Abstract
Background
This nationwide prospective registry study investigated the real-world effectiveness, safety, and persistence of vedolizumab (VDZ) in inflammatory bowel disease (IBD) patients in Taiwan. Disease relapse rates after VDZ discontinuation due to reimbursement restriction were assessed.
Methods
Data were collected prospectively (January 2018 to May 2020) from the Taiwan Society of IBD registry.
Results
Overall, 274 patients (147 ulcerative colitis [UC] patients, 127 Crohn’s disease [CD] patients) were included. Among them, 70.7% with UC and 50.4% with CD were biologic-naïve. At 1 year, 76.0%, 58.0%, 35.0%, and 62.2% of UC patients and 57.1%, 71.4%, 33.3%, and 30.0% of CD patients achieved clinical response, clinical remission, steroid-free remission, and mucosal healing, respectively. All patients underwent hepatitis B and tuberculosis screening before initiating biologics, and prophylaxis was recommended when necessary. One hepatitis B carrier, without antiviral prophylaxis due to economic barriers, had hepatitis B reactivation during steroid tapering and increasing azathioprine dosage, which was controlled with an antiviral agent. No tuberculosis reactivation was noted. At 12 months, non–reimbursement-related treatment persistence rates were 94.0% and 82.5% in UC and CD patients, respectively. Moreover, 75.3% of IBD patients discontinued VDZ due to mandatory drug holiday. Relapse rates after VDZ discontinuation at 6 and 12 months were 36.7% and 64.3% in CD patients and 42.9% and 52.4% in UC patients, respectively.
Conclusions
The findings demonstrated VDZ effectiveness in IBD patients in Taiwan, with high treatment persistence rates and favorable safety profiles. A substantial IBD relapse rate was observed in patients who had mandatory drug holiday.
Keywords: vedolizumab, Crohn’s disease, ulcerative colitis, reimbursement
Key Messages.
What is already known?
Vedolizumab has been approved and reimbursed in Taiwan as both first-line and second-line therapy for moderate-to-severe inflammatory bowel disease (IBD) since 2017, with only second-line short-term effectiveness and safety data being reported in Asian populations.
What is new here?
The VIOLET study was the first nationwide prospective registry study to assess the long-term real-world effectiveness, safety, and persistence of vedolizumab for IBD patients in Taiwan, where tuberculosis and hepatitis B are endemic diseases, and the disease relapse rate after vedolizumab discontinuation due to reimbursement restriction.
How can this study help patient care?
The VIOLET study has shown a high long-term persistence rate without any significant safety signal when patients underwent screening and received prophylaxis in a tuberculosis and hepatitis B endemic area, which could help in clinical decision making of biologic use when managing IBD patients.
Introduction
Over the last 2 decades, anti-tumor necrosis factor (anti-TNF) agents have become the mainstay of therapy for moderate-to-severe Crohn’s disease (CD) and ulcerative colitis (UC).1,2 However, a proportion of patients undergoing anti-TNF therapy experience primary nonresponse (10%-30%) or secondary loss of response (23%-46%).3 Anti-TNF therapy also has potential risks of infection and malignant complications.4,5 These shortcomings of anti-TNF therapy, along with an evolving understanding of the immunopathogenesis of CD and UC, have led to the development of biologics with alternative mechanisms of action.6
Vedolizumab (VDZ) is a gut-selective anti-lymphocyte trafficking humanized monoclonal antibody used for inflammatory bowel disease (IBD) treatment. VDZ binds to α4β7 integrin, which prevents lymphocyte translocation from the blood to the inflamed gut tissue; thus, this biological agent does not cause systemic immunosuppression, thereby potentially providing a more favorable safety profile than systemic immunosuppressants.7 GEMINI long-term safety studies and post hoc analyses have confirmed that VDZ is efficacious and has a favorable safety profile.8-11 It has been approved worldwide since 2014.12
Clinical trials represent only selective patient populations, and the results might not reflect real-world clinical practice. Asia is an endemic region for hepatitis B and tuberculosis (TB)13; however, clinical trials for IBD tend to exclude hepatitis B or TB patients. Multiple studies on the real-world evidence of VDZ effectiveness and safety have been published in Western countries,14-18 and real-world evidence of VDZ therapy in the Asian population is gradually emerging.19-22 However, the emerging evidence is either from hospital-based studies in Taiwan or from second-line therapy studies in Korea. We aimed to conduct a prospective nationwide multicenter registry study in collaboration with the Taiwan Society of Inflammatory Bowel Disease (TSIBD) to determine the safety and effectiveness of VDZ in inducing and maintaining remission as either first-line or second-line therapy, treatment persistence, and IBD relapse after VDZ discontinuation due to the reimbursement policy in Taiwan.
Methods
Study Population and Data Collection
This was a prospective, nationwide registry study, named as the VIOLET (VedolIzumab therapy fOr infLammatory bowEl disease in Taiwan) study, including adult patients (≥18 years of age) with IBD who received at least 1 dose of VDZ for IBD treatment, with up to 1-year follow-up between January 2018 and May 2020. This study was approved by local institutional review boards; all patients provided written informed consent. Patient data were collected across the country from 16 medical centers in Taiwan and were deidentified and transferred to the electronic registry system maintained by the TSIBD using a standardized case report form. The input data were updated quarterly.
VDZ was administered as an intravenous infusion (300 mg) at weeks 0, 2, and 6 and every 8 weeks thereafter, with no dose intensification allowed according to the reimbursement criteria. Treatment effectiveness; concomitant medication; and adverse events (AEs) including infections, infusion reactions, or other potential AEs related to VDZ were recorded at baseline and at 3, 6, 9, and 12 months. Ileocolonoscopy was performed during at least 1 follow-up visit after the initiation of therapy; the primary physician decided the timing based on clinical necessity and patient availability, which is consistent with real-world practice. The use of concomitant immunosuppressant medications or steroids and the duration of continuous medication use were determined by the treating physician.
Variables
Data of baseline characteristics (age at diagnosis, age at VDZ initiation, sex, and smoking status), disease characteristics, disease-related complications or extraintestinal manifestations, phenotype classified according to Montreal subclassifications, and treatment history (steroids, immunomodulators, and anti-TNF agents) were collected. Data of other patient characteristics related to VDZ prescription, such as baseline disease activity, concomitant treatments, reason for VDZ discontinuation, and disease activity after VDZ withdrawal, were also collected. Corticosteroid therapy was defined as the use of any intravenous or oral corticosteroid at any time during the VDZ treatment. Immunomodulator therapy with azathioprine, 6-mercaptopurine, methotrexate, tacrolimus, or cyclosporine was initiated. AEs were also recorded. Infusion reactions were defined as any AE that occurred on the day of or the day after VDZ infusion. AEs were defined as any untoward medical occurrence that did not result in VDZ discontinuation or hospitalization. Severe AEs (SAEs) included events requiring hospitalization, prolongation of existing hospitalization, and interventions to prevent permanent impairment or damage or those causing mortality.
Outcomes and Definition
The effectiveness outcomes of this study were the proportion of individuals achieving a clinical response (CRS), clinical remission (CRM), mucosal healing (MH), and steroid-free remission (SRM) after 6 months and 12 months of VDZ treatment. Secondary outcomes included the safety and treatment persistence of VDZ. Other outcomes were disease activity and concomitant medication used during VDZ treatment and the relapse rate after discontinuing VDZ due to the reimbursement policy.
For UC patients, CRS was defined as ≥3 points and ≥30% reduction in the complete Mayo score from baseline or ≥2 points and ≥25% reduction in the partial Mayo score from baseline. CRM was defined as a total Mayo score of ≤2 points, with no individual subscore being >1 point. MH was defined as a Mayo endoscopy subscore of 0 or 1. For CD patients, CRM was defined as a Crohn’s Disease Activity Index (CDAI) score of <150, and CRS was defined as a decrease in the CDAI score of ≥70 points at 6 and 12 months after VDZ initiation. SRM for patients on steroids at baseline was defined similar to CRM in UC patients, in addition to the patient being free from steroids after treatment. MH was defined as a Crohn’s Disease Endoscopic Index of Severity score of ≤4. The Mayo score and CDAI are regularly used in clinical practice, as their application is required for claiming reimbursement for biologics in Taiwan. All IBD specialists were well educated and trained in the formal assessment of IBD scores under the TSIBD, as this has been a compulsory training in Taiwan since 2014.
VDZ treatment persistence up to month 12 was analyzed using the Kaplan-Meier analysis based on records of VDZ discontinuation.
The safety of VDZ was analyzed based on the occurrence of events such as opportunistic infections, hepatitis B virus (HBV) infection, TB, gastrointestinal tract infections, infusion-related reactions, hypersensitivity, malignancy, and hepatic injury in patients who had available data up to 12 months after VDZ initiation.
In UC patients, relapse after VDZ withdrawal was defined as an Mayo endoscopic score of ≥2, stool frequency of ≥2 (>3-4 per day), or rectal bleeding of ≥2 (50% time). In CD patients, relapse after VDZ withdrawal was defined as an increased CDAI score of ≥70 from the time of VDZ discontinuation or a CDAI score of ≥220.
Reimbursement Criteria for Biologics and Risk Management Plan for Hepatitis B and Tuberculosis in Taiwan
In Taiwan, adalimumab has been the first reimbursed biologic available for moderate-to-severe CD since 2011. Golimumab and adalimumab have been reimbursed for UC since 2016. VDZ and infliximab have been reimbursed for IBD patients since June 2017.23 Because of the limited budget, biologics are under strict reimbursement criteria set by the National Health Insurance (NHI) program. For the biologics to be reimbursed, the patients should have met all the 3 criteria, which are as follows. First, IBD patients should have registered for catastrophic illness. Second, IBD patients should have received conventional therapy (immunosuppressive drugs and/or corticosteroids) for at least 6 months. Third, when conventional therapy failed or during AEs, the disease severity should have met the severity criteria: a CDAI score of ≥300 or a CDAI score of ≥100 with prior CD-related surgery for CD patients and a Mayo score of ≥9 and Mayo endoscopy subscore of ≥2 for UC patients. Another issue is the limitation in terms of the biologic treatment period. For patients who met the aforementioned reimbursement criteria and responded to the treatment, reimbursement was provided for a treatment period of only 12 months; for example, the last dose of VDZ would be administered at the 46th week rather than the 54th week since the initiation of 1 term of reimbursement. After the 12-month treatment period, patients have to either shift to self-financing treatment or discontinue the treatment until 3 months later (mandatory drug holiday) to reapply for reimbursement when disease severity meets the previously mentioned criteria.
As an endemic area (ie, Taiwan) for hepatitis B and TB, the government has set a risk management plan for patients who need biologic treatment. All patients have to undergo hepatitis B and TB screening before initiating the biologic treatment. Patients who have latent TB or active TB must first receive prophylaxis or treatment, which are covered by the NHI. For patients with chronic hepatitis B, antiviral agents could be reimbursed. However, for a hepatitis B carrier with normal liver function, prophylaxis is highly recommended according to the guidelines,24 but patients have to pay for the treatment by themselves.
Statistical Analysis
Continuous variables are presented as mean ± SD or as median (interquartile range [IQR]) if the distribution was skewed, and categorical or binary variables are presented as proportions or percentages. P values were based on a 2-sample t test for continuous variables and a chi-square or Fisher exact test for categorical variables. Effectiveness was analyzed using (1) as-observed analysis in patients with available data at the indicated time point and (2) the last observation carried forward approach for imputation of missing data. Treatment persistence was analyzed using the Kaplan-Meier survival analysis based on records of VDZ discontinuation. Patients who discontinued VDZ due to reimbursement restriction or while in disease remission were censored. The log-rank test was used to compare the persistence of VDZ between CD and UC patients and to compare between biologic-naïve and biologic-exposed patients. Kaplan-Meier survival analysis was also used to assess the risk of relapse after VDZ discontinuation due to the reimbursement policy. Statistical analysis was performed using SPSS for Windows (version 20.0; IBM Corp, Armonk, NY, USA). The tests were 2-tailed with a significance level of .05.
Results
Baseline Clinical Characteristics
A total of 274 patients (UC: n = 147, 61.9% male, 70.7% biologic-naïve; CD: n = 127, 70.9% male, 50.4% biologic-naïve) with active IBD receiving VDZ were included in the analysis. Data of demographic and relevant clinical characteristics are presented in Table 1. At VDZ initiation, the mean age of UC and CD patients was 48.5 ± 14.2 and 39.8 ± 15.4 years, respectively. The male-to-female sex ratio was 1.6 for UC and 2.4 for CD. The age and sex ratio in our study cohort were comparable to those in a previous report from Taiwan.25 The median disease duration before VDZ treatment was 3.9 (IQR, 1.6-8.9) years for UC and 3.1 (IQR, 1.5-6.9) years for CD. We further stratified patients into the biologic-naïve and biologic-exposed groups; the median disease duration before VDZ initiation was 3.0 (IQR, 0.9-7.8) years for UC and 1.7 (IQR, 0.8-4.5) years for CD in the biologic-naïve group and 4.8 (IQR, 2.3-10.0) years for UC and 4.9 (IQR, 2.7-8.9) years for CD in the biologic-exposed group. The disease duration before VDZ initiation was slightly longer than that reported in other studies,26,27 probably related to the strict criteria for biologics reimbursement in Taiwan. In UC patients, the baseline disease activity was 9.2 ± 2.3 based on the Mayo score, pancolitis (51%) was the most common disease location, and 10.7% of patients had undergone previous bowel resection (no patients had undergone prior total colectomy) or surgery for perianal disease. Three (2%) UC patients had a history of malignancy, which was related to IBD in 2 of these patients. In CD patients, the baseline disease activity was 241 ± 89 based on the CDAI, ileocolitis (53.5%) was the most common disease location, the inflammatory type (45.7%) was the most common disease behavior, and 37.0% had undergone previous bowel resection or surgery for perianal disease. At the time of VDZ initiation, more than half of the UC and CD patients were receiving concomitant immunosuppressants and corticosteroids.
Table 1.
Characteristics of the enrolled patients.
| Characteristic | UC (n = 147) | CD (n = 127) |
|---|---|---|
| Male | 91 (61.9) | 90 (70.9) |
| Age (initial VDZ treatment), y | 48.5 ± 14.2 | 39.8 ± 15.4 |
| Body mass index, kg/m2 | 23.0 ± 3.48 | 21.8 ± 4.03 |
| Duration of disease, y | 3.9 (1.6-8.9) | 3.1 (1.5-6.9) |
| Biologic-naïve | 3.0 (0.9-7.8) | 1.7 (0.8-4.5) |
| Biologic-exposed | 4.8 (2.3-10.0) | 4.9 (2.7-8.9) |
| Smoking status | ||
| Current smoker | 6 (4.1) | 11 (8.7) |
| Former smoker | 18 (12.2) | 11 (8.7) |
| Never | 118 (80.3) | 103 (81.1) |
| Disease extent | ||
| Proctitis | 27 (18.4) | — |
| Left-sided colitis | 44 (29.9) | — |
| Pancolitis | 75 (51.0) | — |
| Ileal | — | 42 (33.1) |
| Colonic | — | 17 (13.4) |
| Ileocolonic | — | 68 (53.5) |
| Perianal | — | 8 (6.3) |
| Disease behavior | ||
| Inflammatory | — | 58 (45.7) |
| Stricture | — | 39 (30.7) |
| Penetrating | — | 26 (20.5) |
| Mayo score | 9.2 ± 2.3 | — |
| CDAI | — | 239.5 ± 89.0) |
| Previous surgical history | 16 (10.9) | 47 (37.0) |
| Bowel resections | 9 (6.1) | 43 (33.9) |
| Surgery for perianal disease | 8 (5.4) | 20 (15.7) |
| Previous malignancy history | 3 (2.0) | 0 (0) |
| IBD-related cancer history | 2 (1.4) | 0 (0) |
| HBV infection | 21 (14.3) | 9 (7.1) |
| HBsAg positive | 15 (10.2) | 7 (5.5) |
| QuantiFERON test | ||
| Positive | 5 (3.4) | 4 (3.1) |
| Intermediate | 5 (3.4) | 1 (0.8) |
| Prior anti-TNF exposure | 43 (29.3) | 63 (49.6) |
| Adalimumab | 21 (48.8) | 61 (96.8) |
| Infliximab | 20 (46.5) | 2 (3.2) |
| Golimumab | 2 (4.6) | 0 (0) |
| Concurrent medications at the time of VDZ initiation | ||
| Immunosuppressants | 88 (59.9) | 70 (55.1) |
| Steroids | 108 (73.5) | 68 (53.5) |
Values are n (%), mean ± SD, or median (interquartile range).
Abbreviations: CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; IBD, inflammatory bowel disease; TNF, tumor necrosis factor; UC, ulcerative colitis; VDZ, vedolizumab.
As mentioned, Taiwan is an endemic area for hepatitis B and TB; therefore, hepatitis B and TB screening before biologic treatment is required according to the Taiwan NHI risk management program. In this cohort, 14.3% (n = 21 of 147) and 7.1% (n = 9 of 127) of UC and CD patients had hepatitis B infection, respectively. A positive result for hepatitis B surface antigen test was found before VDZ initiation in 15 (10.2%) UC patients and 7 (5.5%) CD patients. Antiviral prophylaxis was recommended according to the local guidelines but depended on the patients’ decision because patients with baseline normal liver function test results do not fit the reimbursement criteria for antiviral treatment. A positive result for the QuantiFERON test for TB was found in 3.4% (n = 5 of 147) and 3.1% (n = 4 of 127) of the UC and CD patients, respectively; all of them were without active TB as assessed using chest images. Isoniazid prophylaxis had been provided to all patients with a positive result for the QuantiFERON test according to local guidelines.
Overall, 70.7% and 50.4% of the UC and CD patients, respectively, were biologic-naïve at the time of VDZ initiation. Among biologic-exposed patients, 20.9% (n = 9 of 43) of the UC patients and 3.2% (n = 2 of 63) of the CD patients were exposed to 2 anti-TNF agents. Because adalimumab has been used for the longest period in Taiwan, it was the most commonly prescribed biologic in UC and CD patients in this cohort.
Trends in Disease Activity and Medication Use During VDZ Treatment
As displayed in Table 2, in CD patients, CDAI showed a significant time-dependent decrease from 178 ± 87.3 at month 3 (n = 108) to 124 ± 93.9 at month 12 (n = 56) after VDZ initiation when compared with the baseline disease activity (P < .001); C-reactive protein levels decreased from 1.44 ± 2.12 at month 3 (n = 97) to 1.13 ± 1.46 at month 12 (n = 37) after VDZ initiation (P = 0.210); fecal calprotectin levels decreased from 1277 ± 1722 at month 3 (n = 20) to 812 ± 871 at month 12 (n = 8) after VDZ initiation therapy (P = .802) (Supplementary Table 1). In UC patients, the Mayo score significantly decreased from 4.68 ± 2.71 at month 3 to 4.35 ± 3.45 at month 12 after VDZ initiation when compared with the baseline disease activity (P < .001); fecal calprotectin levels decreased from 1324 ± 1734 at month 3 (n = 28) to 989 ± 1342 at month 12 (n = 14) after VDZ initiation (P = .282) (Supplementary Table 1).
Table 2.
Disease activity over time among patients undergoing VDZ therapy.
| Disease Activity | Time | ||||
|---|---|---|---|---|---|
| 0 mo | 3 mo | 6 mo | 9 mo | 12 mo | |
| UC | |||||
| Mayo score | 137, 9.20 ± 2.27 | 116, 4.68 ± 2.71a | 58, 3.71 ± 2.41a | 54, 3.59 ± 2.68a | 37, 4.35 ± 3.45a |
| CD | |||||
| CDAI | 127, 241 ± 89.5 | 108, 178 ± 87.3a | 84, 157 ± 90.7a | 66, 142 ± 98.5a | 56, 124 ± 93.9a |
Values are n, mean ± SD.
Abbreviations: CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; UC, ulcerative colitis; VDZ, vedolizumab.
a P < .001, in comparison with baseline.
Concomitant medications administered during VDZ treatment are summarized in Supplementary Table 1. 5-Aminosalicylic acid was persistently prescribed for UC and CD patients. After 12 months of VDZ therapy, the usage of concomitant immunosuppressants or steroid therapy decreased when compared with that at baseline in UC and CD patients.
CRS, CRM, SRM, and MH Rates at 6 and 12 Months After VDZ Treatment
Treatment effectiveness at 6 and 12 months was analyzed as observed (Table 3). At 6 months, 56.0% (n = 47 of 84), 51.2% (n = 43 of 84), 26.4% (n = 14 of 53), and 12.0% (n = 3 of 25) of the CD patients achieved CRS, CRM, SRM, and MH, respectively. At 12 months, 57.1% (n = 32 of 56), 71.4% (n = 40 of 56), 33.3% (n = 11 of 33), and 30.0% (n = 9 of 30) of the CD patients achieved CRS, CRM, SRM, and MH, respectively. At 6 months, 82.5% (n = 80 of 97), 43.3% (n = 42 of 97), 26.0% (n = 20/77), and 69.0% (n = 40 of 58) of the UC patients achieved CRS, CRM, SRM, and MH, respectively. At 12 months, 76.0% (n = 38 of 50), 58.0% (n = 29 of 50), 35.0% (n = 14 of 40), and 62.2% (n = 23 of 37) of the UC patients achieved CRS, CRM, SRM, and MH, respectively. The effectiveness of VDZ treatment for biologic-naïve and biologic-exposed patients is shown in Table 3. Treatment effectiveness at 6 months and 12 months analyzed using last observation carried forward imputation showed similar trends as those in the as-observed analysis (Supplementary Table 2).
Table 3.
As-observed analysis of CRS, CRM, SRM, and MH in patients with UC and CD after 6 months and 1 year of VDZ therapy.
| Group |
6 mo | 1 y | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CRS | CRM | SRM | MH | CRS | CRM | SRM | MH | ||
| UC | Total | 82.5, 80/97 | 43.3, 42/97 | 26.0, 20/77 | 69.0, 40/58 | 76.0, 38/50 | 58.0, 29/50 | 35.0, 14/40 | 62.2, 23/37 |
| Biologic-naïve | 81.3, 52/64 | 39.1, 25/64 | 28.8, 25/52 | 64.9, 24/37 | 79.4, 27/34 | 55.9, 19/34 | 34.6, 9/26 | 61.5, 16/26 | |
| Biologic-exposed | 84.8, 28/33 | 51.5, 17/33 | 35.0, 14/40 | 76.2, 16/21 | 68.8, 11/16 | 62.5, 10/16 | 35.7, 5/14 | 63.6, 7/11 | |
| CD | Total | 56.0, 47/84 | 51.2, 43/84 | 26.4, 14/53 | 12.0, 3/25 | 57.1, 32/56 | 71.4, 40/56 | 33.3, 11/33 | 30.0, 9/30 |
| Biologic-naïve | 67.4a, 29/43 | 62.8a, 27/43 | 43.3a, 13/30 | 13.3, 2/15 | 64.3a, 18/28 | 82.1, 23/28 | 42.1, 8/19 | 17.6, 3/17 | |
| Biologic-exposed | 43.9a, 18/41 | 39.0a, 16/41 | 4.3a, 1/23 | 10.0, 1/10 | 50.0a, 14/28 | 60.7, 17/28 | 21.4, 3/14 | 46.2, 6/13 | |
Values are %, n/n.
Abbreviations: CD, Crohn’s disease; CRM, clinical remission; CRS, clinical response; MH, mucosal healing; SRM, steroid-free remission; UC, ulcerative colitis; VDZ, vedolizumab.
aBiologic-naïve vs biologic-exposed, P < .05.
Treatment effectiveness of VDZ in combination with an immunomodulator at 6 and 12 months was evaluated in the as-observed analysis (Supplementary Figure 1). In the UC and CD groups, CRS, CRM, SRM, or MH at either 6 months or 12 months in patients receiving combination therapy did not show any significant difference when compared with those in patients receiving VDZ monotherapy. These results suggested that VDZ administered with an immunomodulator provided no additional clinical benefit for the treatment effectiveness of VDZ.
Safety Profile
As displayed in Table 4, VDZ was generally well tolerated during the 1-year treatment period. Treatment-related AEs and SAEs occurred in 12 (4.9%) of 247 and 4 (1.5%) of 247 of patients, respectively. Two UC patients experienced mild infusion-related reactions during the first infusion but no such reactions thereafter. One patient who was an HBV carrier experienced HBV reactivation during regular follow-up. The patient did not receive antiviral prophylaxis owing to normal liver function test results for years and economic barriers. The clinician suspected the hepatitis B reactivation to be related to steroid tapering and azathioprine dose escalation. The hepatitis due to HBV reactivation was controlled with an antiviral agent, and his liver function was back to normal 3 months after the reimbursed antiviral treatment. One patient reported upper respiratory tract infection. Two patients reported gastrointestinal tract infection: 1 had Clostridium difficile–associated diarrhea, and the other had a self-limited infection without the pathogen being identified. Four patients reported SAEs (intractable infection with underlying myelodysplastic syndrome, opportunistic cytomegalovirus infection, intestinal perforation due to an endoscopic procedure, and intra-abdominal abscess). Among these patients, VDZ treatment was discontinued in the patient with intractable infection with underlying myelodysplastic syndrome and in that with cytomegalovirus infection. No new infections or TB reactivation was noted. No new malignancies or cancer relapses were reported. No mortality occurred during the treatment period.
Table 4.
Adverse events, infectious diseases, and other disorders reported during VDZ therapy.
| Adverse Event | UC (n = 147) | CD (n = 127) | Total |
|---|---|---|---|
| Infusion reaction and hypersensitivity | 2 (1.4) | 0 (0) | 2 (0.7) |
| HBV reactivation | 0 (0) | 1 (0.8) | 1 (0.4) |
| HCV reactivation | 0 (0) | 0 (0) | 0 (0) |
| TB reactivation | 0 (0) | 0 (0) | 0 (0) |
| Upper respiratory tract infection | 0 (0) | 1 (0.8) | 1 (0.4) |
| Gastrointestinal tract infection | 0 (0) | 2 (1.6) | 2 (0.7) |
| Others | |||
| Hair loss | 0 (0) | 1 (0.8) | 1 (0.4) |
| Headache | 1 (0.7) | 0 (0) | 1 (0.4) |
| SAE | |||
| Pneumonia | 0 (0) | 0 (0) | 0 (0) |
| Opportunistic infection | 0 (0) | 1 (0.8) | 1 (0.4) |
| Intractable infection | 0 (0) | 1 (0.8) | 1 (0.4) |
| Intra-abdominal abscess | 0 (0) | 1 (0.8) | 1 (0.4) |
| Intestinal perforation | 1 (0.7) | 0 (0) | 1 (0.4) |
| Malignancy | 0 (0) | 0 (0) | 0 (0) |
| Mortality | 0 (0) | 0 (0) | 0 (0) |
Values are n (%).
Abbreviations: CD, Crohn’s disease; HBV, hepatitis B virus; HCV, hepatitis C virus; SAE, serious adverse event; TB, tuberculosis; UC, ulcerative colitis; VDZ, vedolizumab.
Treatment Persistence (Non–Reimbursement Related)
The VDZ persistence rate of patients who had non–reimbursement-related VDZ discontinuation was not significantly different at 6 months (UC: n = 98 [95.1%]; CD: n = 76 [93.1%]; P = .512) and 12 months (UC: n = 31 [94.0%]; CD: n = 34 [82.5%]; P = .06) in the UC and CD patient groups (Figure 1A). The VDZ treatment persistence rate was not significantly different when comparing biologic-naïve and biologic-exposed UC and CD patients during the 1-year follow-up (Figure 1B and 1C). However, biologic-naïve UC patients indicated a higher persistence rate than biologic-exposed UC patients at 6 months (n = 66 [95.5%] vs n = 32 [94.4%]) and 12 months (n = 24 [95.5%] vs n = 7 [91.2%]) (Figure 1B). A similar trend was observed in the biologic-naïve and biologic-exposed CD patients at 6 months (n = 38 [92.1%] vs n = 38 [94.0%], respectively) and 12 months (n = 20 [83.1%] vs n = 14 [81.8%], respectively) (Figure 1C). The lack of a significant difference in the data could be due to the smaller sample size and shorter follow-up time.
Figure 1.
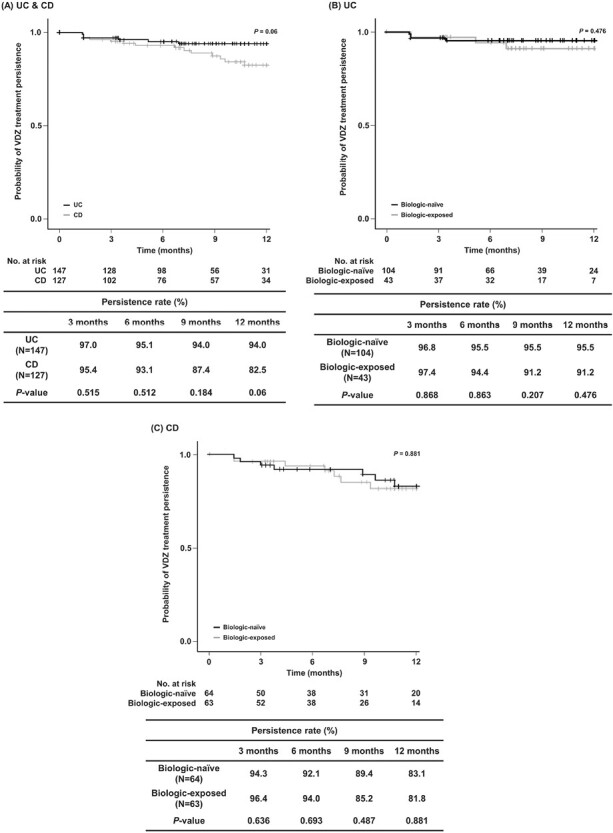
Kaplan-Meier curve showing the cumulative rates of vedolizumab (VDZ) treatment persistence (A) in patients with ulcerative colitis (UC) and Crohn’s disease (CD), (B) biologic-naïve and biologic-exposed patients with UC, and (C) biologic-naïve and biologic-exposed patients with CD at 3, 6, 9, and 12 months.
IBD Relapse after VDZ Discontinuation due to Reimbursement Restriction
A total of 93 (50 UC and 43 CD) patients discontinued VDZ during follow-up. Reimbursement (insurance) restriction was the main reason for discontinuation (n = 70 of 93 [75.3%]), which occurred in 42 (84.0%) UC patients and 28 (65.1%) CD patients. The mean ± SD duration of VDZ use in patients who discontinued VDZ due to reimbursement restriction was 11.4 ± 5.3 and 11.1 ± 3.2 months in the CD and UC groups, respectively. CRM and MH rates at the time of VDZ discontinuation were, respectively, 42.9% and 39.3% in the CD group and 38.0% and 50.0% in the UC group (Table 5). IBD relapse rates at 6 and 12 months after VDZ discontinuation due to reimbursement restriction were 35.7% and 64.3% in the CD group and 42.9% and 52.4% in the UC group, respectively (Figure 2). No significant difference in the relapse rates was observed between the UC and CD patients. For patients with clinical relapse after mandatory drug holiday, retreatment with VDZ resulted in a CRS rate of 93.8% (15 of 16 patients) in UC patients at a mean treatment duration of 6.5 months; in CD patients, the CRS rate was 88.9% (8 of 9 patients) at a mean treatment duration of 10.6 months (Table 5).
Table 5.
Characteristics of IBD patients after VDZ discontinuation due to reimbursement restriction.
| Characteristic | CD (n = 28) | UC (n = 42) |
|---|---|---|
| Male | 31 (72.1) | 34 (68.0) |
| Age at baseline, y | 45.4 ± 19.1 | 50.2 ± 14.3 |
| Duration of VDZ treatment, mo | 11.4 ± 5.3 | 11.1 ± 3.2 |
| Treatment outcome at the time of VDZ discontinuation | ||
| CRM | 12 (42.9) | 16 (38.0) |
| MH | 11 (39.3) | 21 (50.0) |
| Follow-up available after VDZ discontinuation, mo | 8.0 ± 6.9 | 8.6 ± 7.1 |
| VDZ retreatment outcome after relapse | 9 | 16 |
| CRS | 8 (88.9) | 15 (93.8) |
| Duration of VDZ treatment, mo | 10.6 ± 8.7 | 6.5 ± 5.6 |
Values are n (%), mean ± SD, or n.
Abbreviations: CD, Crohn’s disease; CRM, clinical remission; CRS, clinical response; IBD, inflammatory bowel disease; MH, mucosal healing; UC, ulcerative colitis; VDZ, vedolizumab.
Figure 2.
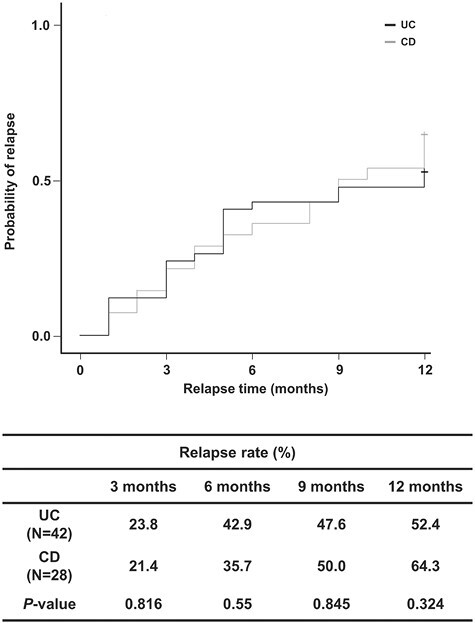
Kaplan-Meier time-to-relapse curve showing the relapse of inflammatory bowel disease at 6 and 12 months after vedolizumab discontinuation. CD, Crohn’s disease; UC, ulcerative colitis.
Discussion
This is the first Asian prospective, nationwide registry study demonstrating the effectiveness and safety of VDZ as first-line and second-line biologic therapy in real-world practice for both the induction and maintenance of UC and CD remission. Among the 274 IBD patients treated with VDZ in this study, almost half of the patients had prior anti-TNF exposure; a significant number of patients had ileal or ileocolonic CD and pancolitis. More than half of the CD patients had stricturing or penetrating disease. After 12 months of VDZ therapy, most of the UC and CD patients successfully achieved CSR or CRM, and more than 60% of the UC patients and 30% of the CD patients achieved MH. We fully agree that MH is the treatment goal for IBD patients and suggest that our colleagues have endoscopy follow-up. However, because of the limited period for biologics reimbursement and because most patients would need to reapply for the biologics reimbursement, in real-world practice, most patients refuse to undergo evaluation (including C-reactive protein, fecal calprotectin, and endoscopy) at 12 months but choose to undergo the evaluation during reapplication. Additionally, fecal calprotectin analysis is not reimbursed in Taiwan, which affected the completeness of the follow-up evaluation. Under such conditions, CRM or CRS in UC was estimated using partial Mayo score. This also demonstrates the difference between the clinical trial setting and real-world practice. Reimbursement-related issues in health insurance affect patient preference, and our study represented actual practice behaviors of physicians in Taiwan.
Considering the safety profile, SAE rates (0.7% and 2.4% in UC and CD patients, respectively) in our study were numerically lower than those reported in the GEMINI 1 and 2 trials (3% and 7% in UC and CD patients, respectively).28,29 The integrated safety analysis of VDZ clinical trials reported that the exposure-adjusted incidence rate of serious infections was 2.7 per 100 person-years.30 Integrated safety analyses of pivotal infliximab and adalimumab trials demonstrated that the incidence rate of serious infections was 6.5 and 6.7 per 100 person-years, respectively.31,32 In the retrospective real-world EVOLVE study, VDZ was associated with significantly fewer SAEs and serious infections than anti-TNF therapy in biologic-naïve IBD patients.33 In a systematic review of real-world VDZ studies, the SAE rates ranged from 0% to 13% and AE rates ranged from 0% to 67%.34 The SAE rate in this study was within the range when compared with that in other real-world studies. One possible explanation for the relatively lower AE rate is the difference in reporting of the AEs between clinical trials and real-world practice. In clinical trials, any AE should be reported, including treatment-related and non–treatment-related AEs, whereas in real-world practice, patients and clinicians tend to report significant and major AEs.
Hepatitis B and TB are endemic in many Asian countries, including Taiwan13; thus, investigating HBV and TB reactivation was a key aspect in our study. Under the risk management plan, we routinely screen and provide prophylaxis for TB with reimbursement. Prophylaxis does not ensure eradication of TB. Reactivation can still occur after using biologics despite prophylaxis. In a study of anti-TNF agents among 6 IBD patients with latent TB, 4 (67%) patients who received isoniazid chemoprophylaxis could not prevent the reactivation of TB after 37.5 months of anti-TNF treatment.35 In our study, all patients received prophylactic treatment for latent TB, and no reactivation was found until the end of follow-up. Therefore, prophylaxis and periodic screening for TB reactivation with VDZ treatment is as important as those with anti-TNF agents.
For hepatitis B, the risk management program allowed us to screen, but the antiviral agent was reimbursed only for chronic hepatitis B patients. For patients with a normal liver function test result at baseline, even though the guidelines strongly recommend prophylaxis, the treatment is self-financed, and the patient’s decision might be affected by economic barriers. We did observe a hepatitis B carrier who had not received prophylaxis owing to economic barriers and suffered from hepatitis B reactivation. However, this patient was also under treatment adjustment including steroid tapering and dose escalation of an immunomodulator (azathioprine). It is well known that the addition of immunosuppressive agents and tapering of steroids could result in an incremental increase in the risk of HBV reactivation.36 Consideration persists over whether IBD patients treated with VDZ should receive concomitant treatment with immunomodulators. In our study, VDZ combined with an immunomodulator had no synergistic benefit in terms of clinical effectiveness when compared with VDZ alone. A previous meta-analysis showed that combination therapy was not associated with an increase in the development of AEs during VDZ therapy (odds ratio, 1.17; 95% confidence interval, 0.75-1.84).17 Another previous meta-analysis reported that combining VDZ with an immunomodulator resulted in no significant difference in CRM or CRS in the induction or maintenance phases of IBD.37 Nevertheless, the patient received the combination therapy due to the reimbursement criteria (conventional therapy for at least 6 months) in Taiwan. Fortunately, the hepatitis B was controlled after the antiviral treatment. This suggests that, in real-world practice, when HBV-infected patients need immunosuppressive treatment, prophylactic antiviral agents would be the first choice; for patients who cannot afford the treatment, regular follow-up of routine liver function tests and HBV DNA titer would be mandatory to detect the reactivation on time and minimize the risk.
In our CD cohort, the CRS rate (57.1%) was lower than the CRM rate (71.4%) after 12 months of VDZ therapy, which may be related to the reimbursement criteria for biologics in Taiwan. Specifically, for CD patients with a surgical history at baseline (37%), a CDAI score of >100 could make them eligible to apply for biologics reimbursement. Therefore, this specific group of patients could potentially not fall into the definition of CRS owing to their disease activity but still meet the definition for CRM (CDAI score <150) after VDZ treatment.
We observed that VDZ treatment persistence rates at 12 months were 94% and 82.5% in the UC and CD groups, respectively, regardless of the reimbursement status. The VDZ treatment persistence rates at 12 months in biologic-naïve IBD patients was higher than those in biologic-exposed patients but without a significant difference. Further long-term follow-up was still needed. Higher persistence in biologic-naïve patients has been reported in other real-world studies.38-40
Regarding the relapse after VDZ withdrawal, this study revealed that at 6 months after VDZ discontinuation, 40% (n = 42 of 70) of IBD patients experienced IBD relapse. In a French retrospective study, 64% of IBD patients treated with VDZ after a median duration of 17.5 (IQR, 10.6-25.4) months experienced relapse within 11 months after VDZ discontinuation.39 The patients included in the French study had achieved steroid-free CRM for at least 3 months. Therefore, lower disease activity and longer time to relapse were observed in that study than in our study. In our study, only one-third of IBD patients achieved SRM after 12 months of VDZ therapy, but VDZ therapy had to be discontinued because of the reimbursement policy. Therefore, the patients in our study experienced a shorter time to relapse, which implies that it is less appropriate to discontinue VDZ before achieving adequate disease control in these patients.
Our study had several limitations. We collected data from different centers, and although we expected the patient populations to be similar given the similarities in geographical regions and the types of medical centers, heterogeneity remained in the data collected and potentially in the populations of these centers. Furthermore, the registry still actively registers patients; only patients with 6 months or 12 months of follow-up were used in our analysis. However, this study also has several strengths. First, this was the first nationwide registry study reporting the long-term effectiveness of VDZ therapy for Asian IBD patients and the safety profile of VDZ in a hepatitis B and TB-endemic area. This was also the first report to demonstrate the IBD relapse rates after a limited duration of VDZ treatment owing to a government-required mandatory drug holiday, providing valuable evidence for opportunities to shape future reimbursement policies.
Conclusions
Our study demonstrated that the effectiveness of VDZ therapy in IBD patients in Taiwan was comparable to that reported in studies conducted in other regions. Our data also showed high treatment persistence and a favorable safety profile for VDZ therapy during a 12-month treatment period. However, patients who discontinued VDZ due to the mandatory drug holiday experienced a substantial IBD relapse rate. This finding suggests that prolonged VDZ treatment until better disease control or deep remission is achieved can further benefit such patients.
Supplementary Material
Acknowledgments
We are grateful to the Taiwan Society of Inflammatory Bowel Disease for providing administrative and technical support. Copyediting support was provided by Cactus Communications and funded by Takeda Pharmaceuticals International AG (Singapore Branch). This study was conducted in accordance with the current version of the Declaration of Helsinki. The institutional review board of National Taiwan University Hospital approved the study (No. 201608018RINB).
Contributor Information
Wei-Chen Lin, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Mackay Memorial Hospital, Taipei, Taiwan.
Wei-Chen Tai, Division of Hepatogastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
Chung-Hsin Chang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan.
Chia-Hung Tu, Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan.
I-Che Feng, Division of Gastroenterology and Hepatology, Chi Mei Medical Center, Tainan, Taiwan.
Ming-Jium Shieh, Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan.
Chen-Shuan Chung, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan.
Hsu-Heng Yen, Division of Gastroenterology, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan.
Jen-Wei Chou, Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
Jau-Min Wong, Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan.
Yu-Hwa Liu, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan.
Tien-Yu Huang, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Chiao-Hsiung Chuang, Department of Internal Medicine, Medical College and Hospital, National Cheng Kung University, Tainan, Taiwan.
Tzung-Jiun Tsai, Division of Gastroenterology and Hepatology, Department of Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
Feng-Fan Chiang, Division of Colon and Rectal Surgery, Department of Surgery, Taichung Veterans General Hospital, Taichung, Taiwan.
Chien-Yu Lu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Wen-Hung Hsu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Fang-Jung Yu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Te-Hsin Chao, Division of Colon and Rectal Surgery, Department of Surgery, Chiayi and Wangiao Branch, Taichung Veterans General Hospital, Taichung, Taiwan.
Deng-Chyang Wu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Ai-Sheng Ho, Division of Gastroenterology, Department of Internal Medicine, Cheng Hsin General Hospital, Taipei, Taiwan.
Hung-Hsin Lin, Division of Colon and Rectal Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan.
Chun-Lung Feng, Division of Gastroenterology and Hepatology, China Medical University Hsinchu Hospital, Hsinchu, Taiwan.
Keng-Liang Wu, Division of Hepato-Gastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
Ming-Wun Wong, Department of Medicine, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation and Tzu Chi University, Hualien, Taiwan.
Chien-Chih Tung, Department of Integrated Diagnostics and Therapeutics, National Taiwan University Hospital, Taipei, Taiwan.
Chun-Chi Lin, Division of Colon and Rectal Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan.
Chia-Chang Chen, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan.
Huang-Ming Hu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan.
Lung-Sheng Lu, Division of Hepato-Gastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Huann-Sheng Wang, Division of Colorectal Surgery, Department of Surgery, Taipei Veterans General Hospital, National Yang Ming Chiao Tung University, Taipei, Taiwan.
I-Chen Wu, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Hsin-Yu Kuo, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Jia-Feng Wu, Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan.
Hsiang Yao Shih, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Yen-Hsuan Ni, Department of Pediatrics, College of Medicine, National Taiwan University, Taipei, Taiwan.
Shu-Lun Tang, Takeda Pharmaceuticals Taiwan, Ltd., Taipei, Taiwan.
Peng-Hsu Chen, Takeda Pharmaceuticals Taiwan, Ltd., Taipei, Taiwan.
Shu-Chen Wei, Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan.
Author Contribution
W.C.L., W.C.T., P.H.C., S.L.T., and S.C.W. conceived and designed the study, drafted the manuscript, and conducted data analysis. All authors contributed to the research and approved the manuscript.
Funding
This study was funded by Takeda Pharmaceuticals Taiwan, Ltd.
Conflicts of Interest
S.C.W. has received consultancy fees from AbbVie, Celltrion, Ferring Pharmaceuticals Inc, Janssen, Pfizer Inc, Takeda, and Tanabe; and speaker fees from AbbVie, Bristol Myers Squibb, Celltrion, Excelsior Biopharma Inc, Ferring Pharmaceuticals Inc, Pfizer Inc, Janssen, Takeda, and Tanabe. S.L.T. and P.H.C. are employed by Takeda Pharmaceuticals Taiwan, Ltd. All other authors disclose no relevant relationships.
References
- 1. Raine T, Bonovas S, Burisch J, et al. . ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2-17. [DOI] [PubMed] [Google Scholar]
- 2. Torres J, Bonovas S, Doherty G, et al. . ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4-22. [DOI] [PubMed] [Google Scholar]
- 3. Roda G, Jharap B, Neeraj N, et al. . Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lemaitre M, Kirchgesner J, Rudnichi A, et al. . Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamamoto-Furusho JK, Al Harbi O, Armuzzi A, et al. . Incidence of suboptimal response to tumor necrosis factor antagonist therapy in inflammatory bowel disease in newly industrialised countries: the EXPLORE study. Dig Liver Dis. 2020;52(8):869-877. [DOI] [PubMed] [Google Scholar]
- 6. Wyant T, Fedyk E, Abhyankar B.. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10(12):1437-1444. [DOI] [PubMed] [Google Scholar]
- 7. Click B, Regueiro M.. A practical guide to the safety and monitoring of new IBD Therapies. Inflamm Bowel Dis. 2019;25(5):831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loftus EV Jr, Colombel JF, Feagan BG, et al. . Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017;11(4):400-411. [DOI] [PubMed] [Google Scholar]
- 9. Vermeire S, Loftus EV Jr, Colombel JF, et al. . Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis. 2017;11(4):412-424. [DOI] [PubMed] [Google Scholar]
- 10. Card T, Ungaro R, Bhayat F, et al. . Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data. Aliment Pharmacol Ther. 2020;51(1):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee R, Chuah SW, Hilmi IN, et al. . Efficacy and safety of vedolizumab in Crohn’s disease in patients from Asian countries in the GEMINI 2 study. Intest Res. 2021;19(1):83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poole RM. Vedolizumab: first global approval. Drugs. 2014;74(11):1293-1303. [DOI] [PubMed] [Google Scholar]
- 13. Banerjee R, Ali RAR, Wei SC, et al. . Biologics for the management of inflammatory bowel disease: a review in tuberculosis-endemic countries. Gut Liver. 2020;14(6):685-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bohm M, Xu R, Zhang Y, et al. . Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn’s disease. Aliment Pharmacol Ther. 2020;52(4):669-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukin D, Faleck D, Xu R, et al. . Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(1):126-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amiot A, Serrero M, Peyrin-Biroulet L, et al. . Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi-centre cohort study. Aliment Pharmacol Ther. 2019;50(1):40-53. [DOI] [PubMed] [Google Scholar]
- 17. Kotze PG, Ma C, Almutairdi A, et al. . Real-world clinical, endoscopic and radiographic efficacy of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48(6):626-637. [DOI] [PubMed] [Google Scholar]
- 18. Kopylov U, Ron Y, Avni-Biron I, et al. . Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the israeli real-world experience. Inflamm Bowel Dis. 2017;23(3):404-408. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Yoon H, Kim N, et al. . Clinical outcomes and response predictors of vedolizumab induction treatment for Korean patients with inflammatory bowel diseases who failed anti-TNF therapy: a KASID prospective multicenter cohort study. Inflamm Bowel Dis. 2021;27(12):1931-1941. [DOI] [PubMed] [Google Scholar]
- 20. Ye BD, Cheon JH, Song KH, et al. . The real-world outcomes of vedolizumab in patients with ulcerative colitis in Korea: a multicenter retrospective study. Therap Adv Gastroenterol. 2021;14:17562848211024717562848211024769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu Y, Chen CC, Ko CW, et al. . Real-world efficacy and safety of vedolizumab among patients with inflammatory bowel disease: a single tertiary medical center experience in Central Taiwan. Adv Dig Med. 2020;8:40-46. [Google Scholar]
- 22. Kuo CJ, Le PH, Tai WC, et al. . The effectiveness and safety of vedolizumab induction for moderate to severe ulcerative colitis for Asia patient: a real practice observational study. J Formos Med Assoc. 2022;121(9):1689-1695. [DOI] [PubMed] [Google Scholar]
- 23. Su HJ, Chiu YT, Chiu CT, et al. . Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. J Formos Med Assoc. 2019;118(7):1083-1092. [DOI] [PubMed] [Google Scholar]
- 24. Wei SC, Chang TA, Chao TH, et al. . Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017;15(3):266-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yen HH, Weng MT, Tung CC, et al. . Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population-based study. Intest Res. 2019;17(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nguyen NH, Kurnool S, Dulai PS, et al. . Short disease duration is associated with increased risk of treatment failure in biologic-treated patients with ulcerative colitis. Inflamm Bowel Dis. 2020;26(9):1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faleck DM, Winters A, Chablaney S, et al. . Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn’s disease but not ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(12):2497-2505.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feagan BG, Rutgeerts P, Sands BE, et al. . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699-710. [DOI] [PubMed] [Google Scholar]
- 29. Sandborn WJ, Feagan BG, Rutgeerts P, et al. . Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2013;369(8):711-721. [DOI] [PubMed] [Google Scholar]
- 30. Colombel J-F, Sands BE, Rutgeerts P, et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. . A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107(7):1051-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burmester GR, Panaccione R, Gordon KB, et al. . Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bressler B, Yarur A, Silverberg MS, et al. . Vedolizumab and anti-tumour necrosis factor α real-world outcomes in biologic-naïve inflammatory bowel disease patients: results from the EVOLVE study. J Crohns Colitis. 2021;15(10):1694-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. . Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53(9):1048-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akyuz F, Cavus B, Iliaz R, et al. . Inflammatory bowel disease and mycobacteria: how much can we trust isoniazid prophylaxis during antitumor necrosis factor therapy? Eur J Gastroenterol Hepatol. 2019;31(7):777-780. [DOI] [PubMed] [Google Scholar]
- 36. Perrillo RP, Gish R, Falck-Ytter YT.. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221-244.e3. [DOI] [PubMed] [Google Scholar]
- 37. Yzet C, Diouf M, Singh S, et al. . No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases: a meta-analysis. Clin Gastroenterol Hepatol. 2021;19(4):668-679.e8. [DOI] [PubMed] [Google Scholar]
- 38. Reenaers C, Cremer A, Dewit O, et al. . Effectiveness and persistence of Vedolizumab in patients with inflammatory bowel disease: results from the Belgian REal-LIfe study with VEdolizumab (Be-RELIVE). Acta Gastroenterol Belg. 2020;83(1):15-23. [PubMed] [Google Scholar]
- 39. Martin A, Nachury M, Peyrin-Biroulet L, et al. . Maintenance of remission among patients with inflammatory bowel disease after vedolizumab discontinuation: a multicentre cohort study. J Crohns Colitis. 2020;14(7):896-903. [DOI] [PubMed] [Google Scholar]
- 40. Mevius A, Brandes A, Hardtstock F, et al. . Persistence with biologic treatment in patients with inflammatory bowel disease: a German claims data analysis. Digestion. 2021;102(2):216-226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


