Abstract
Anemia is a major public health concern. Young children, menstruating adolescent girls and women, and pregnant women are among the most vulnerable. Anemia is the consequence of a wide range of causes, including biological, socioeconomic, and ecological risk factors. Primary causes include: iron deficiency; inherited red blood cell disorders; infections, such as soil-transmitted helminthiasis, schistosomiasis, and malaria; gynecological and obstetric conditions; and other chronic diseases that lead to blood loss, decreased erythropoiesis, or destruction of erythrocytes. The most vulnerable population groups in low- and middle-income countries are often at the greatest risk to suffer from several of these causes simultaneously as low socioeconomic status is linked with an increased risk of anemia through multiple pathways. Targeted and effective action is needed to prevent anemia. Understanding the causes and risk factors of anemia for different population subgroups within a country guides the design and implementation of effective strategies to prevent and treat anemia. A coordinated approach across various expert groups and programs could make the best use of existing data or could help to determine when newer and more relevant data may need to be collected, especially in countries with a high anemia burden and limited information on the etiology of anemia.
Keywords: anemia, anemia of inflammation, iron deficiency anemia, non-nutritional anemia, nutritional anemia
INTRODUCTION
Anemia is defined as a hemoglobin concentration below an age-, sex,-and pregnancy-specific cutoff.1 For nonpregnant women, for example, a hemoglobin concentration of 110–119 g/L is defined as mild anemia, a concentration of 80–109 g/L as moderate anemia, and a hemoglobin concentration below 80 g/L is considered severe anemia. Overall, anemia continues to be a health condition of major public health concern, with young children, menstruating adolescent girls and women, and pregnant women among the most vulnerable population groups. Anemia is associated with poor cognitive and motor development outcomes in children, increased morbidity and mortality in women and children, poor birth outcomes, and decreased work productivity in adults.2–8 Globally, over half a billion women 15–49 years of age and 269 million children aged 6–59 months were estimated to be affected by anemia in 2019.9 The present paper aims to review known causes and risk factors of anemia and to provide reflections on the most pressing issues to be addressed in accelerating reductions in the prevalence and severity of anemia. The paper was prepared as an input paper in support of the Comprehensive framework for integrated action on the prevention, diagnosis, and management of anemia led by the World Health Organization (WHO).
Globally, 30% (95% uncertainty interval [UI] 27–33%) of nonpregnant women 15–49 years of age were estimated to be anemic in 2019, a prevalence level that has remained unchanged from the year 2000 (31% [95% UI 28–34%]).10 In contrast, the prevalence of anemia in pregnant women decreased from 41% (95% UI 39–43%) to 36% (95% UI 34–39%) during that same time period.10 However, the progress on anemia reduction is not sufficient to meet the global anemia target set by the World Health Assembly (i.e., 50% reduction of anemia among women of reproductive age for each country by 2025).10,11 Among children aged 6–59 months, the global prevalence of anemia was 40% (95% UI 36–44%) in 2019, down from 48% (95% UI 45–51%) in 2000, but unchanged since 2010 (40% [95% UI 36–44%]).9,10 In 2019, the highest burden of anemia was found in the WHO Regions of South-East Asia and Africa; the prevalence of anemia among women exceeded 50% in 10 countries and 70% among children in 11 countries.10 Of note, data suggest a shift toward mild anemia; while the prevalence of mild anemia globally and in most regions stagnated from 2000 to 2019, important progress was achieved as the prevalence of moderate and severe anemia declined in most populations and regions.10
Anemia is the consequence of a wide range of causes as well as biological, socioeconomic, and ecological risk factors,6–8 which often act concurrently (Figure 1). The three main underlying physiological mechanisms of anemia are: (1) ineffective erythropoiesis (i.e., inadequate production of erythrocytes); (2) hemolysis (i.e., erythrocytes are destroyed); and (3) blood loss.6–8
FIGURE 1.
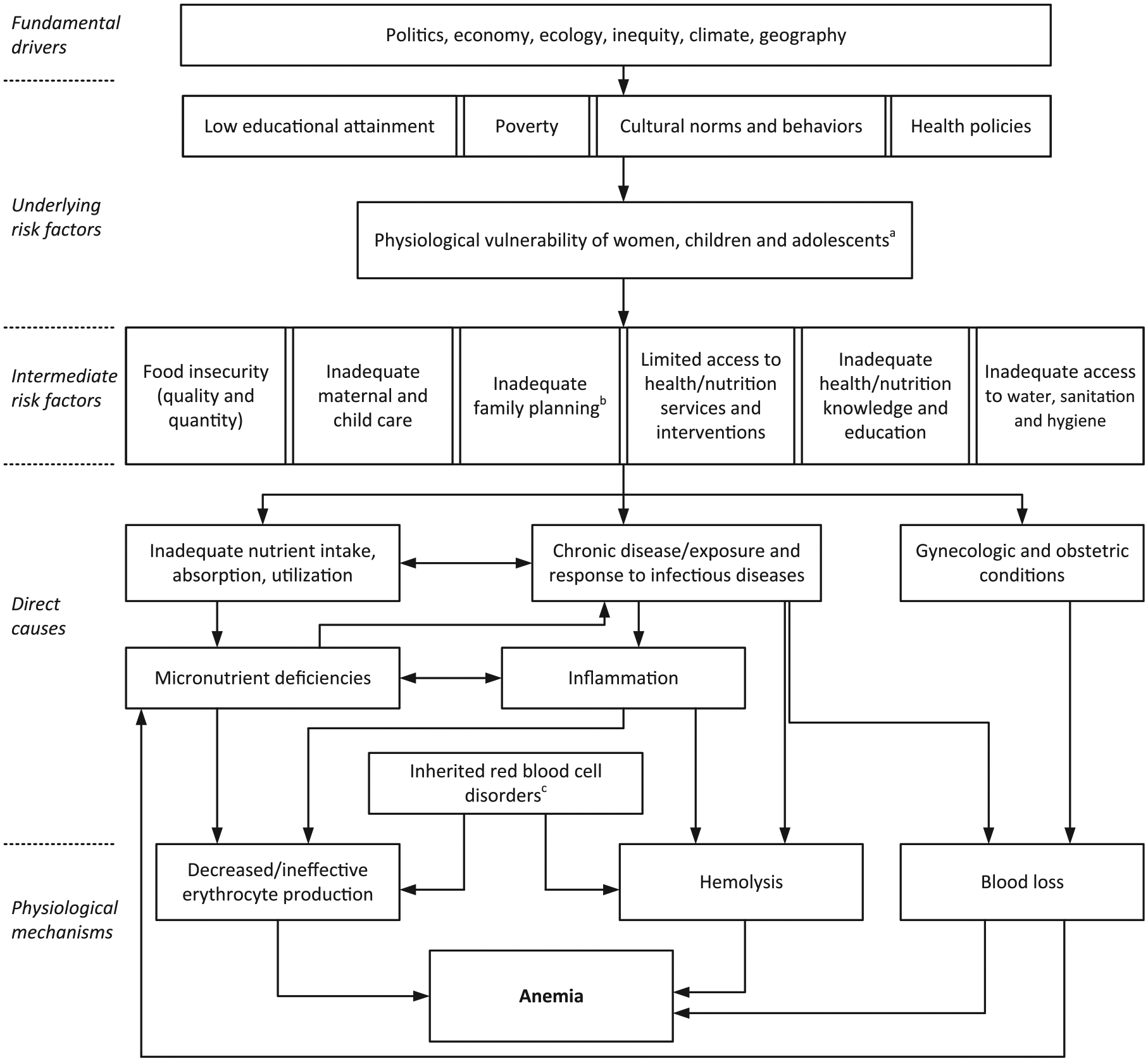
Conceptual framework of anemia etiology. Notes: aHigh nutrient requirement due to growth, pregnancy, and increased susceptibility to infections during childhood and pregnancy. bEarly onset of childbearing, high parity, and short birth spacing. cInherited red blood cell disorders include sickle cell disorders, thalassemias, glucose-6-phosphate dehydrogenase (G6PD) deficiency, and other hemoglobinopathies. Source: Adapted and reproduced with permission from the publisher of reference.6 The original figure by Chaparro and Suchdev6 was developed based on determinants presented in references 53, 84, and 85.
Iron deficiency is considered the most common nutritional deficiency leading to anemia.6 Inadequate dietary iron intake is the primary pathway resulting in iron deficiency anemia. About 60% of the total global burden of anemia in 2019 was estimated to be due to dietary iron deficiency and thus iron deficiency accounted for the most significant cause of anemia-related disability.12 Deficiencies in vitamins A, B2, B6, B12, C, D, E, folate, copper, selenium, and zinc can also result in anemia due to their specific roles in the synthesis of hemoglobin and/or erythrocyte production;6,7,13 however, some of these micronutrients may not play a major role in the burden of anemia globally.6,7 Additional mechanisms include nutrient losses (e.g., iron deficiency secondary to blood loss from parasitic infections, hemorrhage associated with childbirth, or menstrual loss), impaired absorption (e.g., lack of intrinsic factor to facilitate vitamin B12 absorption or high intake of inhibitors, such as phytate that impair iron absorption), and nutrient interactions (e.g., vitamin A deficiency affecting mobilization of iron stores).7 Using data from nationally representative surveys, the project on Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) recently assessed associations between anemia and multiple risk factors, such as iron and vitamin A deficiencies, inflammation, and malaria, and found that the proportion of anemic individuals who were iron deficient ranged from 30% to 71% in the different surveys and was negatively associated with the burden of infection/inflammation present in the population (assessed by elevated C-reactive protein and alpha-1-acid glycoprotein) (Figure 2).14,15
FIGURE 2.

Venn diagrams illustrating the weighted prevalence of iron deficiency, anemia, iron-deficiency anemia, and the proportions of anemic women with iron deficiency in nonpregnant women of reproductive age by category of infection burden: results from the BRINDA project. Results from the BRINDA Project include nationally representative surveys. Iron deficiency was defined as an inflammation-adjusted ferritin concentration <15 μg/L. Anemia was defined as a hemoglobin concentration <120 g/L. Iron-deficiency anemia was defined as a hemoglobin concentration <120 g/L and inflammation-adjusted ferritin concentration <15 μg/L. Countries were categorized by infection burden as follows—low: Georgia and the United States; moderate: Colombia and Mexico (2006 and 2012); and high: Cameroon, Côte d’Ivoire, Liberia, and Laos. Abbreviation: WRA, women of reproductive age. Source: Wirth et al.15
Inherited red blood cell disorders are other common causes of anemia, accounting for about 15% of the global burden of anemia in 2019.12 These include conditions such as: α- and β-thalassemia due to abnormalities of hemoglobin synthesis, sickle cell disorders due to changes in the hemoglobin structure, other hemoglobinopathies due to hemoglobin gene variants, abnormalities of red cell enzymes (glucose-6-phosphate dehydrogenase [G6PD] deficiency), or abnormalities of the red blood cell membrane (hereditary spherocytosis, elliptocytosis, and ovalocytosis).16,17 At least 5% of the world population carries a significant gene variant, but because of localized high carrier prevalence the highest prevalence of inherited red blood cell disorders is found in South-East Asia and Africa.18 This geographical localization of hemoglobinopathies is due to the partial protection against severe malaria. The proportion of anemia due to genetic hemoglobin and red blood cell disorders will likely continue to rise globally, particularly in low- and middle-income countries, as other causes, such as nutritional deficiencies and infectious diseases, are increasingly prevented.19 It is also likely to rise in higher-income countries due to population migration.
Anemia due to infections is another important cause of anemia globally, estimated to account for about 12% of total cases in 2019,12 and this is directly related to the geographical burden of infection. Infections can impair nutrient absorption and metabolism or can cause nutrient loss. Inflammation and chronic disease can also lead to anemia known as anemia of inflammation, which is immune-driven. Proinflammatory cytokines increase the synthesis of an iron-regulating hormone, hepcidin, which causes the sequestration of iron as ferritin, thereby blocking iron transfer to erythrocyte precursor cells in the bone marrow and resulting in decreased erythropoiesis as well as decreased erythrocyte survival.20–23 The exact pathological mechanisms depend on the underlying infection or disease causing the anemia.23 Soil-transmitted helminths, including hookworm (Necator americanus and Ancylostoma duodenale), Trichuris trichiura, and Ascaris lumbricoides, parasitize the gastrointestinal tract causing blood loss, which in turn can result in the loss of iron and the development of anemia.24 Globally, about 1.5 billion people are estimated to be infected with soil-transmitted helminths, with the highest infection rates in areas with poor sanitation in tropical and subtropical regions of sub-Saharan Africa, the Americas, China, and East Asia.25 Schistosomiasis is an infectious disease caused by trematode parasites of the genus Schistosoma. Adult worms live within the veins of the human host, mate, and cause anemia due to inflammation and blood loss.26 Other common infections that can cause anemia are Helicobacter pylori and visceral leishmaniasis.27,28 The primary causes of mild and moderate anemia tend to differ from those that cause severe anemia.6 Malaria is a major cause of anemia and is a primary cause of severe anemia.6 While malaria does not lead to iron loss, it alters iron metabolism through mechanisms that include hemolysis, release of heme, dyserythropoiesis, deposition of iron in macrophages, and inhibition of dietary iron absorption. Anemia is also very common among individuals infected with HIV and tuberculosis.6,16 HIV infection causes anemia through a wide range of mechanisms, including ineffective erythrocyte production, hemolysis, blood loss, and side effects of drug treatment. Chronic inflammation also causes anemia in tuberculosis patients.16
Various other conditions, such as gastrointestinal disease, kidney disorders, and other diseases, lead to blood loss, decreased erythropoiesis, or destruction of erythrocytes, resulting in anemia of inflammation, also referred to as anemia of chronic disease.22 This is considered the most frequent type of anemia among hospitalized and chronically ill patients. Globally, these diverse diseases are estimated to cause about 13% of anemia.12,30 Obesity is characterized by chronic mild inflammation, which has consistently been found to alter hematologic parameters and iron metabolism.31,32 However, even though obesity is associated with features of anemia of inflammation (i.e., alterations in iron homeostasis and hypoferremia),22 studies on associations between obesity and anemia have been inconsistent.31,32
Menstruating adolescent girls and women are among the most vulnerable population groups at risk of developing anemia because of the regular blood loss due to menstruation.7 This is aggravated during adolescence, a period known as the second growth spurt in life.33 During pregnancy, the risk of anemia increases due to the increased iron needs (e.g., for the placenta, the fetus, and expanded maternal blood volume during pregnancy) and potential blood loss during and after childbirth.34 Severe bleeding of postpartum hemorrhage is a risk factor for anemia.35 Every year about 14 million women primarily in low- and middle-income countries suffer from postpartum hemorrhage.36,37 In addition, pre-existing conditions, such as HIV, other infections, and noncommunicable diseases, may further exacerbate the risk of anemia during pregnancy.38
Importantly, the above-described causes can occur concurrently, and the most vulnerable population groups in low- and middle-income countries are often at the greatest risk to suffer from several of these causes simultaneously. Low socioeconomic status is linked with an increased risk of anemia via multiple pathways from poor living conditions, which include: poor water, sanitation, and hygiene; air pollution; smoking; food insecurity; and poor dietary quality (e.g., low dietary diversity predominantly relying on grain-based diets).7,39 Low attainment of formal education is another risk factor for anemia, as less formal education may affect a woman’s ability to access and understand the information provided on health, nutrition, family planning, and/or to earn a higher income, hence affecting nutritional security.7 A recent analysis of the national or subnational decline in anemia prevalence among women of reproductive age and the associated drivers in low- and middle-income countries found that healthcare utilization, especially seeking antenatal care during pregnancy and the use of contraceptives, were strong drivers for anemia reduction.11 Varying from setting to setting, there are health disparities by ethnicity and race,7 with minority groups often at increased risk of anemia likely due to the complex interplay of socioeconomic risk factors. Gender inequality, lack of women’s empowerment, and cultural practices related to early marriage and pregnancy can also contribute to the risk of anemia.7,8 In the above-mentioned, multicountry analyses of national and subnational anemia reduction, greater age at first pregnancy, higher body mass index, more birth spacing, and lower parity were associated with a modest reduction in anemia prevalence.11
MAJOR GAPS IN KNOWLEDGE ON THE CAUSES AND RISK FACTORS OF ANEMIA AND WAYS FORWARD TO USE EXISTING DATA AND COLLECT NEW RELEVANT DATA
To prevent anemia among the most vulnerable population groups and accelerate progress toward the Global Nutrition Target 2 (50% reduction in the prevalence of anemia in women of reproductive age by the year 2025) and the Sustainable Development Goals (SDG) indicator 2.2.3 (prevalence of anemia in women 15–49 years of age, by pregnancy status),40,41 understanding the etiology of anemia is an important aspect in designing effective and targeted programs at the country or subnational level. In the following section, we describe current gaps and explore potential ways forward on improving the use of existing data and identifying data needed to better target anemia programs (Table 1). The points raised were derived from recent reviews of anemia, input from various experts from the WHO interdepartmental working group on anemia, STAGE (Strategic and Technical Advisory Group for Maternal, Newborn, Child, and Adolescent Health and Nutrition), and the Anemia Action Alliance. Approaches to improve the diagnosis of anemia and its causes, and to accelerate interventions and programs to prevent and treat anemia are reviewed elsewhere.42–44
TABLE 1.
Major gaps in knowledge on the causes and risk factors of anemia and ways forward to use existing data and collect new relevant data.
| Gaps in knowledge | Way forward |
|---|---|
| At present, 64 countries have no anemia information. | Consider hemoglobin data collection in nationally representative surveys in countries with outdated, little, or no available data. |
| Hemoglobin is assessed in national surveys primarily among the most vulnerable population groups (children and women of reproductive age). | Consider extending data collection to other population groups (such as older adolescents and older people), as resources permit. |
| Sparsity of data on various causes and risk factors of context-specific anemia. | For newly planned surveys, consider the inclusion of context-specific indicators (see Table 3). |
| Because of the complex interplay of nutritional and non-nutritional causes and risk factors of anemia, relevant data may come from a variety of sources. | Survey planning, collection of data, and interpretation of results require a coordinated, multidisciplinary approach. |
| Available data not always used to full potential. | Sharing of deidentified data and biospecimen and public repository of deidentified datasets may improve the use of existing data. |
| Technical concerns related to hemoglobin assessment. | Gold standard is the determination of hemoglobin in venous blood samples by automated hematology analyzer, although the use of venous or pooled capillary blood samples in point-of-care devices using quality control with international reference standards could be considered.42 |
WHO recently updated the national, regional, and global estimates of anemia for women 15–49 years of age (by pregnancy status) and children aged 6–59 months for the time period 2000–2019.10 Estimates for these population groups from 197 countries were based on 489 data sources collected in 133 countries spanning 1995–2020.10,45 Thus, hemoglobin data from population-representative surveys were lacking for 64 countries. The authors45 state that anemia estimates and trends over time were more reliable when countries had several data sources collected throughout this timeframe. At least three data sources were available for women in 64 countries (covering 72% of women globally) and for children in 63 countries (covering 60% of children globally); however, there were variations by region (Table 2).45 These recently updated global and regional estimates are similar to previous estimates despite updates to data sources and covariates.46 Moreover, anemia prevalence estimates produced for the Global Burden of Disease (GBD) Study 2019 are comparable for women of reproductive age despite differences in inclusion and exclusion criteria, statistical models, and covariates.47 The GBD estimates for children 0–59 months of age are slightly higher than those by Stevens et al. (46% [95% UI 44–48%] vs. 40% [95% UI 36–44%]), and not explained by the difference in the age range alone.10,47 Nevertheless, despite considerable data gaps and related uncertainty in the estimates for countries without recent nationally representative hemoglobin results, these estimates highlight that anemia continues to be a public health problem in many countries. Moreover, anemia prevalence estimates help determine whether the risk of anemia is severe, moderate, or mild in a country and can inform decisions regarding effective strategies to prevent and treat anemia, as well as opportunities to further assess and monitor the anemia situation in representative surveys.
TABLE 2.
Number of surveys reporting anemia prevalence in children 6−59 months of age and women 15−49 years of age used in generating WHO global estimates of anemia 2000−2019, by WHO region.
| WHO region (number of countries) | Americas (35) | African (47) | Eastern Mediterranean (22a) | European (53) | South-East Asia (11) | Western Pacific (27b) |
|---|---|---|---|---|---|---|
| Number of surveys | 94 | 160 | 42 | 51 | 48 | 94 |
| Average number of surveys per country | 2.7 | 3.4 | 1.9 | 1.0 | 4.4 | 3.5 |
| Number (%) of countries in region without survey data | 10 (29%) | 8 (17%) | 6 (27%) | 32 (60%) | 0 | 6 (22%) |
Note: Verified surveys reported in the WHO Micronutrients Database as of March 30, 2022.
Abbreviation: WHO, World Health Organization.
Including Occupied Palestinian Territory.
Taiwan, China counted as part of China.
WHO presently provides global anemia estimates only for women 15–49 years of age, by pregnancy status, and for children aged 6–59 months9 because these are the most vulnerable population groups for anemia. Data on hemoglobin concentration are even more limited for other population groups. Specifically, out of 432 nationally representative surveys carried out since 1990 and reported in WHO’s Micronutrients Database,48 hemoglobin was assessed primarily in the most vulnerable population groups, such as preschool-age children (68% of surveys), pregnant women (56% of surveys), and women of reproductive age (68% of surveys), with fewer surveys among other population groups, such as adolescents (53% of surveys; with about half of these including only older adolescents 15–19 years of age), men (27% of surveys), school-age children (23% of surveys), and older persons (11% of surveys). The decision on extending the survey to other population groups (such as adolescents or older persons) is guided by many factors and could include the assumed anemia risk, the prevalence of relevant causes among different population groups, and costs. Available resources can be a limiting factor in determining the number of indicators collected and the number of population groups assessed.
As described above, causes of anemia are both nutritional and non-nutritional, which are further aggravated by numerous socioeconomic and ecological risk factors caused by inequity (Figure 1). Thus, preventing anemia requires a coordinated, multisectoral, and strategic approach.7 Similarly, the collection and interpretation of data for understanding the causes and burden of anemia within a country or region can require a multidisciplinary approach. While there is extensive information available on malaria in children from sub-Saharan Africa, soil-transmitted helminths infections, and HIV/AIDS among adults,25,49–51 there is a sparsity of data on various other causes of anemia for different population groups. For example, there is less information on malaria among adults or HIV/AIDS among children. Also, there have been few population analyses of the genetic variants of different inherited red blood cell disorders.16,17 Due to this lack of information, it is likely that the prevalence of inherited red blood cell disorders and the respective global burden of anemia due to this cause is presently underestimated. The GBD 2019 study estimates the proportion of total anemia prevalence attributable to 35 causes based on cause-specific hemoglobin shifts, the estimated prevalence of known causes, and the overall hemoglobin distribution for each location, year, and population group from 1990 to the present.47 Because of the data sparsity, the GBD study does not use indicators of iron status to estimate the prevalence of iron deficiency, instead, it relies on counterfactual modeling and attempts to isolate the disease burden due to iron deficiency using hemoglobin as a proxy.52 Systematic data collection of various causes and risk factors of anemia from population-representative surveys could contribute to a better understanding of the complex interplay of causes and risk factors associated with the occurrence of anemia.
Despite the above-mentioned data gap, hemoglobin is among the most frequently assessed indicators in national nutrition surveys.48 However, national survey results are not always easily accessible in the public domain. With considerable efforts, the WHO’s Vitamin and Mineral Nutrition Information System (VMNIS) systematically retrieves and summarizes hemoglobin and other micronutrient status results from population-representative surveys.48 A public data repository of deidentified individual data that includes hemoglobin, as well as recommended indicators of causes and risk factors of anemia (Table 3), from population-representative surveys could facilitate and maximize the use of existing survey data and allow for standardized analyses to generate harmonized and comparable data among and within countries.
TABLE 3.
Indicators of relevant causes and risk factors of anemia to be considered for assessment in surveys, surveillance, or program monitoring.
| Causes and risk factors of anemia | Diagnostic test, biomarker, or characteristics to identify condition | Proposed cutoff values or defining characteristics | Feasibility of collection | Cost |
|---|---|---|---|---|
| Causes | ||||
| Iron deficiency | Ferritin | Infants and children < 5 years (< 12 μg/L); children, adolescents, and adults 5 years and older (< 15 μg/L); pregnant women (< 15 μg/L)67 | Often requires venous blood collection but a sandwich enzyme-linked immunosorbent assay technique enables the collection of pools of capillary blood; cold chain required | $-$$$ |
| C-reactive protein, alpha-1-acid glycoprotein | Inflammation defined as CRP > 5 mg/L or acid glycoprotein > 1 g/L | Biomarkers of inflammation are required for accurate interpretation of ferritin67 | ||
| Infections | Parasitic (malaria, soil-transmitted helminthiasis [STH], schistosomiasis [SCH]) | Usually defined by the presence of infectious organism | Malaria and hematuria can be measured in the field from capillary blood and urine, respectively73 | $-$$ |
| Viral (HIV/AIDS, hepatitis C, respiratory viruses including COVID-19) | Mapping of STH and SCH is based on stool (STH and SCH) and urine (SCH) specimens74 | |||
| Bacterial (tuberculosis, salmonella, H. pylori, others) | Polymerase chain reaction testing diagnostic capacity variable for other infections | |||
| Inherited red blood cell disorders | Abnormalities of hemoglobin synthesis (alpha or beta thalassemia) | Usually defined with molecular tests by genotyping DNA extracted from dried blood cards | May be collected using dried blood cards, so is not dependent on venous blood collection or cold chain | $-$$ |
| Abnormalities of hemoglobin structure (Hb S, C, and E) | Hemoglobin electrophoresis can also be used to detect Hb S, C, and thalassemia | |||
| Abnormalities of red cell enzymes (G6PD deficiency) | Genotyping or phenotyping tests can be used; rapid diagnostic test kits for qualitative phenotyping tests available (typically below 30–40% of normal activity)76 | |||
| RBC membrane disorders (hereditary spherocytosis, elliptocytosis) | RBC membrane disorders can be diagnosed by RBC cytology, flow cytometry, ektacytometry, electrophoresis of RBC membrane proteins, and genetics75 | |||
| Blood loss | Onset of menstruation/menopause Hormonal contraception use Heavy menstruation Uterine fibroids Pregnancy and/or delivery complications | Usually defined by participant recall to questionnaires that include information about these characteristics | Requires the knowledge of cultural norms around reproductive health and birth | $ |
| Deficiencies in other micronutrients | Vitamin A | Retinol < 0.7 μmol/L (although for women is still uncertain)68 | Requires venous blood collection and cold chain, although folate can also be determined using dried blood cards. Limited availability of laboratories with externally validated performance | $$-$$$ |
| Riboflavin | Erythrocyte glutathione reductase activity coefficient > 1.369 | |||
| Folate | Serum folate < 6.8 nmol/L (risk of megaloblastic anemia) or RBC folate < 748 nmol/L (risk of neural tube defects)a,70 | |||
| Vitamin B12 | < 150 pmol/L (risk of megaloblastic anemia)71 | |||
| Risk factors | ||||
| Demographic and physiological status | Age Pregnancy/lactation |
Usually defined by participant recall to questionnaires | Requires the knowledge of cultural norms | $ |
| Socioeconomic characteristics | Income Educational attainment (incl. maternal education) Food insecurity Inequity, women’s empowerment |
Usually defined by participant recall to questionnaires | Requires the knowledge of cultural norms | $ |
| Lack of micronutrients and diversity in diet | Recent intake of animal source foods Recent intake of iron inhibitors (phytates, tannins) Recent consumption of foods fortified with iron, folate, and/or vitamin A Supplementation with iron, folate, and/or vitamin A |
Usually defined by participant recall to questionnaires | Requires the knowledge of common dietary patterns among target population | $ |
| Family planning practices | Onset of childbearing, parity, birth spacing | Usually defined by participant recall to questionnaires | Requires the knowledge of cultural norms | $ |
| Health practices/health services | Antenatal care during previous pregnancy Emergency obstetric and neonatal care Modern contraceptives Malaria prevention practices, if applicable Deworming, if applicable |
Usually defined by participant recall to questionnaires | Requires the knowledge of cultural norms and offered healthcare services | $ |
| Water access, sanitation, and hygiene | WASH practices | Usually defined by participant recall to questionnaires | Requires the knowledge of local practices and programs | $ |
| Social support programs | Poverty alleviation Income support programs |
Defined at regional level or by participant recall to questionnaires | Requires the knowledge of local programs | $ |
Abbreviations: Hb, hemoglobin; RBC, red blood cell; SCH, schistosomiasis; STH, soil-transmitted helminthiasis; WASH, Water Access, Sanitation, and Hygiene.
The cutoff depends on the laboratory method.
Source: Adapted with permission from USAID Advancing Nutrition.16
In recent years, data sharing of deidentified individual-level data has become more common and has resulted in important findings such as those from the BRINDA project,14,15,53–55 and the findings from the Exemplars in Stunting Reduction project.56 Sharing deidentified individual data across surveys, disciplines, and countries may allow for further exploration of available data in secondary analyses. Similarly, sharing properly stored biospecimens for analysis of additional biomarkers, such as indicators of micronutrient status or specific infections, could help elucidate causes and risk factors of anemia in various contexts. Importantly, before sharing and using samples and protected health information, investigators need to comply with relevant Institutional Review Boards and the privacy standards of the respective countries. Examples include the Health Insurance Portability and Accountability Act in the United States and the European Union General Data Protection Regulation.57,58 When data are exchanged or shared, developing material and data transfer agreements to define the conditions for data and specimen sharing between the data owners and researchers is highly recommended and has become a common practice.59,60
Because cross-sectional surveys do not allow causal attribution, data analyses across multiple data sources are recommended to determine the most likely underlying risk factors and causes of anemia.61 It can be challenging to identify various sources and bring all relevant sectors together for a comprehensive review of the data. Initiatives, such as the West African Health Organization (WAHO) and African Population and Health Research Center’s Countdown to 2030 and the National Information Platforms for Nutrition (NiPN), are examples that provide support regionally, or to specific countries, to strengthen the management, in-country capacity, and use of nutrition data for effective national planning and decision-making.62,63 Such initiatives are potential resources to address the challenges with comprehensive data review for anemia prevention and reduction; however, not all regions or countries have access to these types of initiatives. Another challenge to interpreting anemia results is when two national surveys conducted close in time and assess the same population groups, especially young children, result in vastly different anemia prevalence estimates,64 leading to uncertainties regarding the level of the public health problem, actions warranted, and effectiveness of programs. More details on this topic are reviewed by Garcia-Casal et al.42 An additional consideration is the use of appropriate analytic methods to understand the etiology of anemia. For example, attributable fractions of anemia are sometimes used to examine etiology; however, there are multiple approaches and some are more complex than others, or even inappropriate, when anemia is not rare (e.g., Levin’s formula with odds ratio), which can result in potentially biased results.65 Furthermore, there are limitations to note when attributable fraction analyses are conducted using cross-sectional data because there may be reverse causality and it is not possible to draw causal inferences on true risk reduction of anemia.
While existing data can guide program designs and identify potential interventions, existing data can also be used to identify data gaps. If there are data gaps, it may be possible that administrative data or other proxy data could be leveraged to gain some insights into potential risk factors. Identifying data gaps is important to highlight the limits of our current understanding of the etiology of anemia and to guide future data collection.
In addition to leveraging existing data, there is a need to periodically collect new data. This is particularly important for countries with outdated or no data on anemia prevalence in high-risk populations. Countries that might most benefit from new data include those with a potentially high burden of anemia, limited data on the etiology of anemia, or those with recent changes in health programs or economic, social, or natural disasters. Planning and implementing a national survey is a major undertaking and requires a number of considerations and decisions, which are described in detail in the Micronutrient survey manual.66 Moreover, decisions regarding the inclusion of specific indicators may vary depending on the context and should be guided by the known or assumed prevalence of micronutrient deficiencies (including iron), burden of malaria and other parasitic infections, and prevalence of inherited red blood cell disorders (Table 3). An additional consideration for the choice of indicator(s) included in a survey is the availability of prevention or treatment strategies for the respective causes of anemia with the overarching goal that the survey results inform the design and implementation of new strategies aimed at addressing anemia or aid in the monitoring of existing programs. Survey results may also further guide which assessments could be prioritized in future population-representative surveys.
The inclusion of indicators on micronutrient status, particularly iron status, in the survey, depends on the objectives of the survey and whether the proportion of anemia due to iron deficiency is already known.16 There are several biomarkers of iron status that reflect different stages of iron sufficiency, and serum ferritin and soluble transferrin receptor are the most commonly used. WHO has recently updated guidance on the assessment of serum ferritin concentrations to include a recommendation to also measure markers of inflammation (e.g., C-reactive protein and/or α−1 acid glycoprotein) for the purpose of statistically correcting serum ferritin for inflammation.67 While deficiencies of some micronutrients (such as vitamins B6, C, D, E, copper, selenium, and zinc) may not contribute much to the global anemia burden, deficiencies in vitamins A, B2, B12, and folate could potentially be important in some contexts.16,68–71 Although there has been an increase in awareness of the importance of these micronutrient deficiencies due to their adverse health effects, there is a sparsity of information on deficiency prevalence72 and thus estimating the prevalence of various micronutrient deficiencies and their respective anemia-attributable burden remains challenging.52
In malaria-endemic countries, assessing malaria prevalence is warranted. Rapid diagnostic tests are inexpensive (cost approximately 1 US$) and are easy to administer.66,73 The assessment of indicators of parasitic infections depends on the burden of soil-transmitted helminth and schistosome infections.74 The inclusion and analytical approach to assess inherited red blood cell disorders can be informed by the type and geographic burden of inherited red blood cell disorders.75–77 Table 3 provides an overview of indicators of relevant causes and risk factors of anemia for consideration in surveys.
In countries with prevention programs targeting any of the major causes of anemia, such as iron supplementation or fortification, malaria prevention, deworming, or WASH (i.e., Water Access, Sanitation, and Hygiene), the reach, coverage, and quality of these programs should be assessed to determine the programs’ effectiveness.7 Monitoring and evaluation of programs allows for the identification and correction of any programmatic issues to improve program effectiveness. Malaria surveillance, for example, is considered a key aspect of intervention in countries that are malaria-endemic or have eliminated malaria but remain susceptible.73,78 It is important to note that even if a successfully implemented program preventing iron deficiency, malaria, or soil-transmitted helminthiasis and schistosomiasis, has only a minor impact on anemia, these programs are important for their own sake due to the substantial health burden of these causes per se.24,34,49
There are technical concerns related to hemoglobin assessment, such as the type of blood drawn (e.g., venous vs. capillary blood), the quality of the blood sample, and the analytical method and devices used for measuring hemoglobin, among other preanalytic, analytic, and postanalytic considerations.79–82 The gold standard for hemoglobin determination involves the processing of venous blood in an automated hematology analyzer and following appropriate quality control measures and standards,79,80 although the use of venous or pooled capillary blood samples in point-of-care devices with regular quality control against international reference standards could be considered as reviewed by Garcia-Casal et al.42
There are six global nutrition targets that have been endorsed by the World Health Assembly, five of which relate to children (stunting, wasting, overweight, obesity, and low birth weight) and the one target in women 15–49 years of age calls for a reduction in the prevalence of anemia by 2025.40 As 2025 nears, there will be an opportunity to revisit and revise these targets or call for their extension to 2030 to align with the United Nations’ SDGs.41 Although actions that effectively address conditions relating to the current six global nutrition targets may contribute to reducing childhood anemia, the addition of a global target on reducing anemia in children under 5 years of age could also be considered to draw needed attention and action to reducing the global burden of anemia,83 currently estimated at 269 million children.9 In the meantime, countries may decide to set their own national targets as they implement and monitor their programs designed to prevent and manage anemia.
In summary, causes of anemia span across multiple subject areas, including iron and other micronutrient deficiencies, various parasitic infections, inherited red blood cell disorders, gynecological and obstetric conditions, and various chronic diseases, which are further complicated by numerous interconnected socioeconomic and ecological risk factors. A coordinated and strategic approach across various expert groups and programs could make the best use of existing data, or could help to determine when newer and more relevant data may need to be collected among vulnerable population groups, especially in countries with a high anemia burden and limited information on the etiology of anemia with a focus on preventable or treatable causes of anemia. The complex interplay of causes and risk factors of anemia underscores the need for well-coordinated multisectoral action, including poverty reduction, women’s empowerment and equity, as well as improvements in nutrition and health. Such coordinated action may reduce not only the burden of anemia but also other health outcomes.
ACKNOWELDGMENTS
We greatly appreciate valuable comments on the manuscript by Maurice Bucagu; Maria Nieves Garcia-Casal; Antonio Montresor; Özge Tuncalp (World Health Organization, Geneva, Switzerland); Kenneth H. Brown (University of California, Davis, CA, USA); Theresa Gyorkos (McGill University, Montreal, Canada); Goutham Kandru (Gates Ventures, Seattle, WA, USA, on behalf of Exemplars in Global Health); and Pat McMahon (Mothers First, Ireland). Funding support for the preparation of the present paper was provided by the Bill & Melinda Gates Foundation to the Micronutrient Forum (grant number INV-003021). This work was also financially supported by the Food and Nutrition Actions in Health Systems Unit, Department of Nutrition and Food Safety of the World Health Organization, Geneva, Switzerland. The World Health Organization gratefully acknowledges the financial contribution of the International Micronutrient Malnutrition Prevention and Control Program (IMMPaCt) at the Centers for Disease Control and Prevention (CDC), USA; the United States Agency for International Development (USAID), USA; and the Bill & Melinda Gates Foundation, USA.
Footnotes
COMPETING INTERESTS
The spouse of S.Y.H. serves as a consultant to the Micronutrient Forum and to the Bill & Melinda Gates Foundation. All other authors have no competing interests to declare.
DISCLAIMER
This manuscript was developed as an input paper for the Comprehensive framework for integrated action on the prevention, diagnosis and management of anemia led by the World Health Organization (WHO). This paper is being published individually but will be consolidated with other manuscripts as a special issue of Annals of the New York Academy of Sciences, the coordinator of which was Maria Nieves Garcia-Casal. This special issue will serve as working papers providing insights to help diagnose anemia and its causes with acceptable accuracy and precision in individuals and population, and to prioritize and maintain actions to accelerate reductions in the prevalence of anemia. The special issue is the responsibility of the editorial staff of Annals of the New York Academy of Sciences, who delegated to the coordinator preliminary supervision of both technical conformity to the publishing requirements of Annals of the New York Academy of Sciences and general oversight of the scientific merit of each article. This work was supported by the WHO, the CDC, USA; the USAID, USA; and the Bill & Melinda Gates Foundation, USA. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the WHO, the CDC, the USAID, the sponsors, publisher, or editorial staff of Annals of the New York Academy of Sciences.
REFERENCES
- 1.World Health Organization. (2011). Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. Geneva: World Health Organization. [Google Scholar]
- 2.Haas JD, & Brownlie T (2001). Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. Journal of Nutrition, 131, S676–S690. discussion 688S-690S. [DOI] [PubMed] [Google Scholar]
- 3.Scott S, Chen-Edinboro L, Caulfield L, & Murray-Kolb L (2014). The impact of anemia on child mortality: An updated review. Nutrients, 6, 5915–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung J, Rahman MM, Rahman MS, Swe KT, Islam MR, Rahman MO, & Akter S (2019). Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Annals of the New York Academy of Sciences, 1450, 69–82. [DOI] [PubMed] [Google Scholar]
- 5.Larson LM, Kubes JN, Ramírez-Luzuriaga MJ, Khishen S, Shankar AH, & Prado EL (2019). Effects of increased hemoglobin on child growth, development, and disease: A systematic review and meta-analysis. Annals of the New York Academy of Sciences, 1450, 83–104. [DOI] [PubMed] [Google Scholar]
- 6.Chaparro CM, & Suchdev PS (2019). Anemia epidemiology, patho-physiology, and etiology in low- and middle-income countries. Annals of the New York Academy of Sciences, 1450, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. (2017). Nutritional anemias: Tools for effective prevention and control. Geneva: World Health Organization. [Google Scholar]
- 8.World Health Organization. (2020). Global anemia reduction efforts among women of reproductive age: Impact, achievement of targets and the way forward for optimizing efforts. Geneva: World Health Organization. [Google Scholar]
- 9.World Health Organization. (2022). WHO Global anemia estimates, 2021 Edition. Global anemia estimates in women of reproductive age, by pregnancy status, and in children aged 6–59 months. https://www.who.int/data/gho/data/themes/topics/anemia_in_women_and_children
- 10.Stevens GA, Paciorek CJ, Flores-Urrutia MC, Borghi E, Namaste S, Wirth JP, Suchdev PS, Ezzati M, Rohner F, Flaxman SR, & Rogers LM (2022). National, regional, and global estimates of anemia by severity in women and children for 2000–19: A pooled analysis of population-representative data. Lancet Global Health, 10, e627–e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owais A, Merritt C, Lee C, & Bhutta ZA (2021). Anemia among women of reproductive age: An overview of global burden, trends, determinants, and drivers of progress in low- and middle-income countries. Nutrients, 13, 2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute for Health Metrics and Evaluation. (2019). The Global Burden of Disease. http://www.healthdata.org/gbd
- 13.Green R, & Datta Mitra A (2021). Anemia resulting from other nutritional deficiencies. In Kaushansky K, Lichtman MA, & Prchal JT (Eds.), Williams hematology (10th ed.). New York: McGraw-Hill Professional. https://accessmedicine.mhmedical.com/content.aspx?bookid=2962§ionid=252528194 [Google Scholar]
- 14.Engle-Stone R, Aaron GJ, Huang J, Wirth JP, Namaste SM, Williams AM, Peerson JM, Rohner F, Varadhan R, Addo OY, Temple V, Rayco-Solon P, Macdonald B, & Suchdev PS (2017). Predictors of anemia in preschool children: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. American Journal of Clinical Nutrition, 106, S402–S415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirth JP, Woodruff BA, Engle-Stone R, Namaste SM, Temple VJ, Petry N, Macdonald B, Suchdev PS, Rohner F, & Aaron GJ (2017). Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. American Journal of Clinical Nutrition, 106(Suppl 1), 416S–427S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.USAID Advancing Nutrition Anemia Task Force. (2022). Technical report on anemia. Washington, DC: United States Agency International Development. [Google Scholar]
- 17.Weatherall DJ (2010). The inherited diseases of hemoglobin are an emerging global health burden. Blood, 115, 4331–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modell B (2008). Global epidemiology of hemoglobin disorders and derived service indicators. Bulletin of the World Health Organization, 2008, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weatherall DJ, & Clegg JB (2001). Inherited hemoglobin disorders: An increasing global health problem. Bulletin of the World Health Organization, 79, 704–712. [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss G, & Goodnough LT (2005). Anemia of chronic disease. New England Journal of Medicine, 352, 1011–1023. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth E, & Ganz T (2014). Anemia of inflammation. Hematology Oncology Clinics of North America, 28, 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss G, Ganz T, & Goodnough LT (2019). Anemia of inflammation. Blood, 133, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques O, Weiss G, & Muckenthaler MU (2022). The role of iron in chronic inflammatory diseases: From mechanisms to treatment options in anemia of inflammation. Blood, 140, 2011–2023. [DOI] [PubMed] [Google Scholar]
- 24.Ness TE, Agrawal V, Bedard K, Ouellette L, Erickson TA, Hotez P, & Weatherhead JE (2020). Maternal hookworm infection and its effects on maternal health: A systematic review and meta-analysis. American Journal of Tropical Medicine and Hygiene, 103, 1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. (2022). Soil-transmitted helminth infections. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections
- 26.Colley DG, Bustinduy AL, Secor WE, & King CH (2014). Human schistosomiasis. Lancet, 383, 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto Y, Cheng J, Omachi S, & Morimoto A (2017). Prevalence, severity, and pathogeneses of anemia in visceral leishmaniasis. Parasitology Research, 116, 457–464. [DOI] [PubMed] [Google Scholar]
- 28.Santos MLC, Brito BBD, Silva FAFD, Sampaio MM, Marques HS, Silva NOE, Queiroz DMDM, & Melo FFD (2020). Helicobacter pylori infection: Beyond gastric manifestations. World Journal of Gastroenterology, 26, 4076–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spottiswoode N, Duffy PE, & Drakesmith H (2014). Iron, anemia and hepcidin in malaria. Frontiers in Pharmacology, 5, 125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassebaum NJ, & GBD 2013 Anemia Collaborators. (2016). The global burden of anemia. Hematology Oncology Clinics of North America, 30, 247–308. [DOI] [PubMed] [Google Scholar]
- 31.Purdy JC, & Shatzel JJ (2021). The hematologic consequences of obesity. European Journal of Haematology, 106, 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cepeda-Lopez AC, & Baye K (2020). Obesity, iron deficiency and anemia: A complex relationship. Public Health Nutrition, 23, 1703–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris SA, Frongillo EA, Black MM, Dong Y, Fall C, Lampl M, Liese AD, Naguib M, Prentice A, Rochat T, Stephensen CB, Tinago CB, Ward KA, Wrottesley SV, & Patton GC (2022). Nutrition in adolescent growth and development. Lancet, 399, 172–184. [DOI] [PubMed] [Google Scholar]
- 34.Pasricha S-R, Tye-Din J, Muckenthaler MU, & Swinkels DW (2021). Iron deficiency. Lancet, 397, 233–248. [DOI] [PubMed] [Google Scholar]
- 35.Anger H, Durocher J, Dabash R, & Winikoff B (2019). How well do postpartum blood loss and common definitions of postpartum hemorrhage correlate with postpartum anemia and fall in hemoglobin? PLoS ONE, 14, e0221216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroli G, Cuesta C, Abalos E, & Gulmezoglu AM (2008). Epidemiology of postpartum hemorrhage: A systematic review. Best Practice & Research. Clinical Obstetrics & Gynaecology, 22, 999–1012. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. (2018). WHO recommendations: Uterotonics for the prevention of postpartum hemorrhage. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 38.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, & Alkema L (2014). Global causes of maternal death: A WHO systematic analysis. Lancet Global Health, 2, e323–e333. [DOI] [PubMed] [Google Scholar]
- 39.Accinelli RA, & Leon-Abarca JA (2017). Solid fuel use is associated with anemia in children. Environmental Research, 158, 431–435. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. (2014). Global nutrition targets 2025: policy brief series (WHO/NMH/NHD/14.2). Geneva: World Health Organization. [Google Scholar]
- 41.United Nations. (2021). Sustainable Development Goals. https://www.un.org/sustainabledevelopment/
- 42.Garcia-Casal MN, Dary O, Jefferds ME, & Pasricha S-R (2022). Diagnosis of anemia: Challenges selecting methods, addressing underlying causes and implementing actions at public health level. Annals of the New York Academy of Sciences, 00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez de Romaña D, Golan J, Mildon A, Jefferds ME, Rogers LM, & Arabi M (2022). Preventive and therapeutic interventions to address anemia. Annals of the New York Academy of Sciences, 00–00. [Google Scholar]
- 44.Mildon A, Lopez de Romaña D, Jefferds ME, Rogers LM, & Arabi M (2022). Integrating and coordinating programs for the management of anemia across the life course. Annals of the New York Academy of Sciences, 00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. (2021). WHO methods and data sources for mean hemoglobin and anemia estimates in women of reproductive age and pre-school age children 2000–2019. Geneva: World Health Organization. [Google Scholar]
- 46.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, & Ezzati M (2013). Global, regional, and national trends in hemoglobin concentration and prevalence of total and severe anemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Global Health, 1, e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GBD 2019 Diseases and Injuries Collaborators. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet, 396, 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. (2021). WHO micronutrient database. Vitamin and mineral nutrition information system. https://www.who.int/teams/nutrition-food-safety/databases/vitamin-and-mineral-nutrition-information-system/ [Google Scholar]
- 49.World Health Organization. (2021). World malaria report 2021. Geneva: World Health Organization. [Google Scholar]
- 50.Montresor A, Mupfasoni D, Mikhailov A, Mwinzi P, Lucianez A, Jamsheed M, Gasimov E, Warusavithana S, Yajima A, Bisoffi Z, Buonfrate D, Steinmann P, Utzinger J, Levecke B, Vlaminck J, Cools P, Vercruysse J, Cringoli G, Rinaldi L, … Gyorkos TW (2020). The global progress of soil-transmitted helminthiases control in 2020 and World Health Organization targets for 2030. PLOS Neglected Tropical Diseases, 14, e0008505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. (2021). HIV/AIDS. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- 52.Hess SY, Mclain AC, Frongillo EA, Afshin A, Kassebaum NJ, Osendarp SJM, Atkin R, Rawat R, & Brown KH (2021). Challenges for estimating the global prevalence of micronutrient deficiencies and related disease burden: A case study of the Global Burden of Disease Study. Current Developments in Nutrition, 5, 5012002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Namaste SML, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ, Northrop-Clewes CA, & Suchdev PS (2017). Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. American Journal of Clinical Nutrition, 106, S359–S371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoltzfus RJ, & Klemm R (2017). Research, policy, and programmatic considerations from the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. American Journal of Clinical Nutrition, 106, 428S–434S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.BRINDA. (2017). Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia. https://brinda-nutrition.org/
- 56.Akseer N, Vaivada T, Rothschild O, Ho K, & Bhutta ZA (2020). Understanding multifactorial drivers of child stunting reduction in exemplar countries: A mixed-methods approach. American Journal of Clinical Nutrition, 112, S792–S805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US Department of Health and Human Services & National Institute of Health. (2020). How can covered entities use and disclose protected health information for research and comply with the privacy rule? https://privacyruleandresearch.nih.gov/pr_08.asp
- 58.Staunton C, Slokenberga S, & Mascalzoni D (2019). The GDPR and the research exemption: Considerations on the necessary safeguards for research biobanks. European Journal of Human Genetics, 27, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitton T, Nielsen J, & Nicol D (2019). Terms of engagement: Transfer of biological materials for research in Australia. Journal of Law, Medicine & Ethics, 27, 338–354. [PubMed] [Google Scholar]
- 60.Bubela T, Guebert J, & Mishra A (2015). Use and misuse of material transfer agreements: Lessons in proportionality from research, repositories, and litigation. PLoS Biology, 13, e1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VanderWeele TJ, Lash TL, & Rothman KJ (2020). Causal interference and scientific reasoning. In Lash TL, VanderWeele TJ, & Haneause S (Eds.), Modern epidemiology. Philadelphia, PA: Wolters Kluwer Health. [Google Scholar]
- 62.Countdown to 2030. (2020). Women’s, children’s & adolesents’ health. https://www.countdown2030.org/
- 63.NiPN. (2022). Strengthening capacity to analyse data to track progress, inform policies, and improve programs for better nutrition. https://www.nipn-nutrition-platforms.org/
- 64.Stevens GA, Flores-Urrutia MC, Rogers LM, Paciorek CJ, Rohner F, Namaste S, & Wirth JP (2022). Associations between type of blood collection, analytical approach, mean hemoglobin and anemia prevalence in population-based surveys: A systematic review and meta-analysis. Journal of Global Health, 12, 04088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ko Y, Williams AM, Peerson JM, Luo H, Flores-Ayala R, Wirth JP, Engle-Stone R, Young MF, & Suchdev PS (2022). Approaches to quantify the contribution of multiple anemia risk factors in children and women from cross-sectional national surveys. PLoS Global Public Health, 2(10), e0001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention, World Health Organization, Nutrition International. (2020). Micronutrient survey manual. Geneva: World Health Organization. [Google Scholar]
- 67.World Health Organization. (2020). WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 68.World Health Organization. (2011). Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: World Health Organization. [Google Scholar]
- 69.Pentieva K (2021). Riboflavin. In Gibson RS (Ed.), Principles of nutritional assessment (3rd ed.). https://nutritionalassessment.org/riboflavin/ [Google Scholar]
- 70.World Health Organization. (2015). Serum and red blood cell folate concentrations for assessing folate status in populations. Geneva: World Health Organization. [Google Scholar]
- 71.De Benoist B (2008). Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food and Nutrition Bulletin, 29, S238–S244. [DOI] [PubMed] [Google Scholar]
- 72.Brown KH, Moore SE, Hess SY, Mcdonald CM, Jones KS, Meadows SR, Manger MS, Coates J, Alayon S, & Osendarp SJ (2021). Increasing the availability and utilization of reliable data on population micronutrient (MN) status globally: The MN Data Generation Initiative. American Journal of Clinical Nutrition, 114, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. (2018). Malaria surveillance, monitoring and evaluation: A reference manual. Geneva: World Health Organization. [Google Scholar]
- 74.Khurana S, Singh S, & Mewara A (2021). Diagnostic techniques for soil-transmitted helminths — Recent advances. Research and Reports in Tropical Medicine, 12, 181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Da Costa L, Suner L, Galimand J, Bonnel A, Pascreau T, Couque N, Fenneteau O, & Mohandas N (2016). Diagnostic tool for red blood cell membrane disorders: Assessment of a new generation ektacytometer. Blood Cells, Molecules and Diseases, 56, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organization. (2018). Guide to G6PD deficiency rapid diagnostic testing to support P. vivax radical cure. Geneva: World Health Organization. [Google Scholar]
- 77.Taher AT, Weatherall DJ, & Cappellini MD (2018). Thalassaemia. Lancet, 391, 155–167. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. (2022). WHO guidelines for malaria. https://app.magicapp.org/#/guideline/LwRMXj/section/jzy6YE
- 79.Neufeld LM, Larson LM, Kurpad A, Mburu S, Martorell R, & Brown KH (2019). Hemoglobin concentration and anemia diagnosis in venous and capillary blood: Biological basis and policy implications. Annals of the New York Academy of Sciences, 1450, 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karakochuk CD, Hess SY, Moorthy D, Namaste S, Parker ME, Rappaport AI, Wegmüller R, & Dary O (2019). Measurement and interpretation of hemoglobin concentration in clinical and field settings: A narrative review. Annals of the New York Academy of Sciences, 1450, 126–146. [DOI] [PubMed] [Google Scholar]
- 81.Rappaport AI, Karakochuk CD, Hess SY, Whitehead RD Jr., Namaste SML, Dary O, Parker ME, Neufeld LM, Larson LM, Newton S, Wegmuller R, & Moorthy D (2020). Variability in hemoglobin concentration by measurement tool and blood source: An analysis from seven countries. Journal of Clinical Pathology, 74, 657–663. [DOI] [PubMed] [Google Scholar]
- 82.Whitehead RD, Mei Z, Mapango C, & Jefferds MED (2019). Methods and analyzers for hemoglobin measurement in clinical laboratories and field settings. Annals of the New York Academy of Sciences, 1450, 147–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zlotkin S, & Dewey KG (2021). Perspective: Putting the youngest among us into the nutrition “call for action” for food fortification strategies. American Journal of Clinical Nutrition, 114, 1257–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasricha S-R, Drakesmith H, Black J, Hipgrave D, & Biggs B-A (2013). Control of iron deficiency anemia in low- and middle-income countries. Blood, 121, 2607–2617. [DOI] [PubMed] [Google Scholar]
- 85.Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, & Subramanian S (2011). Anemia in low-income and middle-income countries. Lancet, 378, 2123–2135. [DOI] [PubMed] [Google Scholar]


