Abstract
BACKGROUND
Lower CD4+ cell count in people with HIV infection (PWH) is associated with increased cardiovascular disease (CVD) risk. Whether subsets of CD4+ T helper cells are linked with CVD is unclear.
OBJECTIVES
The aim of this study was to explore the association between peripherally circulating CD4+ T cell subsets and incident CVD.
METHODS
Data from 1,860 participants (1,270 PWH) without prevalent CVD from the VACS (Veterans Aging Cohort Study), a prospective, observational cohort of veterans with and without HIV infection, were analyzed. T cell subsets were quantified in baseline samples using flow cytometry. Incident CVD events were identified using International Classification of Diseases-9th Revision and International Classification of Diseases-10th Revision diagnosis and procedure codes. Participants were followed from baseline date (2005-2006) to the first of CVD incidence, death, or September 30, 2016. Cox proportional hazards regression was used to model associations between these T cell subsets and the risk for incident CVD while adjusting for demographics and other CVD risk factors.
RESULTS
The median participant age at baseline was 51.6 years. Most were male (94%) and of Black race (69.1%). There were 344 incident CVD events (219 in PWH) during follow-up (median 9.8 years). In PWH, higher proportions (per SD increment) of T helper type 17 cells (adjusted HR: 1.19; 95% CI: 1.08-1.31), T effector memory cells re-expressing CD45RA (adjusted HR: 1.19; 95% CI: 1.07-1.34), and CD28null cells (adjusted HR: 1.18; 95% CI: 1.03-1.34) were significantly associated with an increased risk for incident CVD. Among those without HIV infection, no T cell subsets were significantly associated with CVD.
CONCLUSIONS
Among PWH, T helper type 17 cells, senescent cells, and CD4+ T effector memory cells re-expressing CD45RA were significantly associated with incident CVD that was not explained by CVD risk factors.
Keywords: cardiovascular disease, circulating T cells, HIV, peripheral
People with HIV infection (PWH) have higher risks for coronary artery disease, ischemic stroke, and heart failure than the general population.1,2 The multifactorial mechanisms driving this excess risk for cardiovascular disease (CVD) include HIV-specific factors, such as inflammation,3 that become elevated with HIV infection; side effects of antiretroviral therapy such as dyslipidemia and insulin resistance4; a high prevalence of smoking and other CVD risk factors; and nontraditional risk factors such as hepatitis C.2 Although the relationship between inflammation and cardiovascular risk in PWH and people without HIV infection has been described,5 granular data on how alterations in specific adaptive immune cell subsets contribute to this excess risk for CVD are limited.
Prior studies among PWH report that low total CD4+ cell count is associated with an increased risk for CVD events.6 Studies of PWH, people without HIV infection, and murine models implicate certain CD4+ T cell subsets (eg, T helper type 1 [Th1] cytokine-producing CD4 cells) in subclinical measures of atherosclerosis (eg, carotid atherosclerosis, coronary artery calcium)7,8 and cardiac fibrosis.9 In contrast, higher levels of regulatory T (Treg) cells (CD4+CD25+FoxP3+), which down-regulate inflammatory responses, are associated with lower levels of atherosclerosis.10 Findings linking Th17 (CD4+IL17+) to atherosclerosis are inconsistent.11 Furthermore, chronic infections, including HIV infection12 and cytomegalovirus (CMV),2,13 increase the number of CD4+28null T cells, a measure of cellular senescence, and terminally differentiated CD4 T cells, CD4+ T effector memory cells re-expressing CD45RA (TEMRA) (CD4+CD45RA+CD28−CD57+).14 Like HIV infection, CMV seropositivity, which is more common among PWH than people without HIV infection, has been linked to incident CVD events15 and is associated with the expansion of potentially proatherosclerotic T cell subsets. Whether these CD4+ subsets are associated with incident CVD events in humans is unclear.
The objective of this study was to examine the associations between Th1, Th17, Treg, and senescent (CD28neg and TEMRA) CD4+ T cells and incident CVD among PWH and people without HIV infection to better understand the role of these cells in CVD in general and to help explain the excess CVD risk observed among PWH.
METHODS
STUDY SAMPLE.
The VACS (Veterans Aging Cohort Study) Survey Cohort is a prospective, observational, longitudinal cohort study of veterans living with and without HIV infection receiving clinical care from U.S. Veterans Health Administration. Those with and without HIV infection are group-matched on age, race/ethnicity, sex, and geographic location.16,17 The VACS Biomarker Cohort consists of a subset of VACS Survey Cohort participants who agreed to provide blood specimens, including peripheral blood mononuclear cells, between 2005 and 2007.3 Because of the voluntary nature of participation in the Biomarker Cohort, the matching present in the VACS Survey Cohort was not retained in the Biomarker Cohort. Information regarding demographic and clinical data are extracted from the U.S. Department of Veterans Affairs (VA) corporate data warehouse and from self-reported surveys administered at annual VACS visits. The Veterans Health Administration Vital Status File contains mortality data from multiple VA and non-VA data sources, including the Veterans Benefits Administration Integrated Benefits System Death File (formally known as the Beneficiary Identification Records Locator Subsystem Death File), Medicare Vital Status File, and the Social Security Administration Death Master File. The Institutional Review Boards of Vanderbilt University Medical Center, Tennessee Valley Health System, the West Haven VA Medical Center, and Yale University approved this study.
For this analysis we included all VACS Biomarker Cohort participants without prevalent CVD at baseline, defined as the date the participant provided the blood specimen. Participants were followed from the baseline date to the earliest of CVD incidence, death, or censoring on September 30, 2016, to correspond to the end of available Medicare administrative data. From a starting sample of 2,389 VACS Biomarker Cohort participants, we excluded participants with prevalent CVD on or before their baseline dates or within 180 days postbaseline to account for delayed reporting. As we have done in our prior work,6 prevalent CVD was defined as the presence of acute myocardial infarction, unstable angina, coronary heart disease (including revascularization), heart failure, or ischemic stroke identified using International Classification of Disease-9th Revision (ICD-9) and/or Current Procedural Terminology codes from VA, VA fee-for-service, or Centers for Medicare and Medicaid Services administrative files (n = 506). We further excluded participants with no further clinical encounters after baseline (n = 11), those with indeterminate HIV status (ie, had a negative result on HIV screening but underwent testing for HIV viral load or CD4 T cell count [n = 10]), and those who seroconverted during the follow-up period (n = 3).
IMMUNE CELL PHENOTYPING.
T cell subsets are expressed as percentage of CD4+ cells using flow cytometry. Detailed methods, including specific antibodies (Supplemental Table 1) used, are provided in the Supplemental Appendix and have been previously published.18 Gating strategies are shown in Supplemental Figures 1 to 3.
MONOCYTE ACTIVATION, INFLAMMATORY, AND COAGULATION MARKERS.
We examined 3 biomarkers: the monocyte activation marker soluble CD14, the inflammatory marker interleukin (IL)-6, and the coagulation marker D-dimer. These biomarker variables have been used in previous VACS Biomarker Cohort projects.3 Methods for these biomarkers are provided in the Supplemental Appendix.
INCIDENT CVD EVENTS.
The primary outcome of interest was total incident CVD, defined as the composite of the following incident events: acute myocardial infarction, unstable angina, coronary artery revascularization (ie, percutaneous coronary intervention or coronary artery bypass graft), ischemic stroke, and heart failure using ICD-9, International Classification of Diseases-10th Revision, and Current Procedural Terminology codes from VA, VA fee-for-service, and Medicare administrative files through September 2016, as detailed in Supplemental Table 2.
BASELINE COVARIATES.
Covariate data were obtained closest to the date of blood sample collection. Age, sex, and race/ethnicity were determined through administrative data. Hypertension was categorized as no hypertension (blood pressure <140/90 mm Hg and no antihypertensive medication), controlled hypertension (<140/90 mm Hg with antihypertensive medication), or uncontrolled hypertension (≥140/90 mm Hg). Renal function was measured using estimated glomerular filtration rate). Diabetes (yes or no) was defined by a previously validated metric using glucose values, diabetes medication use, and/or at least 1 inpatient or 2 outpatient ICD-9 codes for diabetes.19 Hepatitis C virus (HCV) seropositivity was defined as a positive HCV antibody test result or at least 1 inpatient or 2 outpatient ICD-9 codes for this diagnosis. “HCV negative” indicates negative HCV antibody test result(s) only, “chronic HCV” indicates positive HCV RNA test result(s), “HCV exposure” indicates positive HCV antibody test result(s) but no positive HCV RNA test result(s), and “never tested in the VA” indicates that no HCV laboratory test results were available from the VA.20 Laboratory results, including hemoglobin, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and triglyceride level, were obtained from the VA corporate data warehouse. Body mass index was defined by 1 outpatient measurement of weight and height collected during routine clinical care. Smoking status was self-reported on the VACS survey closest to the date of blood draw. The Alcohol Use Disorders Identification Test–Concise (AUDIT-C) and alcohol abuse or dependence ICD-9 codes were used to create a 4-level alcohol-use variable: not current, not hazardous (AUDIT-C score <4), hazardous or heavy episodic (AUDIT-C score ≥4), and abuse or dependence (ICD-9 code). Cocaine use was assessed by self-report and by the presence of cocaine-use disorder ICD-9 codes. CMV immunoglobulin G concentration was determined using an enzyme-linked immunosorbent assay from Diamedix, and CMV seropositivity was defined as CMV immunoglobulin G concentration >8 EU/mL. Performance characteristics of the assays, correlation plots, and distribution of T cells by CMV titer are described in the Supplemental Appendix, Supplemental Figure 4, and Supplemental Table 3. B cell proportions (CD19+ cells) were added as an additional covariate.
HIV status was ascertained on the basis of 2 criteria: 1) at least 1 inpatient or 2 outpatient ICD-9 codes for AIDS (042), asymptomatic HIV infection (V08), or diagnosis-related group codes (488-490) in the VA electronic medical record; and 2) participant inclusion in the VA Immunology Case Registry. HIV variables were CD4+ cell count, HIV-1 RNA level, and antiretroviral therapy use. CD4+ cell count and HIV-1 RNA level, measured as part of routine clinical care, were determined from VA electronic medical record data obtained closest to the blood collection date (up to 180 days afterward). Baseline antiretroviral therapy use (yes or no) was determined from VA pharmacy data 180 days prior to or up to 7 days after the blood collection date and was categorized into the following drug classes: nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors.
STATISTICAL ANALYSIS.
Baseline characteristics are presented as median (IQR) for continuous variables and as frequencies with percentages for categorical variables stratified by HIV and total CD4+ cell count status. Cox proportional hazards regression models were used to estimate the HR and 95% CI for each 1-SD increase in each immune cell. The proportional hazards assumption was tested using Schoenfeld residuals and including the interaction term of the covariates by time. We constructed 4 Cox models to estimate the association of T cells and incident CVD risk. Each model was constructed for the total cohort and then for PWH and people without HIV infection separately. For models analyzing the total cohort, HIV status was a covariate. Model 1 was adjusted for age and race/ethnicity. Model 2 was additionally adjusted for CMV antibody status, hypertension, diabetes, lipid levels, body mass index, hepatitis C, hemoglobin, smoking status, and estimated glomerular filtration rate. Model 3 built upon model 2 and additionally adjusted for alcohol use and cocaine abuse. Model 4 further included IL-6, D-dimer, and soluble CD14. Model 4 was our primary model. As a sensitivity analysis, we incorporated time-updated HIV-1 RNA and total CD4 T cell count (PWH only). Because the CVD components may have unique underlying pathophysiology, we also used incident heart failure and atherosclerotic CVD (acute myocardial infarction, ischemic stroke, or coronary artery revascularization) as 2 separate outcome variables.
Missing data were imputed using multiple imputation by chained equations with 5 separate imputed datasets on the basis of predictive mean matching using the mice library (version 3.11.0) of the R programming language.21 Cox survival models were fitted in each imputed dataset and then combined to obtain pooled HRs and SEs. P values and 95% CIs presented in this paper were not adjusted for multiplicity, and therefore inferences drawn from these statistics may not be reproducible. All analyses were performed using R version 4.0.2 (The R Project for Statistical Computing).
RESULTS
PARTICIPANT CHARACTERISTICS.
A total of 1,860 VACS Biomarker Cohort participants free of prevalent CVD were included in this analysis. The median age was 51.6 years, and most participants were male (94%) and of Black race (69.1%). There was higher prevalence of obesity, diabetes, and serum low-density lipoprotein >160 mg/dL among people without HIV infection and a higher prevalence of hepatitis C, serum high-density lipoprotein cholesterol <40 mg/dL, and serum triglyceride > 200 mg/dL among PWH; prevalence increased with decreasing CD4 count (Table 1).
TABLE 1.
Baseline Characteristics Stratified by HIV Status and CD4 Cell Count
| HIV Negative (n = 590) |
HIV Positive | ||||
|---|---|---|---|---|---|
| HIV Positive (N = 1,269) |
CD4+ Count >500 cells/mm3 (n = 452) |
CD4+ Count 200-500 cells/mm3 (n = 585) |
CD4+ Count <200 cells/mm3 (n = 232) |
||
| Age, y | 52.1 (46.8-56.6) | 51.4 (46.3-57.0) | 51.8 (46.1-57.5) | 51.9 (47.1-57.4) | 50.2 (45.5-54.9) |
| Male | 519 (88.0) | 1,230 (96.9) | 437 (96.5) | 567 (96.9) | 226 (97.4) |
| Race/ethnicity | |||||
| White | 103 (17.5) | 236 (18.6) | 89 (19.6) | 117 (20.0) | 30 (12.9) |
| Black | 412 (69.8) | 873 (68.7) | 303 (66.9) | 389 (66.5) | 181 (78.0) |
| Hispanic/othera | 75 (12.7) | 161 (12.7) | 61 (13.5) | 79 (13.5) | 21 (9.1) |
| BMIb >30 kg/m2 | 253 (42.9) | 201 (15.8) | 88 (19.4) | 82 (14.0) | 31 (13.4) |
| Hypertension | |||||
| None | 134 (22.7) | 413 (32.5) | 139 (30.7) | 178 (30.4) | 96 (41.4) |
| Controlled | 314 (53.2) | 581 (45.7) | 215 (47.5) | 275 (47.0) | 91 (39.2) |
| Uncontrolled | 142 (24.1) | 274 (21.6) | 132 (22.6) | 132 (22.6) | 45 (19.4) |
| Total cholesterolb >200 mg/dL | 166 (28.1) | 345 (27.2) | 148 (32.7) | 162 (27.7) | 35 (15.1) |
| LDL-Cb >160 mg/dL | 42 (7.1) | 58 (4.6) | 22 (4.9) | 30 (5.1) | 6 (2.6) |
| HDL-Cb <40 mg/dL | 176 (29.8) | 530 (41.7) | 166 (36.6) | 243 (41.5) | 121 (52.2) |
| Triglycerideb >200 mg/dL | 95 (16.1) | 338 (26.6) | 123 (27.2) | 161 (27.5) | 54 (23.3) |
| Diabetes | 145 (24.6) | 204 (16.1) | 73 (16.1) | 102 (17.4) | 29 (12.5) |
| Smokingb | |||||
| Never | 148 (25.4) | 322 (25.4) | 132 (29.1) | 138 (23.6) | 52 (22.4) |
| Current | 292 (49.5) | 629 (49.5) | 196 (43.3) | 295 (50.4) | 138 (59.5) |
| Former | 148 (25.1) | 317 (25.0) | 125 (27.6) | 150 (25.6) | 42 (18.1) |
| eGFRb <60 mL/min/1.73 m2 | 37 (6.3) | 67 (5.3) | 24 (5.3) | 51 (5.3) | 12 (5.2) |
| HCV status | |||||
| Negative | 389 (65.9) | 726 (57.2) | 279 (61.6) | 317 (54.2) | 130 (56.0) |
| Positive | 135 (22.9) | 437 (34.4) | 138 (30.5) | 214 (36.6) | 85 (36.6) |
| Exposed | 26 (4.4) | 68 (5.4) | 18 (4.0) | 40 (6.8) | 10 (4.3) |
| Unknown | 40 (6.8) | 38 (3.0) | 17 (3.8) | 14 (2.4) | 7 (3.0) |
| Anemia (Hb <12 g/dL) | 32 (5.4) | 130 (10.2) | 25 (5.5) | 57 (9.7) | 48 (20.7) |
| Alcohol abuse/dependence | 200 (33.9) | 491 (38.7) | 102 (22.6) | 150 (25.6) | 85 (36.6) |
| Cocaine-use disorder | 243 (41.2) | 447 (35.2) | 136 (30.0) | 204 (34.9) | 107 (46.1) |
| CMV statusb positive | 403 (68.3) | 1,160 (91.3) | 416 (91.8) | 533 (91.1) | 211 (90.9) |
| HIV-1 RNA >500 copies/mL | — | 432 (34.0) | — | — | — |
| CD4 count, cells/mm3 | |||||
| <200 | — | 232 (18.3) | — | — | — |
| 201-499 | — | 585 (46.1) | — | — | — |
| >500 | — | 452 (35.7) | — | — | — |
| ART | — | 1,075 (84.6) | — | — | — |
| NRTI | 1,047 (82.5) | ||||
| NNRTI | 451 (35.5) | ||||
| PI | 662 (52.2) | ||||
Values are median (IQR) or n (%). P values for differences in baseline characteristics using Wilcoxon rank sum tests for continuous variables and chi-square tests for categorical variables were all <0.01 except for race/ethnicity (P = 0.06), smoking (P = 0.02), and eGFR (P = 0.15). aIndian, Black, Asian, mixed race, Hawaiian, and missing. bNumber missing: BMI, n = 5 (0.3%); total cholesterol, n = 20 (1.1%); LDL-C, n = 62 (3.3%); HDL-C, n = 54 (2.9%); triglyceride, n = 32 (1.7%); hypertension, n = 2 (0.1%); smoking status, n = 4 (0.2%); eGFR, n = 4 (0.2%); hepatitis C, n = 1 (0.1%); and CMV status, n = 22 (1.2%).
ART = antiretroviral therapy; BMI = body mass index; CMV = cytomegalovirus; eGFR = estimated glomerular filtration rate; Hb = hemoglobin; HCV = hepatitis C virus; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; NNRTI = non-nucleoside reverse transcriptase inhibitor; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor.
PROPORTIONS OF SOLUBLE BIOMARKERS, IMMUNE CELLS BY HIV STATUS, AND CD4 T CELL COUNT.
Th1, Th2, and Th17 cells, TEMRA, and senescent and Treg cells were lower among people without HIV infection; in PWH, proportions of T cells increased as CD4 count declined. These differences were all significant (P < 0.01 for all, except Th1 with P = 0.06) (Table 2). A similar trend was observed for IL-6, D-dimer, and soluble CD14, with the lowest values observed in people without HIV infection and the highest in PWH, particularly in immunocompromised persons (CD4 count <200 cells/mm3; P < 0.01) (Table 2).
TABLE 2.
Baseline Biomarker and Immune Cells by HIV Status and CD4 Count
| HIV Negative (n = 590) |
HIV Positive | ||||
|---|---|---|---|---|---|
| HIV Positive (N = 1,269) |
CD4+ Count >500 cells/mm3 (n = 452) |
CD4+ Count 200-500 cells/mm3 (n = 585) |
CD4+ Count <200 cells/mm3 (n = 232) |
||
| IL-6, pg/mL | 1.66 (1.08-2.87) | 1.98 (1.37-3.24) | 1.59 (1.20-2.42) | 2.10 (1.44-3.32) | 2.47 (1.71-4.47) |
| D-dimer, μg/mL | 0.29 (0.20-0.45) | 0.25 (0.15-0.45) | 0.22 (0.14-0.37) | 0.25 (0.15-0.45) | 0.37 (0.22-0.64) |
| sCD14, mg/mL | 1.72 (1.45-2.03) | 1.70 (1.43-2.03) | 1.61 (1.37-1.90) | 1.71 (1.46-2.02) | 1.86 (1.55-2.30) |
| Th1 cells, % CD4+ | 16.4 (10.8-23.8) | 16.1 (10.6-24.2) | 15.4 (9.9-22.1) | 16.1 (11.2-24.3) | 18.1 (10.0-28.0) |
| Th2 cells, % CD4+ | 2.7 (1.8-4.0) | 3.9 (2.5-5.7) | 3.3 (2.2-4.7) | 4.0 (2.8-5.6) | 5.7 (3.4-9.0) |
| Th17 cells, % CD4+ | 1.9 (1.4-3.0) | 3.0 (2.0-4.7) | 2.4 (1.6-3.5) | 3.1 (2.1-4.5) | 5.0 (3.0-8.3) |
| TEMRA, % CD4+ | 2.3 (1.2-4.4) | 4.8 (2.4-9.7) | 3.5 (1.9-7.0) | 4.8 (2.5-9.0) | 11.3 (5.3-21.5) |
| Senescent cells, % CD4+ | 6.0 (3.1-12.2) | 13.1 (7.1-24.3) | 10.5 (6.3-17.9) | 12.4 (7.0-23.2) | 25.3 (13.8-46.9) |
| Treg cells, % CD4+ | 3.2 (1.9-5.7) | 4.6 (2.6-8.6) | 3.9 (2.3-6.9) | 4.7 (2.8-8.3) | 7.4 (3.2-14.2) |
| B cells, % lymphocytes | 20.4 (14.1-27.6) | 16.5 (10.9-24.8) | 16.2 (10.8-23.9) | 17.0 (11.2-24.4) | 16.8 (10.3-28.5) |
Values are median (IQR). Number missing: Th1 cells, n = 212 (11.4%); Th2 cells, n = 212 (11.4%); Th17 cells, n = 212 (11.4%); TEMRA, n = 60 (3.2%); senescent cells, n = 60 (3.2%); Treg cells, n = 66 (3.5%); B cells, n = 55 (3%); IL-6, n = 31 (1.7%); D-dimer, n = 27 (1.5%); and sCD14, n = 24 (1.3%). Missing data for the immune cell subsets were generally due to technical error in the assays.
IL = interleukin; sCD14 = soluble CD14; TEMRA = T effector memory cells re-expressing CD45RA; Th = T helper type; Treg = regulatory T.
ASSOCIATIONS BETWEEN IMMUNE CELLS AND CVD RISK.
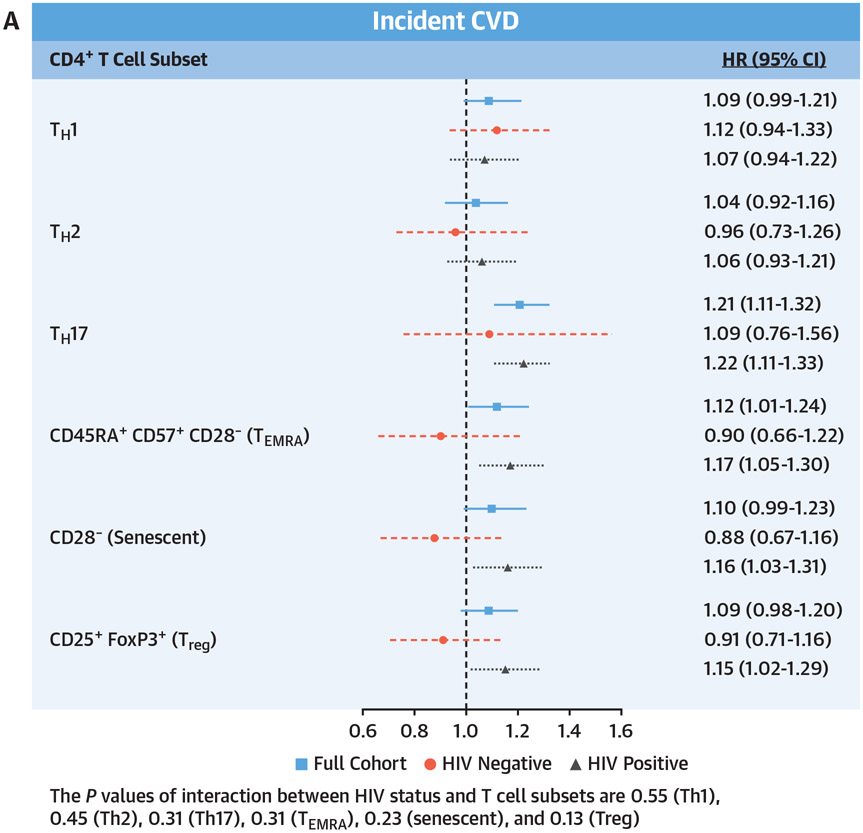
During a median follow-up period of 9.8 years, there were 344 incident CVD events. Incidence rates per 1,000 person-years were lower among PWH compared with people without HIV infection (19.9 vs 23.7, respectively). In the total cohort, after adjustment for traditional and nontraditional CVD risk factors, higher proportions (per SD increment) of Th17 (HR: 1.20; 95% CI: 1.09-1.33; P < 0.001), TEMRA (HR: 1.16; 95% CI: 1.04-1.29; P < 0.001), senescent (HR: 1.14; 95% CI: 1.02-1.28; P = 0.02), and Th1 (HR: 1.12; 95% CI: 1.01-1.24; P = 0.04) CD4+ T cells were significantly associated with an increased risk for incident CVD (Central Illustration, Supplemental Table 4).
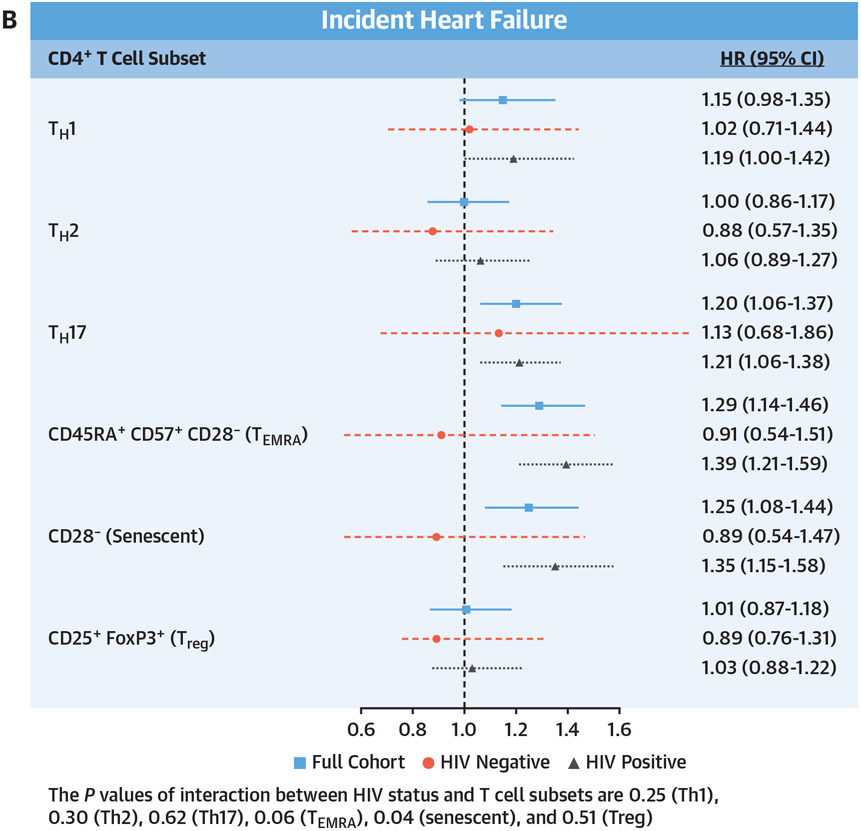
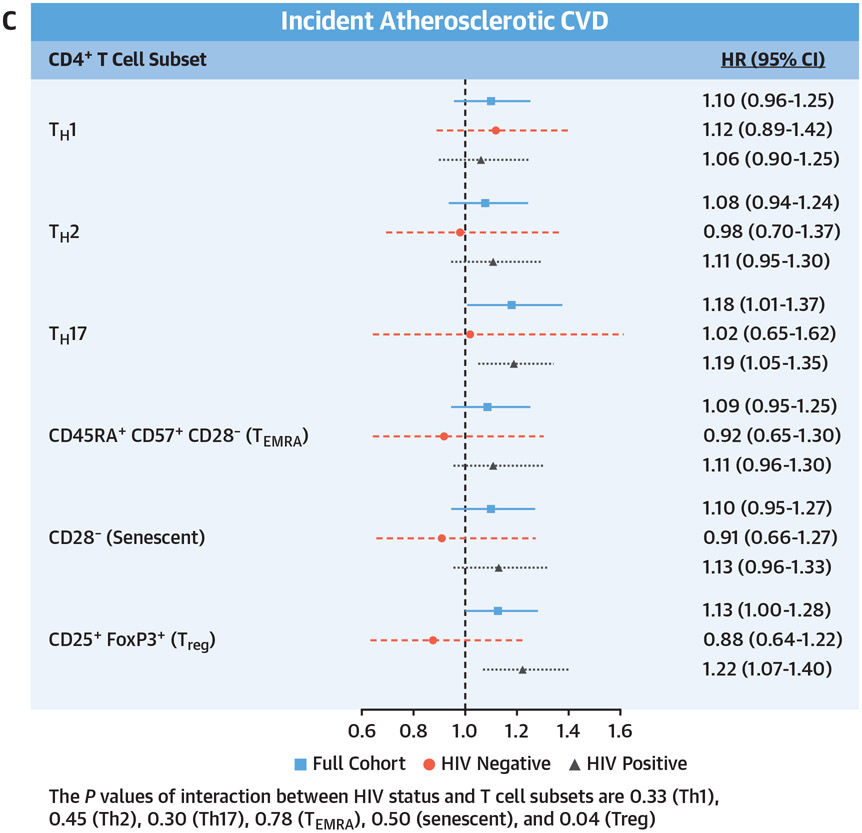
CENTRAL ILLUSTRATION. CD4 Subsets and Incident Cardiovascular Disease, Heart Failure, and Atherosclerotic Cardiovascular Disease.
Adjusted Cox proportional hazards model assessing the relationship of baseline T cell subsets and (A) composite cardiovascular disease (CVD) (including heart failure, acute myocardial infarction, unstable angina, coronary artery revascularization, and ischemic stroke), (B) incident heart failure, and (C) incident atherosclerotic CVD (including acute myocardial infarction, unstable angina, coronary artery revascularization, and ischemic stroke). Adjusted for age, race/ethnicity, cytomegalovirus antibody status, hypertension, diabetes, lipid levels, body mass index, hepatitis C virus status, hemoglobin, smoking status, estimated glomerular filtration rate, history of alcohol abuse, cocaine abuse, interleukin-6, D-dimer, and soluble CD14. HRs are reported per SD increment with 95% CIs, and no corrections for multiple testing were applied.
When the cohort was stratified by HIV status, the associations between Th17, TEMRA, and senescent CD4+ T cells and CVD among PWH were similar to the overall cohort (Central Illustration, Supplemental Table 4). Further adjustment for time-updated HIV viral load and total CD4 cell count did not diminish these associations (Table 3). For people without HIV, none of the T cell subsets was significantly associated with incident CVD. Furthermore, the measures of association between TEMRA cells and senescent CD4+ T cells approached 1 (HRs: 0.99 [95% CI: 0.73-1.33] and 0.99 [95% CI: 0.74-1.31]). In contrast, the magnitude of association for Th17 and CVD among people without HIV infection was similar to that among PWH (HR: 1.16; 95% CI: 0.81-1.68). The P values for the interaction between HIV status and T cell subsets were 0.55 (Th1), 0.45 (Th2), 0.31 (Th17), 0.31 (TEMRA), 0.23 (senescent), and 0.13 (Treg).
TABLE 3.
Association of Immune Cells With Risk for Cardiovascular Disease Among People With HIV
| HR (95% CI) per SD Increment | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | P Value | Adjusted Model | P Value | Adjusted Model + Baseline VL | P Value | Adjusted Model + Baseline Nadir CD4 |
P Value | Adjusted Model + Baseline VL, Nadir CD4, ART Class |
P Value | Adjusted Model + Time-Updated VL |
P Value | Adjusted Model + Time-Updated VL and CD4 |
P Value | |
| Th1 cells | 1.13 (0.99-1.28) | 0.07 | 1.09 (0.95-1.24) | 0.21 | 1.11 (0.97-1.27) | 0.13 | 1.08 (0.94-1.24) | 0.26 | 1.09 (0.96-1.25) | 0.19 | 1.06 (0.93-1.21) | 0.39 | 1.04 (0.91-1.19) | 0.52 |
| Th2 cells | 1.07 (0.94-1.21) | 0.31 | 1.05 (0.91-1.21) | 0.50 | 1.09 (0.95-1.26) | 0.23 | 1.05 (0.91-1.22) | 0.5 | 1.05 (0.9-1.21) | 0.56 | 1.06 (0.92-1.22) | 0.41 | 1.03 (0.89-1.19) | 0.66 |
| Th17 cells | 1.19 (1.08-1.3) | <0.001 | 1.19 (1.08-1.31) | <0.001 | 1.19 (1.08-1.33) | <0.001 | 1.19 (1.06-1.33) | <0.001 | 1.17 (1.05-1.3) | <0.001 | 1.17 (1.06-1.30) | 0.01 | 1.15 (1.04-1.27) | 0.01 |
| TEMRA | 1.15 (1.03-1.28) | 0.01 | 1.19 (1.07-1.34) | <0.001 | 1.20 (1.06-1.36) | <0.001 | 1.19 (1.05-1.35) | 0.01 | 1.19 (1.04-1.36) | 0.01 | 1.18 (1.05-1.34) | 0.01 | 1.17 (1.03-1.32) | 0.01 |
| Senescent cells | 1.15 (1.02-1.29) | 0.03 | 1.18 (1.03-1.34) | 0.01 | 1.18 (1.03-1.35) | 0.02 | 1.17 (1.02-1.34) | 0.03 | 1.16 (1.01-1.34) | 0.03 | 1.16 (1.01-1.32) | 0.03 | 1.14 (1.00-1.30) | 0.06 |
| Treg cells | 1.15 (1.02-1.29) | 0.02 | 1.12 (0.99-1.26) | 0.07 | 1.1 (0.96-1.26) | 0.17 | 1.1 (0.96-1.27) | 0.16 | 1.09 (0.95-1.25) | 0.22 | 1.10 (0.97-1.26) | 0.14 | 1.11 (0.97-1.26) | 0.14 |
Adjusted model includes age, race/ethnicity, cytomegalovirus status, hypertension, diabetes, lipid levels, body mass index, hepatitis C virus status, hemoglobin, smoking status, estimated glomerular filtration rate, history of alcohol abuse, cocaine abuse, interleukin-6, D-dimer, and soluble CD14. ART class includes nucleoside/nucleotide reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, and protease inhibitor. SDs of the immune cells: Th1 cells, 11.7; Th2 cells, 3.2; Th17 cells, 3.7 TEMRA, 8.1; senescent cells, 16.0; and Treg cells, 6.9. No corrections for multiple testing were applied.
ART = antiretroviral therapy; VL = viral load; other abbreviations as in Table 2.
As shown in the Central Illustration, for the total cohort, higher proportions of Th17, TEMRA, and senescent CD4+ T cells were significantly associated with an increased risk for incident heart failure, similar to the results for incident total CVD. However, for atherosclerotic CVD, Th17 and Treg CD4+ T cells were the only T cell subsets significantly associated with this outcome. In stratified analyses, these same significant associations were also present among PWH but not people without HIV infection.
DISCUSSION
In a large cohort of people living with and without HIV, each SD increment in peripheral circulating T helper cell subsets (Th1, Th17, TEMRA, and senescent) was associated with an increased risk for total incident CVD. Similarly, Th17 cells, TEMRA, and senescent cells were also significantly associated with heart failure. In contrast, only Th17 and Treg helper cells were significantly associated with atherosclerotic CVD.
In stratified analyses, the association between these T cell subsets and CVD among PWH was similar to that in the overall cohort, with the exception of Th1 cells. Among people without HIV infection, the associations were not significant, although these findings may have been influenced by the smaller sample size of the non-HIV-infected population. Importantly, the associations between these T cell subsets and incident CVD were not attenuated by traditional and nontraditional CVD risk factors, other measures of comorbidity or substance use, or biomarkers of inflammation, altered coagulation, or monocyte activation.
Prior studies examining the role of T cells and CVD were confined largely to experimental models in mice and human studies focusing on atherosclerotic plaque.22,23 In those studies, T cells represented a large proportion of immune cells within atherosclerotic plaques. Within these plaques, T cell subsets are often categorized as “proatherosclerotic,” “atheroprotective,” or both. For example, CD4+ Th1 and senescent cells are generally “proatherosclerotic,” as they produce interferon-gamma and tumor necrosis factor–alpha and are associated with subclinical atherosclerosis and inflammation.8 Th2 and Treg cells are generally thought to be atheroprotective. Th2 cells inhibit Th1 cell differentiation, are inversely associated with subclinical atherosclerosis,8 and produce IL-4, IL-5, and IL-13, which can decrease vascular cell adhesion molecule expression by endothelial cells and activate M2 macrophages.24,25 Treg cells produce transforming growth factor–beta and IL-10, which promote plaque stabilization and a reduction in inflammation.26 Th17 cells can be “proatherosclerotic” in the presence of cytokines, such as interferon-gamma, IL-1β, and tumor necrosis factor–alpha, increasing IL-6 and IL-8 production.27 Whether these T cells, measured in peripheral circulation (ie, not inside an atherosclerotic plaque), are associated with incident CVD is less clear but important to investigate because peripheral circulation is the more clinically accessible compartment.
Among PWH, a reduction in total CD4+ cell count is associated with an increased risk for incident acute myocardial infarction, ischemic stroke, and heart failure.6,28,29 Studies also reveal a significant association between peripheral circulating T cell subsets (eg, subsets of CD4+ T cells) and prevalent CVD and subclinical atherosclerosis.10,11,30 To our knowledge, this is the first study of this magnitude to report that greater proportions of specific peripheral circulating CD4+ T cell subsets are significantly associated with incident total CVD, incident heart failure, and atherosclerotic CVD in PWH. Importantly, only Th17 cells were significantly associated with all 3 phenotypes, suggesting that they may play a key role in the risk for future CVD among PWH. Surprisingly, Treg CD4+ T cells were associated with an increased risk for atherosclerotic CVD. These cells are thought to be “anti-inflammatory” and thus cardioprotective. The balance between Th17 and Treg cells appears to be driven by transforming growth factor–beta, which up-regulates Treg and Th17 cells but favors the Th17 pathway in the presence of IL-6,31 which is elevated in PWH. However, in this case, it is possible that an increase in such cells is a compensatory measure for the increase in other proinflammatory processes co-occurring in PWH or represents a process whereby Treg cells can become dysfunctional (ie, during acute myocardial infarction) and thus are not effective in controlling inflammation. Furthermore, the proportions of these cells were highest among PWH who had lower total CD4+ cell counts and lowest among people without HIV infection. Among PWH with CD4+ cell counts <200 cells/mm3, for example, CD4+CD28− cells represent one-quarter of circulating T cells compared with only 6% of circulating T cells among people without HIV infection (Table 2). Such profound differences in the proportion of T cells that are known to be proinflammatory may help partially explain the excess risk for CVD among PWH, particularly among those with lower total CD4 cell counts.
Among people without HIV infection, results from prior studies examining peripheral circulating T cells and incident CVD events are inconsistent.11 Our earlier work in the MESA (Multi-Ethnic Study of Atherosclerosis) and CHS (Cardiovascular Health Study) cohorts revealed no association between peripheral blood circulating proinflammatory T cell subsets and incident myocardial infarction.32 Our findings in VACS, albeit with a smaller cohort, are consistent with those observed in CHS and MESA. Differences between prior studies and our findings by HIV status may be partially explained by the lack of data on T cell antigen specificity (eg, lipoprotein A, HIV, and CMV) and chemokine-chemokine receptor interactions (eg, C-C motif chemokine ligand 5 [CCL5]–C-C chemokine receptor type 5 [CCR5] axis), an admitted limitation of this study given potential within-subset functional heterogeneity. Not all T cells within a given subset are necessarily proatherosclerotic or atheroprotective. Depending upon the antigen presented or the localized cytokine milieu, a given T cell may be biased toward atherosclerosis, neutral, or biased toward atheroprotective activity.33 In experimental models, antigen specificity is critical for assessing the proatherogenic nature of an individual T cell.34 Whether this antigen specificity contributes to the development of specific types of CVD and by extension may help explain the different T cell profiles for heart failure and atherosclerotic CVD observed in this study is unclear. Both CMV and HIV have been proposed as atherosclerosis relevant antigens. Reports suggest that CMV biases T cell differentiation toward activated, terminally differentiated phenotypes, which may in turn lead to CVD.35 Such a mechanism is consistent with epidemiologic findings linking CMV to heightened CVD risk.36,37 Similarly, chemokines (eg, CCL5) are common in atherosclerotic plaque and the chemokine-chemokine receptor interaction (eg, CCL5-CCR5) may play a role in T cell recruitment to atherosclerotic plaques. The CCR5 receptor, which HIV uses to enter CD4+ T cells, has been shown, when blocked by maraviroc, a CCR5 antagonist, to reduce atherosclerotic lesions in proatherosclerotic mice and to reduce atherosclerotic progression in humans.38 Thus, for PWH, HIV infection, coinfection with CMV, and the CCR5 receptor may all play critical roles in biasing the adaptive immune system toward atherosclerosis and subsequent CVD.
Our findings may have important implications for elucidating the underlying mechanisms driving the excess risk for CVD and help improve risk stratification for PWH. Cellular senescence is a hallmark of aging resulting from repeated immune cell activation and overuse of the immune system. Our findings add support to the hypothesis that accelerated senescence, as captured by TEMRA and CD28null CD4 cells, is associated with increased risk for CVD. Furthermore, our finding that this association is independent of CVD risk factors suggests that these T cell subsets may aid in CVD risk prediction. Coronary heart disease risk prediction tools, even with HIV-specific factors such as total CD4 count, often underperform among PWH.1 Whether incorporation of proinflammatory T cell subsets improves risk prediction is not known.
Our finding that Th17 cells were significantly associated with incident CVD, including heart failure and atherosclerotic CVD, among PWH adds support to the hypothesis that Th17 cells are preferentially up-regulated in the presence of chronic inflammation, as measured by IL-6.31 Our Th17 cell findings are also supported by the association of IL-17A with flow-mediated dilation in PWH.39 The Th17 cell associations with CVD in PWH may also be the result of altered gut microbiota, which has been shown to produce myosin heavy chain 6–specific Th17 cells that drive lethal cardiomyopathy.40
It is worth noting that the VACS represents an important group of PWH: those who are older and possess a higher burden of comorbid disease and substance use. As comorbidities and substance use are linked to CVD among PWH, it is possible that these health conditions may have influenced our results. However, as presented in the Central Illustration, Table 3, and Supplemental Table 4, our findings were robust even after adjustment for demographics, traditional and nontraditional risk factors, substance use, and biomarkers of inflammation, monocyte activation, and altered coagulation.
STUDY LIMITATIONS.
First, as our population was older, was mostly male, and had a high burden of comorbid disease, our findings may not be generalizable to the general population or all PWH, including women. Second, our cardiovascular outcomes were determined using ICD-9 and International Classification of Diseases-10th Revision codes, so it is possible that some misclassification occurred (ie, some true cardiovascular events were not captured by these codes). However, we used validated codes, and this finding would have biased our results toward the null.
Third, we used cryopreserved cells rather than fresh samples. However, there are data, including those from MESA and CHS, demonstrating the validity of this approach.32 Fourth, we collected T cell subsets only at baseline, not during follow-up. Thus, we did not incorporate alterations in T cell proportions over time.
Fifth, we did not observe a significant association between T cell subsets and CVD among people without HIV infection. However, the sample size for those without HIV infection was smaller than for PWH, and we did not observe any significant interaction between HIV status and any of the T cell subsets; thus it remains unclear whether these T cell subsets play a role in the development of CVD among people without HIV infection.
Last, we did not examine CD8 T cells in this analysis. Our choice was based on large epidemiologic studies that linked CD4 but not CD8 T cells to incident CVD events among PWH.41
CONCLUSIONS
Peripheral circulating CD4 T cell subsets were significantly associated with incident CVD, including both heart failure and atherosclerotic CVD, among PWH. The proportions of these cells were highest among PWH who had low CD4 cell counts. This association persisted even after adjustment for CVD risk factors, substance use, and biomarkers of inflammation, altered coagulation, and monocyte activation. Whether this association exists among people without HIV infection is unclear. Future studies should further explore the role of adaptive immunity, including T cell antigen specificity and chemokine-chemokine receptor interactions, as possible mechanisms for the excess risk for CVD among PWH and whether inclusion of these T cell subsets improves CVD risk prediction.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Lower total CD4+ cell count alone does not explain the excess risk for CVD in PWH. Instead, the relative abundance of certain CD4+ T cell subsets is associated with increased cardiovascular risk among PWH.
TRANSLATIONAL OUTLOOK:
Future investigations should seek strategies that target specific types of CD4+ T cells to modify the inflammatory response to HIV infection.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This material is based upon work supported by the VA, the Veterans Health Administration, and the Office of Research and Development, National Institute on Alcohol Abuse and Alcoholism (grants U24-AA020794, U01-AA020790, U24-AA022001, and U10 AA013566), and grant R01HL125032. Dr So-Armah’s work on this project was supported by National Institutes of Health grants K01HL134147, R61DA047032, and P30AI042853. The funders had no role in the study design or the collection, analyses, and interpretation of data. The views expressed in this paper are those of the authors and do not necessarily reflect the position or policy of the VA. Dr Beckman is a consultant for AstraZeneca, Bristol Myers Squibb, Amgen, Merck, Sanofi, Antidote Pharmaceutical, and Boehringer Ingelheim; and serves on data and safety monitoring committees for Bayer and Novartis. Dr McDonnell is an employee and shareholder of 10x Genomics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AUDIT-C
Alcohol Use Disorders Identification Test–Concise
- CCL5
C-C motif chemokine ligand 5
- CCR5
C-C chemokine receptor type 5
- CMV
cytomegalovirus
- CVD
cardiovascular disease
- HCV
hepatitis C virus
- ICD-9
International Classification of Diseases-9th Revision
- IL
interleukin
- PWH
people with HIV infection
- TEMRA
T effector memory cells re-expressing CD45RA
- Th
T helper type
- Treg
regulatory T
- VA
U.S. Department of Veterans Affairs
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental methods, references, figures, and tables, please see the online version of this paper.
REFERENCES
- 1.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So-Armah K, Benjamin LA, Bloomfield GS, et al. HIV and cardiovascular disease. Lancet HIV. 2020;7:e279–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So-Armah KA, Tate JP, Chang CH, et al. Do biomarkers of inflammation, monocyte activation, and altered coagulation explain excess mortality between HIV infected and uninfected people? J Acquir Immune Defic Syndr. 2016;72:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. [DOI] [PubMed] [Google Scholar]
- 5.Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugherty A, Rateri DL. T lymphocytes in atherosclerosis: the yin-yang of Th1 and Th2 influence on lesion formation. Circ Res. 2002;90:1039–1040. [DOI] [PubMed] [Google Scholar]
- 8.Tracy RP, Doyle MF, Olson NC, et al. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevers T, Salvador AM, Velazquez F, et al. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. 2017;214:3311–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albany CJ, Trevelin SC, Giganti G, Lombardi G, Scotta C. Getting to the heart of the matter: the role of regulatory T-cells (Tregs) in cardiovascular disease (CVD) and atherosclerosis. Front Immunol. 2019;10:2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol. 2015;35:258–264. [DOI] [PubMed] [Google Scholar]
- 12.Bernal E, Martinez M, Torres A, et al. T cell senescence predicts subclinical atherosclerosis in HIV-infected patients similarly to traditional cardiovascular risk factors. Antiviral Res. 2019;162:163–170. [DOI] [PubMed] [Google Scholar]
- 13.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157:175–179. [DOI] [PubMed] [Google Scholar]
- 14.Patin E, Hasan M, Bergstedt J, et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19:302–314. [DOI] [PubMed] [Google Scholar]
- 15.Pera A, Caserta S, Albanese F, et al. CD28null pro-atherogenic CD4 T-cells explain the link between CMV infection and an increased risk of cardiovascular death. Theranostics. 2018;8:4509–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44:S13–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44:S25–S30. [DOI] [PubMed] [Google Scholar]
- 18.Bailin SS, McGinnis KA, McDonnell WJ, et al. T lymphocyte subsets associated with prevalent diabetes in veterans with and without human immunodeficiency virus. J Infect Dis. 2020;222:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 22.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. [DOI] [PubMed] [Google Scholar]
- 24.Cardilo-Reis L, Gruber S, Schreier SM, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4: 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Liu R, Yu Q, Dong L, Bi Y, Liu G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019;452:14–22. [DOI] [PubMed] [Google Scholar]
- 26.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–118. [DOI] [PubMed] [Google Scholar]
- 27.Allam G, Abdel-Moneim A, Gaber AM. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed Pharmacother. 2018;106:1412–1418. [DOI] [PubMed] [Google Scholar]
- 28.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sico JJ, Kundu S, So-Armah K, et al. Depression as a risk factor for incident ischemic stroke among HIV-positive veterans in the Veterans Aging Cohort Study. J Am Heart Assoc. 2021;10:e017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angin M, Klarenbeek PL, King M, et al. Regulatory T cells expanded from HIV-1-infected individuals maintain phenotype, TCR repertoire and suppressive capacity. PLoS ONE. 2014;9:e86920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. [DOI] [PubMed] [Google Scholar]
- 32.Olson NC, Sitlani CM, Doyle MF, et al. Innate and adaptive immune cell subsets as risk factors for coronary heart disease in two population-based cohorts. Atherosclerosis. 2020;300:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milioti N, Bermudez-Fajardo A, Penichet ML, Oviedo-Orta E. Antigen-induced immunomodulation in the pathogenesis of atherosclerosis. Clin Dev Immunol. 2008;2008:723539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spyridopoulos I, Martin-Ruiz C, Hilkens C, et al. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell. 2016;15:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg A, Gianella S, Nakazawa M, Trout R, Spector SA. Association of cytomegalovirus DNA and immunologic markers of cardiovascular disease. Open Forum Infect Dis. 2019;6:ofz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Peng G, Bai J, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6:e005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipriani S, Francisci D, Mencarelli A, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation. 2013;127:2114–2124. [DOI] [PubMed] [Google Scholar]
- 39.Wanjalla CN, Temu TM, Mashayekhi M, et al. IL-17A is associated with flow-mediated dilation and IL-4 with carotid plaque in persons with HIV. AIDS. 2022;36(7):963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil-Cruz C, Perez-Shibayama C, De Martin A, et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881–886. [DOI] [PubMed] [Google Scholar]
- 41.Badejo OA, Chang CC, So-Armah KA, et al. CD8+ T-cells count in acute myocardial infarction in HIV disease in a predominantly male cohort. Biomed Res Int. 2015;2015:246870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.