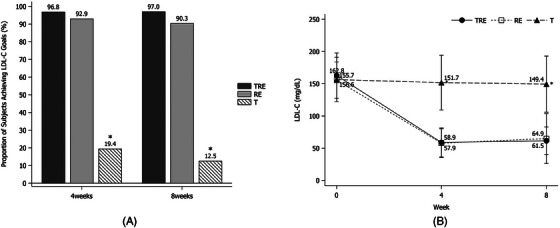
(A) The LDL‐C treatment goal achievement rate according to the NCEP ATP III Guideline (Group 1: < 160 mg/dL, Group 2, 3: < 130 mg/ dL, Group 4: < 100 mg/dL) at 4 and 8 weeks of treatment (* p < .0001 vs. TRE by Chi‐square test). (B) The mean LDL‐C from baseline to 4 and 8 weeks of treatment (* p < .0001 vs. TRE by independent t‐test). LDL‐C, low‐density lipoprotein cholesterol; RE, rosuvastatin/ezetimibe; T: telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe.
3.3. Safety
Safety analysis was performed on persons who took at least one dose of the investigational drug. Among the 99 persons in the safety analysis set, 16 (16.16%) experienced 24 treatment‐emergent adverse events (TEAE). In the TRE group, six persons (18.18%, 10 cases) were reported, four (12.12%, six cases) in the RE group and six (18.18%, eight cases) in the T group. There was no significant difference among the three groups (p = .7422). Most of the 24 cases were lower than moderate in severity (20 mild, 3 moderate, and 1 severe). A severe AE reported in the TRE group was “Large intestine polyp” (one person, 3.03%, one case), but it was judged as “not related” to the investigational drug, and the person recovered. Thirteen persons (13.13%) experienced 17 ADRs after treatment (Table 4). In the TRE group, five persons (15.15%, six cases) were reported, four (12.12%, six cases) in the RE group, and four (12.12%, five cases) in the T group. There was no significant difference among the three groups (p = 1.000). The most common ADR was “Headache” (four persons, 4.04%, four cases), followed by “Alanine aminotransferase increased” and “Aspartate aminotransferase increased.” The most reported ADRs were “Nervous system disorders” and “Investigations” in the TRE and RE groups and “Gastrointestinal Disorders” in the T group. Most ADRs were previously reported for each agent. No SAE was reported. One person (3.03%) dropped out owing to “Alanine aminotransferase increased” in the TRE group. This AE was mild, and the person recovered.
TABLE 4.
Summary of treatment‐emergent adverse events (TEAE) in the study
| Variable | TRE (No. = 33) | RE (No. = 33) | T (No. = 33) | p † |
|---|---|---|---|---|
| TEAEs | 6 (18.18)[10] | 4 (12.12)[6] | 6 (18.18)[8] | .7422C |
| Intensity | ||||
| Mild | 6 (18.18)[9] | 3 (9.09)[5] | 5 (15.15)[6] | – |
| Moderate | 0 | 1 (3.03)[1] | 2 (6.06)[2] | – |
| Severe | 1 (3.03)[1] | 0 | 0 | – |
| SAEs | 0 | 0 | 0 | – |
| ADRs | 5 (15.15)[6] | 4 (12.12)[6] | 4 (12.12)[5] | >.999F |
| Gastrointestinal disorders | 1 (3.03)[1] | 0 | 3 (9.09)[3] | .3196F |
| Conspiration | 0 | 0 | 1 (3.03)[1] | >.999F |
| Dyspepsia | 0 | 0 | 1 (3.03)[1] | >.999F |
| Epigastric discomfort | 0 | 0 | 1 (3.03)[1] | >.999F |
| Vomiting | 1 (3.03)[1] | 0 | 0 | >.999F |
| Nervous system disorders | 2 (6.06)[2] | 2 (6.06)[2] | 0 | .5418F |
| Headache | 2 (6.06)[2] | 2 (6.06)[2] | 0 | .5418F |
| Investigations | 2 (6.06)[3] | 1 (3.03)[3] | 0 | .7709F |
| Alanine aminotransferase increased | 2 (6.06)[2] | 1 (3.03)[1] | 0 | .7709F |
| Aspartate aminotransferase increased | 1 (3.03)[1] | 1 (3.03)[1] | 0 | >.999F |
| Blood creative phosphokinase increased | 0 | 1 (3.03)[1] | 0 | >.999F |
| Ear and labyrinth disorders | 0 | 1 (3.03)[1] | 0 | >.999F |
| Vertigo positional | 0 | 1 (3.03)[1] | 0 | >.999F |
| General disorders and administration site | 0 | 0 | 1 (3.03)[1] | >.999F |
| Chest discomfort | 0 | 0 | 1 (3.03)[1] | >.999F |
| Skin and subcutaneous tissue disorders | 0 | 0 | 1 (3.03)[1] | >.999F |
| Pruritus | 0 | 0 | 1 (3.03)[1] | >.999F |
| Serious ADRs | 0 | 0 | 0 | – |
Data are presented as the number of patients (%) [number of cases].
Abbreviations: ADR, adverse drug reaction; RE, rosuvastatin/ezetimibe; T, telmisartan; TRE, telmisartan/rosuvastatin/ezetimibe; SAE, serious adverse event.
*TEAEs: AE with a start date on or after administration of study drug or preexisting conditions that worsened on or after study drug administration.
p value for Chi‐square test (C) or Fisher's exact test (F).
4. DISCUSSION
This study was a randomized, double‐blind, multicenter, therapeutic confirmatory, phase III study to compare and evaluate the efficacy and safety of combination therapy with TRE in dyslipidemia patients with hypertension. The LS mean (SE) change in msSBP and percentage change in LDL‐C from baseline to 8 weeks of treatment were significantly decreased in the TRE group compared to the RE and T groups, respectively. Furthermore, the rate of achievement of the target BP or LDL‐C level was also significantly higher in the TRE group during the 8‐week follow‐up. Safety analysis revealed no significant differences among the three groups.
The combination of an angiotensin II receptor blocker (ARB) and lipid‐lowering agents is frequently prescribed for their additive risk reduction of CVD. 10 FDC with ARB and statins could have additional beneficial effects other than apparent BP reduction and lipid profile control.
Telmisartan is an ARB with potent selectivity for the angiotensin II type I receptor. Once daily administration of this medication efficiently lowers BP because of its long half‐life. 21 Moreover, it is well tolerated and effectively lowers CVD risks and mortality in high‐risk patients. 21 , 22 In the ONTARGET (The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) investigation, telmisartan demonstrated comparable effectiveness to ramipril in individuals with vascular disease or high‐risk diabetes. 23
Rosuvastatin is an HMG‐CoA reductase inhibitor with a high tissue selectivity. It inhibits cholesterol synthesis by inhibiting the conversion of HMG‐CoA to mevalonic acid. The HMG‐CoA reductase inhibitory effect lasts for 24 h because of its long half‐life and has the highest potency among statins on the market to date. 24 , 25 , 26 However, there are reports that the rate of renal excretion with rosuvastatin is higher than with atorvastatin, a lipophilic statin, and that atorvastatin is more helpful in preserving renal function than rosuvastatin. 27 , 28 However, rosuvastatin has high hepatic selectivity, exhibits higher binding interactions with HMG‐CoA reductase, and displays a notable affinity for the enzyme's active site. Furthermore, rosuvastatin has pleiotropic effects independent of HMG‐CoA reductase inhibition, including anti‐inflammatory, improvements in endothelial function, and antithrombotic and antioxidant effects. 29 The JUPITER study and HOPE‐3 study indicated that rosuvastatin significantly reduced the risk of CVD by reducing the inflammatory biomarkers such as high‐sensitivity C‐reactive protein (CRP) in healthy participants with elevated CRP and in participants with intermediate risk of CVD. 30 , 31
In this study, the TRE group showed a substantial BP‐lowering effect compared with the T group. The results showed that TRE combination therapy showed additional BP‐lowering effects without serious AEs. In a previous study, the combination of telmisartan/amlodipine and rosuvastatin provided statistically significant BP‐lowering effects compared with telmisartan/amlodipine therapy.17These additional BP‐lowering effects are assumed to be caused by rosuvastatin.
Previous studies have suggested that statins can amplify the BP‐lowering effects of ARBs. 10 , 32 , 33 In a recent retrospective observational study, statin use was associated with better ambulatory BP control, 34 and a meta‐analysis of prospective randomized, controlled trials of statin therapy showed that significant reduction of SBP in patients taking statins compared to control group. 35 The mechanism by which statin reduces BP is presumed to be due to pleiotropic effects such as suppression of vascular smooth muscle cell proliferation, reduction of angiotensin II‐type 1 receptor, and vasodilation by increasing nitric oxide bioavailability. 15 , 36 A study using a rabbit model of high cholesterol diet‐induced atherosclerosis demonstrated that the combination of statins and ARBs synergistically exerted an early antiatherosclerotic effect compared to administering each drug individually by reducing plaque burden. 37 The combination of statins with ARBs have also been reported to prevent atherosclerosis activities 38 and reduce carotid intimal thickness. 39 Compared to each single medication therapy, FDC therapies with ARB and statins have shown equivalent efficacies with no additional AEs. 40 , 41
Statins are widely used for preventing atherosclerotic CVDs, and high‐dose statins are more commonly recommended because the current guidelines lower target LDL‐C compared to previous ones. 15 , 16 , 17 However, the maximum dose of rosuvastatin often does not reduce LDL‐C levels to the target range. 42 In addition, clinical trials have reported that rosuvastatin doses above a certain point are associated with an increased risk of AEs, including myopathy. 43 Therefore, additional lipid‐lowering medications are frequently used to reach target LDL‐C levels. 44
Ezetimibe selectively reduces cholesterol absorption in the small intestine with a low incidence of AEs. 45 , 46 IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) has proven that the statin/ezetimibe combination not only lowers LDL‐cholesterol compared to statin monotherapy but also reduces CVD. 47 Therefore, ezetimibe is recommended in combination with statins. In a previous study, patients who received ezetimibe in addition to statins had an additional 15.2% reduction in LDL‐C levels compared to those who received a statin alone. 48 The combination therapy of ezetimibe and statin can effectively lower LDL‐C even at low doses of statins, so it is a good strategy to avoid side effects of high doses of statins. 49 , 50 A previous study showed that RE together significantly lowered LDL‐C levels compared to rosuvastatin alone. 49 In this study, TRE significantly lowered LDL‐C levels compared to RE at 8 weeks. Thus, the combination of TRE can reduce LDL‐C efficiently without serious AEs.
Furthermore, a previous study showed that ezetimibe‐rosuvastatin with telmisartan therapy is effective and safe compared to either ezetimibe‐rosuvastatin double therapy or telmisartan monotherapy. 51 The triple combination yielded improvements in the primary endpoints of msSBP and LDL‐C levels compared to their respective control groups. The efficacy results were consistent with this study, and no clinically significant differences were observed in the safety outcomes in both studies.
There are a few limitations to this study. The study duration was not long enough to evaluate lipid profiles, and a relatively small number of Korean patients were enrolled. Thus, this study limited the generalization of these results to prolonged treatment periods and other ethnic. Despite these limitations, the data show that TRE for 8 weeks in dyslipidemia patients with essential hypertension significantly improved BP and lipid profile.
5. CONCLUSIONS
Combination therapy of TRE was superior in lowering BP and improving lipid profiles compared to RE combination or T alone. No significant difference was observed among the three groups in safety evaluation; thus, the combination of TRE can be safely administered. Therefore, the combined administration of TRE in dyslipidemia patients with essential hypertension controls BP and improves lipid metabolism more effectively.
AUTHOR CONTRIBUTIONS
C.J. Lee and S. M. Kang wrote and revised the manuscript. S. M. Kang contributed to the study design and S.M. Kang, W.C. Kang, S.H. Ihm, I.S. Sohn, J.S. Woo, J.W. Kim, S.J. Hong, J.H. Choi, J.W. Suh, J.B. Seo, J.H. Doh, J.W. Son, J.H. Park, J.H. Lee, Y.J. Hong, J.H. Heo, and J.H. Shin contributed to data collection and analysis.
CONFLICT OF INTEREST STATEMENT
The authors have indicated that they have no conflicts of interest regarding the content of this article.
ACKNOWLEDGMENTS
This study was sponsored by Chong Kun Dang Pharmaceutical Company, Seoul, Korea.
Lee CJ, Kang WC, Ihm SH, et al. Efficacy and safety of combination therapy with telmisartan, rosuvastatin, and ezetimibe in patients with dyslipidemia and hypertension: A randomized, double‐blind, multicenter, therapeutic confirmatory, phase III clinical trial. J Clin Hypertens. 2024;26:262–273. 10.1111/jch.14778
DATA AVAILABILITY STATEMENT
The data are the property of the authors and can be made available upon reasonable request to the corresponding author.
REFERENCES
- 1. World Health Organization . (2021, June). “Cardiovascular diseases (CVDs).” https://www.who.int/en/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds)
- 2. Hurtubise J, McLellan K, Durr K, et al. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr Atheroscler Rep. 2016;18(12):82. [DOI] [PubMed] [Google Scholar]
- 3. Leggio M, Lombardi M, Caldarone E, et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res. 2017;40(12):947‐963. [DOI] [PubMed] [Google Scholar]
- 4. Jin ES, Shim JS, Kim SE, et al. Dyslipidemia Fact Sheet in South Korea, 2022. J Lipid Atheroscler. 2023;12(3):237‐251. doi: 10.12997/jla.2023.12.3.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HC, Cho SMJ, Lee H, et al. Korean Society of Hypertension (KSH)—Hypertension Epidemiology Research Working Group. Korea hypertension fact sheet 2020: analysis of nationwide population‐based data. Clin Hypertens. 2021;27(1):8. doi: 10.1186/s40885-021-00166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahlöf B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol. 2010;105(1):3A‐9A. Suppl. [DOI] [PubMed] [Google Scholar]
- 7. Bangalore S, Shahane A, Parkar S, et al. Compliance and fixed‐dose combination therapy. Curr Hypertens Rep. 2007;9(3):184‐189. [DOI] [PubMed] [Google Scholar]
- 8. Blank R, LaSalle J, Reeves R, et al. Single‐pill therapy in the treatment of concomitant hypertension and dyslipidemia (the amlodipine/atorvastatin gemini study). J Clin Hypertens (Greenwich). 2005;7(5):264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hong SJ, Jeong HS, Cho JM, et al. Efficacy and safety of triple therapy with telmisartan, amlodipine, and rosuvastatin in patients with dyslipidemia and hypertension: the Jeil Telmisartan, Amlodipine, and Rosuvastatin Randomized Clinical Trial. Clin Ther. 2019;41(2):233‐248. e9. [DOI] [PubMed] [Google Scholar]
- 10. Kim TS, Rha SW, Kim SY, et al. Efficacy and tolerability of telmisartan/amlodipine and rosuvastatin coadministration in hypertensive patients with hyperlipidemia: a phase III, multicenter, randomized, double‐blind study. Clin Ther. 2019;41(4):728‐741. [DOI] [PubMed] [Google Scholar]
- 11. Lee HY, Kim SY, Choi KJ, et al. A randomized, multicenter, double‐blind, placebo‐controlled study to evaluate the efficacy and the tolerability of a triple combination of amlodipine/losartan/rosuvastatin in patients with comorbid essential hypertension and hyperlipidemia. Clin Ther. 2017;39(12):2366‐2379. [DOI] [PubMed] [Google Scholar]
- 12. Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. 2022;387(11):967‐977. [DOI] [PubMed] [Google Scholar]
- 13. Deppe S, Böger RH, Weiss J, et al. Telmisartan: a review of its pharmacodynamic and pharmacokinetic properties. Expert Opin Drug Metab Toxicol. 2010;6(7):863‐871. [DOI] [PubMed] [Google Scholar]
- 14. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3024–3025] [published correction appears in J Am Coll Cardiol. 2015 Dec 22;66(24):2812]. J Am Coll Cardiol. 2014;63(25):2889‐2934. Pt B. [DOI] [PubMed] [Google Scholar]
- 15. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system [published correction appears in. Circ Res. 2018;123(8):e20]. Circ Res. 2017;120(1):229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carswell CI, Plosker GL, Jarvis B. Rosuvastatin. Drugs. 2002;62(14):2075‐2087. [DOI] [PubMed] [Google Scholar]
- 17. Kosoglou T, Statkevich P, Johnson‐Levonas AO, et al. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467‐494. [DOI] [PubMed] [Google Scholar]
- 18. Chilbert MR, VanDuyn D, Salah S, et al. Combination therapy of ezetimibe and rosuvastatin for dyslipidemia: current insights. Drug Des Devel Ther. 2022;16:2177‐2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143‐3421. [PubMed] [Google Scholar]
- 20. Yang YJ, Lee SH, Kim BS, et al. Combination therapy of rosuvastatin and ezetimibe in patients with high cardiovascular risk. Clin Ther. 2017;39(1):107‐117. [DOI] [PubMed] [Google Scholar]
- 21. Battershill AJ, Scott LJ. Telmisartan: a review of its use in the management of hypertension [published correction appears in Drugs. 2006;66(15):1987]. Drugs. 2006;66(1):51‐83. [DOI] [PubMed] [Google Scholar]
- 22. Ruilope LM. Telmisartan for the management of patients at high cardiovascular risk. Curr Med Res Opin. 2011;27(8):1673‐1682. [DOI] [PubMed] [Google Scholar]
- 23. ONTARGET Investigators , Yusuf S, Teo KK, et al. ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547‐1559. doi: 10.1056/NEJMoa0801317 [DOI] [PubMed] [Google Scholar]
- 24. Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565‐581. [DOI] [PubMed] [Google Scholar]
- 25. Luvai A, Mbagaya W, Hall AS, Barth JH. Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol. 2012;6:17‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin PD, Warwick MJ, Dane AL, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25(11):2822‐2835. [DOI] [PubMed] [Google Scholar]
- 27. de Zeeuw D, Anzalone DA, Cain VA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 2015;3(3):181‐190. doi: 10.1016/S2213-8587(14)70246-3 [DOI] [PubMed] [Google Scholar]
- 28. Han E, Kim G, Lee JY, et al. Comparison between atorvastatin and rosuvastatin in renal function decline among patients with diabetes. Endocrinol Metab (Seoul). 2017;32(2):274‐280. doi: 10.3803/EnM.2017.32.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system [published correction appears in Circ Res. 2018 Sep 28;123(8):e20]. Circ Res. 2017;120(1):229‐243. doi: 10.1161/CIRCRESAHA.116.308537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359(21):2195‐2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 31. Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2021‐2031. doi: 10.1056/NEJMoa1600176 [DOI] [PubMed] [Google Scholar]
- 32. Kanbay M, Yildirir A, Bozbas H, et al. Statin therapy helps to control blood pressure levels in hypertensive dyslipidemic patients. Ren Fail. 2005;27(3):297‐303. [PubMed] [Google Scholar]
- 33. Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110(24):3687‐3692. [DOI] [PubMed] [Google Scholar]
- 34. Spannella F, Filipponi A, Giulietti F, et al. Statin therapy is associated with better ambulatory blood pressure control: a propensity score analysis. J Hypertens. 2020;38(3):546‐552. doi: 10.1097/HJH.0000000000002276 [DOI] [PubMed] [Google Scholar]
- 35. Briasoulis A, Agarwal V, Valachis A, Messerli FH. Antihypertensive effects of statins: a meta‐analysis of prospective controlled studies. J Clin Hypertens (Greenwich). 2013;15(5):310‐320. doi: 10.1111/jch.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strazzullo P, Kerry SM, Barbato A, et al. Do statins reduce blood pressure?: a meta‐analysis of randomized, controlled trials. Hypertension. 2007;49. 792e798. [DOI] [PubMed] [Google Scholar]
- 37. Lee SG, Lee SJ, Thuy NVP, et al. Synergistic protective effects of a statin and an angiotensin receptor blocker for initiation and progression of atherosclerosis. PLoS One. 2019;14(5):e0215604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Straznicky NE, Howes LG, Lam W, et al. Effects of pravastatin on cardiovascular reactivity to norepinephrine and angiotensin II in patients with hypercholesterolemia and systemic hypertension. Am J Cardiol. 1995;75(8):582‐586. [DOI] [PubMed] [Google Scholar]
- 39. Rizos CV, Liberopoulos EN, Tellis K, et al. Combining rosuvastatin with angiotensin‐receptor blockers of different PPARγ‐activating capacity: effects on high‐density lipoprotein subfractions and associated enzymes. Angiology. 2015;66(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 40. Kim SH, Jo SH, Lee SC, et al. Blood pressure and cholesterol‐lowering efficacy of a fixed‐dose combination with irbesartan and atorvastatin in patients with hypertension and hypercholesterolemia: a randomized, double‐blind, factorial, multicenter phase III study. Clin Ther. 2016;38(10):2171‐2184. [DOI] [PubMed] [Google Scholar]
- 41. Park JS, Shin JH, Hong TJ, et al. Efficacy and safety of fixed‐dose combination therapy with olmesartan medoxomil and rosuvastatin in Korean patients with mild to moderate hypertension and dyslipidemia: an 8‐week, multicenter, randomized, double‐blind, factorial‐design study (OLSTA‐D RCT: oLmesartan rosuvaSTAtin from Daewoong). Drug Des Devel Ther. 2016;10:2599‐2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stein EA, Amerena J, Ballantyne CM, et al. Long‐term efficacy and safety of rosuvastatin 40 mg in patients with severe hypercholesterolemia. Am J Cardiol. 2007;100(9):1387‐1396. [DOI] [PubMed] [Google Scholar]
- 43. Abed W, Abujbara M, Batieha A, et al. Statin induced myopathy among patients attending the National Center for Diabetes, endocrinology, & genetics. Ann Med Surg (Lond). 2022;74:103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281‐1357. [DOI] [PubMed] [Google Scholar]
- 45. Davidson MH. Ezetimibe: a novel option for lowering cholesterol. Expert Rev Cardiovasc Ther. 2003;1(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 46. Zeitouni M, Sabouret P, Kerneis M, et al. 2019 ESC/EAS Guidelines for management of dyslipidaemia: strengths and limitations. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):324‐333. [DOI] [PubMed] [Google Scholar]
- 47. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387‐2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 48. Bays HE, Davidson MH, Massaad R, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up‐titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study). Am J Cardiol. 2011;108(4):523‐530. [DOI] [PubMed] [Google Scholar]
- 49. Kim W, Yoon YE, Shin SH, et al. Efficacy and safety of ezetimibe and rosuvastatin combination therapy versus those of rosuvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2018;40(6):993‐1013. [DOI] [PubMed] [Google Scholar]
- 50. Rhee MY, Kim KJ, Kim SH, et al. Ezetimibe and rosuvastatin combination treatment can reduce the dose of rosuvastatin without compromising its lipid‐lowering efficacy. Clin Ther. 2019;41(12):2571‐2592. [DOI] [PubMed] [Google Scholar]
- 51. Song ZY, Kim MH, Lee HC, et al. Efficacy and safety of coadministered ezetimibe‐rosuvastatin plus telmisartan in South Korean patients with dyslipidemia and hypertension: a multicenter, randomized, double‐blind, active‐controlled, Phase III trial. J Clin Med. 2023;12(6):2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are the property of the authors and can be made available upon reasonable request to the corresponding author.


