Abstract
Skin cancer (SC) poses a global threat to the healthcare system and is expected to increase significantly over the next two decades if not diagnosed at an early stage. Early diagnosis is crucial for successful treatment, as the disease becomes more challenging to cure as it progresses. However, identifying new drugs, achieving clinical success, and overcoming drug resistance remain significant challenges. To overcome these obstacles and provide effective treatment, it is crucial to understand the causes of skin cancer, how cells grow and divide, factors that affect cell growth, and how drug resistance occurs. In this review, we have explained various therapeutic approaches for SC treatment via ligands, targeted photosensitizers, natural and synthetic drugs for the treatment of SC, an epigenetic approach for management of melanoma, photodynamic therapy, and targeted therapy for BRAF-mutated melanoma. This article also provides a detailed summary of the various natural drugs that are effective in managing melanoma and reducing the occurrence of skin cancer at early stages and focuses on the current status and future prospects of various therapies available for the management of skin cancer.
1. Introduction
Cancer is characterized by deregulated cell growth comprising different disease groups.1 It originates from a combination of epigenetic and genetic abnormality that leads to the turn-off of anti-oncogenes and the switch-on of oncogenes/proto-oncogenes.2 There were 19.3 million new cases of cancer globally in 2020 and about 10 million cancer-related deaths occur yearly.3,4 Globally, skin cancer (SC) is the fastest-growing cancer type, which is characterized by an aggressive, persistent, multifaceted cancer.5,6 The various kinds of skin tumors have been designated following the cells from which they develop, with squamous cell carcinoma (SCC) and cutaneous melanoma (CM) as well as basal cell carcinoma (BCC) among the most prevalent and well-characterized.7 A majority of cutaneous tumors (around 90%) are nonmelanoma skin cancer (NMSC).8 When therapy is insufficient or slowed down, NMSC may be locally damaging even though they are typically treatable and seldom lead to mortality or advanced stages. On the other hand, CM, which comprises nearly one percent of skin tumors that pose the greatest risk of mortality, is responsible for 90% of skin-tumor-related fatalities.9
Epidermal cells are the main cause of NMSC, which exhibits typical epidemiology (for example, a higher incidence in Caucasian people). On the other hand, MCC, which is hypothesized to result from Merkel cells, is more common in equatorial regions and is more common in people of white ancestry.10 Although there are several factors involved in the pathogenesis of BCC, SCC, and MCC, exposure of the skin to environmental cancer-causing agents is the most common cause of risk. Progenitor cells may immediately undergo a cancerous change due to ultraviolet radiation (UVR).11−13 Other risk factors for the growth of BCC and SCC involve co-occurring illnesses and therapies (such as psoriasis), repeated contact with the human papillomavirus, drug-induced suppression of immunity in patients with transplants, and specific medications for the management of various kinds of cancer (particularly melanoma).14−16 The growth of NMSC is favorably influenced by poor socioeconomic and demographic positions, as shown by numerous research.17,18 A frequent occurrence in MCC is the inclusion of the Merkel cell polyomavirus (MCP-yV) inside the genome of tumor cells. In recent years, the molecular characteristics underlying the MCP-yV-induced cancerous alterations in Merkel cells have now been clarified.19 Even though SCC and MCC are characterized via a large neoantigen burden, dysregulation of the Wnt/Hedgehog system has been suggested as a potentially crucial factor in the formation of BCC.19−21
The most lethal skin tumor, however, is CM, which makes up just 1% of skin cancers yet is responsible for 90% of fatalities brought on by skin cancer. Over 320,000 fresh diagnoses of CM were reported globally in 2020, leading to 57,000 deaths and over 1.2 million new cases of NMSC, based on the most current GLOBOCAN projections.13 Due to difficulties with NMSC identification and documentation, the final figure could be significantly underestimated.22 The elevated skin tumor rate of death is primarily due to delayed diagnosis, which is triggered by nonspecific symptoms,23 lack of reliable screening techniques,24 a lack of precise and sensitive biomarkers for prompt detection, prognosis, and therapy monitoring,25 and a lack of knowledge of the mechanisms underlying resistance to drugs in these tumors.26 Therefore, over the past two years, the COVID-19 pandemic, which has taken a lead role in everyday clinical practice, has limited the availability of medical services and delayed the detection of individuals with CM and other skin tumors, leading to higher rates of illness and death, and as a consequence, more financial strain on medical facilities.27 There is a need to identify safer biomarkers to speed up identification, prognosis, and response to therapy among individuals with advanced-stage skin malignancies given their poor prediction.28
2. Skin Cancer Occurs Due to the Involvement of Various Factors
The DNA of skin cells is damaged due to overexposure to UV radiation and chemical alteration to certain pyrimidine dimers, which occurs because UV rays are absorbed by the nucleotides.29 SC is mostly caused by an amalgamation of both modifiable (such as environmental) and nonmodifiable (such as genetic) factors. The most frequent SC risk factor is modifiable.30
2.1. Modifiable Risk Factors
UV exposure has the greatest impact on the risk of SC. Production of melanin is stimulated due to UV exposure to melanocytes, and the skin appears tanned indicating impairment to the skin, DNA, and skin cells; if strong, exposure leads to a sun tan, which signifies cell mortality.31
2.2. Categories of UV Radiation
UV radiation has three subcategories UVA, UVB, and UVC.30 The ozone layer does not absorb UVA, thus they deeply penetrate the skin via an epidermal junction. The shorter wavelength is UVB rays. UVA and UVB produce skin tans.32,33 Swelling, erythema, and pain are caused due to overexposure to UVB radiation. The shortest rays are UVC radiation, and the ozone layer absorbs these rays.34
2.3. Role of UV Exposure
The progression of various types of melanoma is linked with UV radiation exposure patterns.35 Long-term susceptibility such as occupational outdoor exposure is frequently linked to SCC and BCC SC.36,37 The superficial BCC usually appears on the trunk.38 As compared to SCC, melanoma is commonly associated with irregular exposure.39 Traditionally outdoor workers are believed to have a higher risk of developing SCC and BCC.40 Combining 15 studies, a mutual analysis displayed increased melanoma risk for outdoor workers working in areas where exposure to UV is higher.30
2.4. Occupational UV Exposure
The risk of melanoma increases due to occupational exposure to UV rays among outdoor workers. It has been demonstrated by various studies that outdoor workers are at augmented risk of SCC and BCC.41 Research does not find an augmented risk of SC among outside workers.42 Still, studies examining UV-intense areas for outdoor workers have displayed that workers have a high chance of having SC on the neck and head.43
3. Role of Genetic Factors in Skin Cancer
Genetic factors (family history) trigger melanoma risk. The person’s features induce the risk of melanoma like blonde or red hair, light-colored eyes, naturally fair skin tone, nevi or moles, and dermal areas that burn, freckle, and redden.44 Skin diseases such as melanoma and nonmelanoma SC are caused by a variety of skin diseases, including altered protein synthesis caused by genetic factors that contribute to the development of SC.45,46 Immunosuppressed patients are more likely to develop cutaneous malignancies than healthy people. An integrated multidisciplinary approach involving dermatologic surgery, radiation oncology, and medical oncology is necessary for the management of such patients.47 There are connections between certain viral infectious diseases, such as AIDS, and SC. It has been noted that AIDS patients have a 3- to 5-fold higher risk of having nonmelanoma SC.48 Furthermore, it is found that the incidence of BCC is 11.4-fold higher in HIV-positive hemophiliac patients than in the general population. HIV patients with SCC have a 50% mortality rate between the ages of 6–84 months and a high risk of metastasis.49 About 90% of NMSC in immunosuppressed patients and up to 50% in immunocompetent patients were found to contain DNA originating from cutaneous or b-HPV types, according to molecular studies that reveal the complicity.50 It is also believed that these viruses may contribute to the pathogenesis of nonmelanoma SC indirectly.51 It has been discovered that patients with xeroderma pigmentosa are more likely to experience sunburn, freckling, and childhood skin.52 SC, including melanoma and nonmelanoma SC, frequently have dysregulated signaling pathways linked to the regulation of gene expression. One such dysregulation is the PTCH1 gene mutation, which causes uncontrollable skin cell proliferation and multiplies BCCs.53 Similarly, in men, a CDKN2A gene mutation is the most frequently found cause, whereas, in women, MDM2 gene mutations are predisposed to melanoma development at an earlier age.54
4. Role of Epigenetics in Skin Cancer
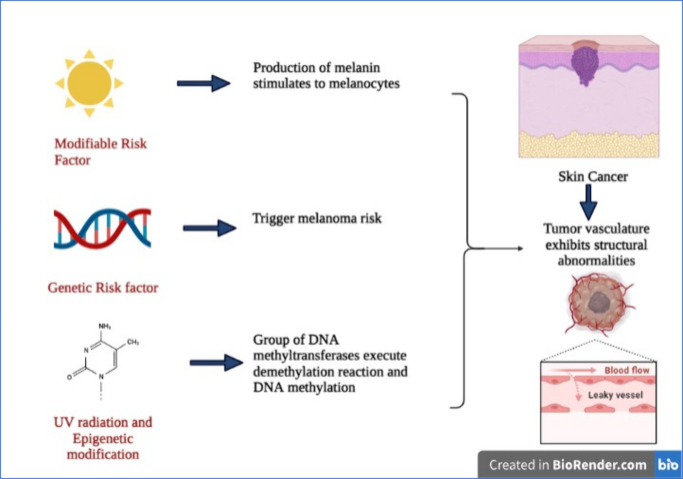
In the year 1942, biologist Conrad H. Waddington first described the term “epigenetics” as the “study of the heritable alteration in the expression of the gene, mediated via mechanisms excluding variations in the primary nucleotide sequence of a gene”.55,56 Histone modifications, chromatin remodeling, and noncoding RNA (ncRNA) mediation are the main forms of epigenetic modification.1,57 Groups of DNA methyltransferases (DNMTs) execute demethylation reactions and DNA methylation.58 Transfer of the methyl group from S-adenosyl methionine (SAM), a one-carbon metabolite, to the fifth position of cytosine catalyzed these enzymes, which form into 5-methylcytosine (5mC).59 From various DNMTs, during the replication phase, DNMT1 performs the methylation reactions, although DNMT-3a/b carries out the de novo reaction. Furthermore, methylation of DNA is distributed mainly at 10% in CpG islands (CGIs) and CpG-dense regions at 60–80%.60 One study found that CGIs primarily reside in the transcriptionally active regions and are unaffected by methylation through a variety of mechanisms like occupied TFs, RNA pol II, or H3K4me3, and nucleosome positioning is driven by DNMT blockage.61 It becomes transcriptionally silenced if CGI becomes condensed in the promoter region. In contrast, via a sequence of enzymes, i.e., 10–11 translocases (TETs) known as TET1/2/3-governed-reactions, DNA that has been methylated can be changed back to its original cytosine base.1 The 5mC (5-methylcytosine) can be oxidized by TETs to yield 5fC (5-formylcytosine), 5hmC (5- hydroxymethylcytosine), and 5caC (5-carboxylcytosine).62 Base excision repair transformed 5caC and 5fC to a cytosine base by coupling with thymine DNA. However, from any of the above intermediates, the mechanism can also be changed by a replication-governed mechanism to cytosine synthesis.63 Moreover, by recruiting suppressing transcription factors or binding or recruiting proteins, DNA methylation can repress gene expression, consequently revealing rare proof of DNA-methylation-governed transcriptional activation,1,64 shown in Figure 1.
Figure 1.
Formation of the tumor and its entry through the leaky vasculature of cells and major causes of skin cancer (created with BioRender.com).
5. Risk Factors and Metastasis Risk of Skin Cancer
5.1. Immunosuppression
For patients with immunosuppressed states having rapid growth of cutaneous squamous cell carcinoma, it generally recurs locally in the second year after excision in 13% of patients, and patients have a 5–8% risk of metastasis. For older patients with tumors on the skin of their head and neck, the prognosis is typically worse if there are multiple tumors present and if the patient has a history of excessive sun exposure.65 Immunosuppression in patients is mainly diagnosed by blood tests.66
5.2. Testicular Germ-Cell Tumors (TGCT)
Among young adult men, testicular germ-cell tumors (TGCT) are the most prevalent cancer. Previous research suggested that TGCT survivors are more likely to develop SC. Among TGCT survivors, the standardized incidence rates for SC and melanoma were 1.93 (95% CI, 1.62–2.29, P = 0.0001) and 1.81 (95% CI, 1.57–2.08, P = 0.0001), respectively.67
5.3. Administration of Anti-Epileptic Agents/Drugs (AEDs)
The majority of anti-epileptic medications were not linked to SC. SCC was linked to the use of carbamazepine (OR, 1.88; 95% CI, 1.42–2.49) and lamotrigine (OR, 1.57; CI, 1.12–2.22), with carbamazepine showing evidence of a dose–response relationship. The estimated absolute risks were low; for instance, one additional SCC required 6335 person-years of high cumulative exposure to carbamazepine.68 Compared to other AEDs, lamotrigine and carbamazepine most likely increase the skin’s sensitivity to sunlight. According to reports, these two AEDs have the ability to photosensitize due to their photochemical characteristics and ability to cause photosensitivity reactions. AEDs and other photosensitizing medications may raise the risk of skin cancer by making people more sensitive to UV radiation.68,69
5.4. Human Papillomavirus (HPV) Infection and Human Immunodeficiency Virus (HIV) Infection
After adjusting for sex, age, and comorbidities, the adjusted hazard ratio of SC for patients with HPV in comparison to controls was 2.45 (95% CI, 1.44–4.18, P < 0.01). A patient with HPV infection had a significantly higher risk of SC if they were older than 40 years, according to the subgroup analysis.70 Patients with HIV are more likely to develop BCC and SCC. When compared to HIV-uninfected individuals, SCC had the highest correlation and statistical significance, with a prevalence ratio of 5.1. HIV-positive people were 80% more likely to develop skin cancer than HIV-negative people (95% CI, 1.3–2.4, P = 0.001). Patients’ ages were associated with a 45-fold increase in risk (95% CI, 3.3–15.9, P = 0.001). After adjusting for patient age, sex, and race, the likelihood of developing cancer was 6.4 times higher than that of the other (95% CI, 49–84, P = 0.001). HIV infection increases the likelihood of acquiring HPV, which includes oncogenic viruses, because of immunosuppression treatment and high-risk behavior.71
5.5. Smoking
Current smoking increased the risk of SCC (pooled RR = 1.32; 95% CI, 1.15, 1.52) but decreased the risk of BCC (pooled RR = 0.85; 95% CI, 0.75, 0.96) and MM (pooled RR = 0.72; 95% CI, 0.64, 0.82). There was no evidence of publication bias, and no one study significantly influenced the combined findings. While former smoking was not linked to an increased risk of SC, similar results were found for heavy smoking.72 The induction of specific mutations in the p53 gene could exert its carcinogen effect on human skin.72,73 A second possible effect of smoking in the development of cutaneous SCC could be the loss of immune surveillance since smoking has been shown to suppress immunologic functions,74 and immunosuppressed patients are known to have an increased risk of SCC.75
5.6. Li–Fraumeni Syndrome
An inherited cancer syndrome called Li–Fraumeni syndrome (LFS) is marked by the early onset of several different cancers. A germline TP53 gene mutation is connected to LFS. There were 71 patients (59% of whom were female) and 33 families in total. A median age of 41 (25–65) years was reached by 10 patients (14%) who had a total of 19 SC. A person’s lifetime risk of developing SC is 10.4% (95% CI, 4.4–23.5%) at age 40, 25.2% (95% CI, 12.3–47.6%) at age 60, and 44.6% (95% CI, 22.9–73.9%) at age 70,76 summarized in Table 1.
Table 1. Risk Factors of Skin Cancers, Identification Test, and Skin Metastasis Risk.
| risk factors | identification | skin metastasis risk | references |
|---|---|---|---|
| immunosuppression | blood tests | recur locally in 13% of patients and have a 5–8% risk of metastasis | (65) |
| ultraviolet (UV) radiation | histopathology | DNA base pairing mainly UV–B and UV–C, damage double bonds of pyrimidines | (77) |
| testicular germ-cell tumors (TGCT) | biopsy | SC and incidence melanoma among TGCT patients were 1.93 (95% CI, 1.62–2.29, P < 0.0001) and 1.81 (95% CI, 1.57–2.08, P < 0.0001) | (67) |
| administration of anti-epileptic agent/drugs (AEDs) | histopathology | use of some AEDs involving phenobarbital (OR 0.49, 95% CI, 0.25–0.95), pregabalin (OR 0.61, 95% CI, 0.34–1.09), clonazepam (OR 0.71, 95% CI, 0.42–1.20) and valproic acid (OR 0.75, 95% CI, 0.51–1.09) | (68) |
| human papillomavirus (HPV) infection | histopathology | the adjusted hazard ratio (HR) of SC for patients with HPV relative to controls was 2.45 after adjusting for sex, age, and comorbidities (95% CI, 1.44–4.18, P < 0.01) | (70) |
| smoking | histopathology | smoking is related to a high risk of SCC (pooled R, R = 1.32, 95% CI, 1.15, 1.52), but the risk with BCC and MM is very low (pooled R, R = 0.85, 95% CI, 0.75, 0.96 and pooled R, R = 0.72, 95% CI, 0.64, 0.82, respectively) | (72) |
| Li–Fraumeni syndrome | genetic biomarker | the risk of SC at the age of 40 is 10.4% (95% CI, 4.4–23.5%), at age 60 it is 25.2% (95% CI, 12.3–47.6%), and at age 70 this risk is 44.6% (95% CI, 22.9–73.9%) | (76) |
6. Pathogenesis of Skin Cancer
SC pathophysiology is diverse. The development of nonmelanoma SC and malignant melanoma is due to UV radiation.78 UV radiation is further subdivided as mentioned below.79
-
I.
UVA (Ultraviolet A)
-
II.
UVB (Ultraviolet B)
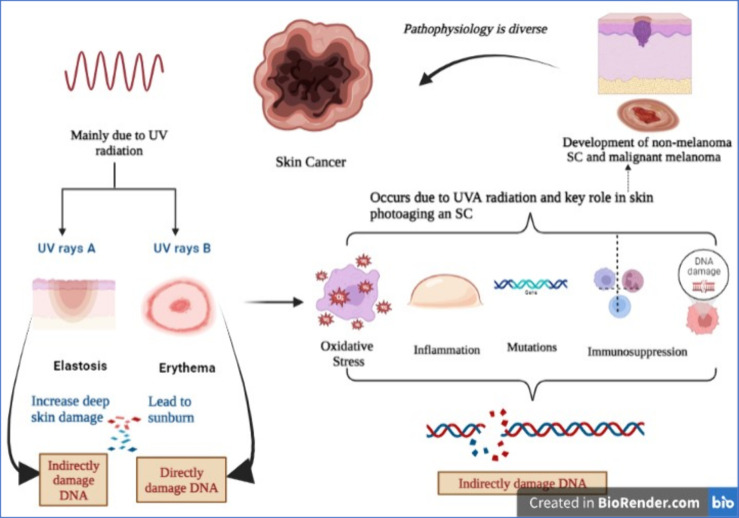
UVA radiation increases deep skin damage (elastosis) by passing deeper into the skin than UVB rays.80 UVB radiation leads to sunburn or erythema. DNA damage, immunosuppression, gene mutations, inflammatory responses, and oxidative stress occur due to UVA radiation, and all of these play a key role in skin photoaging and SC.81 The DNA is directly damaged by UVB radiation while UVA indirectly damages the DNA, which occurs by damage to cellular membranes and free radicals.82 An interrelation between UV-radiation-induced immunosuppression and SC genesis is demonstrated by researchers. UV radiation is carcinogenic because it promotes tumor formation by promoting mutations in anti-oncogenes and also initiates tumor development.83 UVA radiation plays a significant role in the carcinogenesis of stem cells of the skin and by inflammatory responses and tumorigenesis, and UVB promotes DNA damage.84 In the skin when UV radiation penetrates the DNA of the epidermal layer, keratinocytes absorb much of its energy. According to the assumption of researchers, the photoreceptor in the skin is DNA, and UV rays triggering the formation of a cyclobutane pyrimidine dimer is the early molecular step that decreases immunity.85 The mechanism of having SC by UV-radiation-induced damage is complex and intricate. UV radiation leads to mutations to p53 (anti-oncogene genes) that repair DNA or promote cell death of cells whose DNA is damaged. So, they are not involved in the DNA repair process if p53 genes are altered/mutated.86 This deregulation of apoptosis promotes unchecked keratinocyte mitosis, and the development of SC begins. UV-radiation-induced free radical damage is a prime mechanism of carcinogenesis, and patients are predisposed to skin tumors due to their genetically determined ability to metabolize free radicals.87 The role of antioxidants played by the enzyme glutathione S-transferase (GST) is to decrease the toxic effects of ROS.88 The enzyme glutathione S-transferase polymorphisms (GSTP) is expressed broadly in the epidermis and dermis of the skin, and the development of SC is a vital mediator. The GSTP gene deletion increased susceptibility to the developed SC.89 Alterations in color, size, shape, mole surfaces, and other skin lesions or new growth on the skin are important signs of cutaneous carcinoma development.90 Generally, the alterations that are found over a few days are not tumors, but alterations that last for a month or more should be evaluated by a physician,91 as shown in Figure 2.
Figure 2.
An illustrative image describes the pathogenesis of skin cancer, which is mainly observed due to UV rays (UVA and UVB); direct or indirect damage to DNA then produces oxidative stress, inflammation, mutation, and immunosuppression (created with BioRender.com).
7. Therapeutic Strategies for the Treatment of Skin Cancer
7.1. Ligands
Targeting antitumor medications to solid tumors at the specific therapeutic concentration is still challenging.92 To address this issue, a promising approach is to use ligands that specifically target and enter tumor cells and/or cells within the tumor microenvironment while avoiding noncancerous cells. Various ligands can be utilized for targeting like antibodies, nanobodies, peptides, proteins, etc.93
7.2. Approaches to Detect New Ligands
The discovery of targeting approaches that have minimum interaction with the healthy tissue is still needed for finding novel targeting molecules that have high specificity for cancers.94 It is highly desirable to develop new ligands with multiple targeting capabilities to combat the difficulty and aggressivity of the tumor microenvironment.93 “Phage display technology” is a technique for recognizing new peptides and antibodies that target a specific receptor. It was developed in the year 1985.95
7.3. Therapeutic Approaches for SC Treatment via a Ligand
The radio-sensitizing and photosensitizing properties of gold nanoparticles conjugated to 5-aminolevulinic acid (5ALA) were demonstrated.96 CLEC2A is the ligand of the NKp65-triggering NK cell receptor. It was found in sporadic dermal cancer-linked fibroblasts. Dermal fibroblasts that express CLEC2A are crucial for the immune control of the skin.97 A new ruthenium-consisting PS was developed by groups of researchers that modulate biological and photophysical properties to better meet PDT requirements.98 Interestingly, it was discovered that cells in the mitotic phase were more impacted and had a unique apoptosis mechanism.99 In ALA-based PDT, ALA esters or 5-aminolevulinic acid (ALA) are utilized as pro-drugs to promote the synthesis of the powerful PS protoporphyrin IX (PpIX).100 The production of ROS and toxic responses is caused by the activation of PpIX by light. Studies have shown that butyric acid (GABA) transporters are included in ALA uptake and its methyl ester (MAL) into cells.101 Therefore, the creation of inhibitors that specifically target particular GAT subtypes and the homology models may be a benefit in the design of therapeutic inhibitors that can be used to reduce the pain caused by ALA.102 Branched polyethylenimine (BPEI)-modified Eu3+ and YVO4:Bi3+ NCs (nanocrystals) were prepared, an epidermal growth factor (EGF) and folic acid (FA) were bound to the BPEI-coated Eu3+, and the YVO4:Bi3+ NCs showed low cytotoxicity and efficient targeting of fluorescent NCs to the targeted overexpressed folate receptor in HeLa cells or EGFR in A431 cells.103 5-Fluorouracil (5-FU) combined with calcipotriol increases HLA class II, thymic stromal lymphopoietin (TSLP), and the natural killer cell group 2D (NKG2D), and there are benefits of combining calcipotriol with 5-FU therapy in maximizing CD4+ T-cell-mediated immunity activation against SC and other cancers.104
7.4. Targeted Photosensitizers
7.4.1. Folate (FA) and Transferrin (TF)
The most used targeting ligands are FA and TF, included in photodynamic therapy (PDT). While the targeted delivery of photosensitizers (PSs) is more often investigated in nanocarriers, the production of bioconjugates with enhanced selectivity for cancer cells is described by investigators.105 Although improved internalization by cancer cells is lacking studies, this work for the manufacture of new FA-targeted PS conjugates can serve as a guideline.106 Platinum porphyrin and FA form a complex from carboxylic acid activation, permitting its amide bond formation with the linker and yielding a novel FA-targeted PS selective for FRα-positive cell lines. Confocal microscopy studies confirm the endocytosis of targeted PS by HeLa cells, which contrasts with the FRα-negative cell line.93,107 Phototoxicity assays show the PS’s selectivity, with a 78% reduction in viability of the FR-positive cell line compared to the FR-negative line, a difference of 25%.93 Furthermore, transferrin, FA, and different endogenous ligands have been researched for anti-tumor-targeted PDT.108 FRα is less overexpressed than biotin receptors in various cancer cell lines of distinct histological origins.109 It is signified by elevated selectivity and increased aggregation in tumor cells via Biotin-targeted PS.110
7.4.2. Nanobody and Antibody
Antibodies targeting PSs and other fractions create a division of molecules that are utilized for the delivery of PSs, and with the advancement of customized medicine, it has gained popularity.111 The probable synthetic approaches for the advancement of tetrapyrrole-based antibody–PS conjugates are cysteine (maleimide conjugation) or conjugation through lysine (isothiocyanate and amide conjugation), alkyne–azide cycloaddition promoted by strain but without copper, and SNAP–Tag conjugation and chemistry (copper-catalyzed alkyne–azide cycloaddition).93,112
7.4.3. Peptide-Targeted PS
The targeted delivery of PS promotes aqueous solubility with the aid of small peptides that increase therapeutic efficacy and phototoxicity.113 Solid- or solution-phase approaches provoke activations of carboxylic acid. Phage display against EGFR identified the peptide GE11 that attracted the attention of various researchers.114
7.5. Natural Compounds Used in the Management of Skin Cancer
7.5.1. Saffron
Saffron (Crocus sativus L.) is a traditional medicine that belongs to the family Iridaceae.115 Crocin is an active constituent of saffron that is widely used to treat various types of cancers; on the other hand, it arrests the cell cycle in leukemia cells.116 Moreover, its apoptotic property and the mechanism of crocin was studied on skin cancer cells (A431 and SCL-1). It decreases the expression of STAT/JAK circuits while restricting the cell cycle in G0/G1 and inhibiting cell propagation of human skin cancer cells.117
7.5.2. Green Tea Polyphenols (GTPs)
GTPs are obtained from Camellia sinensis (family Theaceae) and contain anticancer (prostate cancer), antimicrobial, antidiabetic, anti-atherosclerotic, and anti-obesity activity.118−120 The researcher found that regular administration of green tea polyphenols reduces the occurrence of nonmelanoma SC via triggering DNA repair, preventing inflammation, and inhibiting IL-1β production, leading to suppression of melanoma skin cancer growth. Furthermore, it is also found that GTPs induce miR-29 attenuation and block tumor growth by suppressing DNA hypermethylation.121
7.5.3. Diosmetin
Diosmetin is a naturally occurring flavonoid mainly derived from citrus fruits.122 Over the past three decades, diosmetin has been shown to have a variety of therapeutic effects like anticancer activity on breast cancer as well as antioxidant, antibacterial, and anti-inflammatory activity.123,124 The previous study demonstrated that diosmetin prevents the development of a tumor and suppresses angiogenesis in SC. The results of this study revealed that diosmetin blocks the migration of SC cells and also induces apoptosis via regulating caspase circuits.125
7.5.4. Cantharidin (CTD)
Cantharidin is a poisonous monoterpene obtained from blister beetles that belong to the order Coleoptera and family Meloidae.126 It can be used to treat various types of cancer like hepatic cancer, leukemia, breast cancer, and other diseases.127 It was found that it suppresses the MAPK (p38, JNK, and ERK) signaling pathway via decreasing NF-κB and AKT, resulting in down-regulation of MMP-2/-9 and uPA protein expression in SC cells.128
7.5.5. Neem
Neem is a natural compound belonging to the family Meliaceae. It is recognized by its botanical name Azadirachta indica. It possesses the potential to treat parasitic diseases, skin diseases, oral squamous cell carcinoma, and sexually transmitted diseases and has anti-inflammatory and antibacterial activity.129,130 Moreover, neem can be used to treat nonmelanoma cells by promoting apoptosis of cancer cells through regulating caspase-3, Bax, caspase-9, and Bcl-2 expression.131
7.5.6. Cocoa
Cocoa is obtained from plant seeds Theobroma cacao L. of the family Sterculiaceae.132 Cocoa is a traditional medicine that can be used in the treatment of malaria, worm expellers, and wound healing.133 The research found that cocoa can decrease the cell viability and cell growth of melanoma cell lines, i.e., B16-F10 and A-375, by inducing oxidative stress in cells.134
7.5.7. Myricetin
Myricetin is a naturally occurring component found in various fruits, tea, and wine. Various families like Anacardiaceae, Pinaceae, Primulaceae, Polygonaceae, and Myricaceae are the major sources of myricetin. The study showed that myricetin possesses an anti-inflammatory, antidiabetic, and anticancer effect on colon cancer as well as anti-obesity properties, and it is hepatoprotective.135 Myricetin also blocks the development of the Cyclin B/CDK1 complex in SC cells. Moreover, myricetin triggers anti-oncogenes (p53) and promotes the expression of CDK inhibitors (p27 and p21), thus restricting the cell division of SC cells.136
7.5.8. Silymarin
Silymarin is a obtained from the Silybum marianum of the family Asteraceae and is also recognized as milk thistle.137,138 A study showed that it is a promising molecule that manages various types of disorders including prostate cancer and liver, cardiac, and neurological diseases.139 The study showed that silymarin protects against skin cancer which is induced due to UVB radiation.141 Moreover, it induces the repairing of cyclobutane pyrimidine dimers and increases the DNA damage of nonmelanoma SC cells through the up-regulation of p53.140
7.5.9. Zyflamend
Zyflamend is an herbal extract derived from turmeric, rosemary, green tea, and ginger. It is used to treat pancreatic cancer and inflammation.141,142 It promotes caspase-9 cleavage in SC cells. It was also found that zyflamend inhibits angiogenesis, invasion, metastasis, and tumor cell proliferation via regulating inflammatory pathways.143
7.5.10. Rutin
Rutin is a flavonoid derived from Ruta graveolens, of the family Rutaceae. It is present in apricots, tea, grapefruit seeds, cherries, plums, buckwheat, orange, grapes, and onion.144 It is a natural compound that consists of various pharmacological activities including antimicrobial, anti-inflammatory, and anticancerous effects on lung, liver, and cervical cancer.145,146 The potency of rutin is evaluated by the researcher on the SC cell line (A375). They found that rutin causes cell mortality of SC cells via promoting autophagy and apoptosis.147
7.5.11. Isoliquiritigenin
Isoliquiritigenin is obtained from Glycyrrhiza uralensis of the family Leguminosae.148 It has various therapeutic benefits like antidiabetic, antivirus, anti-aging, anti-inflammatory, and antioxidative properties. It is also used to treat oral cancer by promoting apoptosis and the arrest of the cell cycle during phase G2.149 Moreover, it can inhibit the growth of skin cancer cells and promote cell apoptosis. Thus, it is a promising candidate that can inhibit the multiplication of melanoma cells via suppressing miR-301b.150
7.5.12. Phloretin
Phloretin is a naturally occurring dihydrochalcone obtained from various vegetables and fruits, mostly found in Manchurian apricots and apple tree leaves.151 It possesses various therapeutic activities including immunosuppressant, hepatoprotective, antioxidant, cardioprotective, and antidiabetic properties.152 The study showed that phloretin reduces sunburn which occurs due to UV radiation by suppressing the expression of matrix metalloproteinase-9 and inhibiting thymidine dimer formation. UV radiation is a major cause of SC, thus it is a promising therapeutic target for SC.153
7.5.13. Apigenin
Apigenin is obtained from Teucrium polium L., Melissa officinalis, and Salvia officinalis and belongs to the Lamiaceae family.154,155 It is a traditional herb which is used to treat various types of cancers like prostate cancer, cervical cancer, leukemia, pancreatic cancer, breast cancer, liver cancer, and lung cancer.156 A current study showed that apigenin decreases the incidence of SC. On the basis of the findings of this study, we concluded that apigenin is a novel lead compound that enhances the expression of the tumor suppressor gene (P53) and promotes cell apoptosis.157
7.5.14. Baicalein
Baicalein is a flavonoid obtained from the leaves of Thymus vulgaris and Oroxylum indicum and the roots of Scutellaria baicalensis.158 In numerous human cancer cells, including melanoma cancer, baicalein has been demonstrated to suppress cell proliferation and trigger apoptosis.159,160 Nevertheless, baicalein has also been shown to decrease osteosarcoma cell metastasis in vitro and in vivo, along with prostate, lung, breast, and liver tumors.161 It significantly reduced invasion and migration in B16F10 melanoma cells by inhibiting the activity of MMP-2 and -9. Furthermore, it enhances tight junction strengthening linked with the suppression of claudin expression, which is related to the inactivation of the PI3K/Akt signaling pathway.160 Current findings suggest that baicalein is a promising therapeutic candidate for melanoma therapy.
7.5.15. Thymoquinone (TQ)
TQ is the biologically active constituent found in black cumin (Nigella sativa) seeds.162 Several studies have demonstrated the effectiveness of TQ in treating neoplasms, including skin, ovarian, breast, colon, prostate, liver, and cervical malignancies.162−164 To sum up, this study’s results showed that TQ blocked the β-catenin signaling pathway, which in turn prevented melanogenesis in B16F10 murine melanoma cells, as well as zebrafish melanogenesis. TQ did not appear to be harmful to normal melanocytes, but it was toxic to both of the melanoma cell lines that were investigated.165 Effective anticancer therapies depend on the production of TQ and TQ-loaded nanocarriers of melanoma cells obtained from various phases of the disease.166 The results of this investigation could be helpful in the future for developing treatments for melanoma and pigmented lesions.165
7.5.16. Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a flavonoid found in large amounts in Hedyotis diffusa and Lonicera japonica, among many other plants, and has been utilized extensively in the treatment of melanoma.167 It is widely known that luteolin has antitumor effects on a variety of human cancers.168 Additionally, it has been observed that luteolin inhibits EMT in cancer cell lines including PC3 and A431.169 The chemotherapeutic activity of luteolin was evaluated by the researcher on a melanoma cell line (B16F10 cells). By regulating the β3 integrin, luteolin prevents hypoxia-induced EMT in malignant melanoma cells both in vitro and in vivo. This suggests that luteolin could be used as a chemotherapeutic and chemopreventive molecule for melanoma,172 as shown in Table 2 and Figure 3.
Table 2. Natural Drugs and Their Effects along with a Mechanism for the Treatment of Skin Cancer.
| sr. no. | compound | effect | mechanism | references |
|---|---|---|---|---|
| 1 | saffron | decreases the expression of STAT/JAK circuits | restricts the cell cycle in G0/G1 and inhibits cell propagation | (116,117) |
| 2 | green tea polyphenols | suppresses DNA hypermethylation | blocks tumor growth | (121) |
| 3 | diosmetin | regulates caspase circuits | induces apoptosis | (123) |
| 4 | cantharidin | suppresses MAPK (p38, ERK, and JNK) | down-regulates MMP-2/-9 and uPA protein expression | (128) |
| 5 | neem | regulates caspase-3, Bax, caspase-9, and Bcl-2 expression | promotes apoptosis | (131) |
| 6 | cocoa | induces oxidative stress | decreases the cell viability and cell growth | (134) |
| 7 | myricetin | triggers anti-oncogenes (p53) | restricts the cell division | (136) |
| 8 | silymarin | up-regulation of p53 | increases the DNA damage | (140) |
| 9 | zyflamend | initiates caspase-9 cleavage | inhibits angiogenesis, invasion, metastasis, and proliferation | (143) |
| 10 | rutin | promotes autophagy and apoptosis | induces cell mortality | (147) |
| 11 | isoliquiritigenin | inhibits miR-301b | inhibits cell multiplication | (150) |
| 12 | phloretin | suppresses expression of matrix metalloproteinase-9 and inhibits thymidine | - | (153) |
| 13 | apigenin | enhances expression of p53 | promotes cell apoptosis | (157) |
| 14 | baicalein | down-regulates MMP-9 and MMP-2 expression | suppresses invasion and migration | (160) |
| 15 | thymoquinone | down-regulates DNMT1 | up-regulates BRCA1 | (162) |
| 16 | luteolin | inhibits epithelial–mesenchymal transition | blocks the progression of SC cells | (170) |
Figure 3.
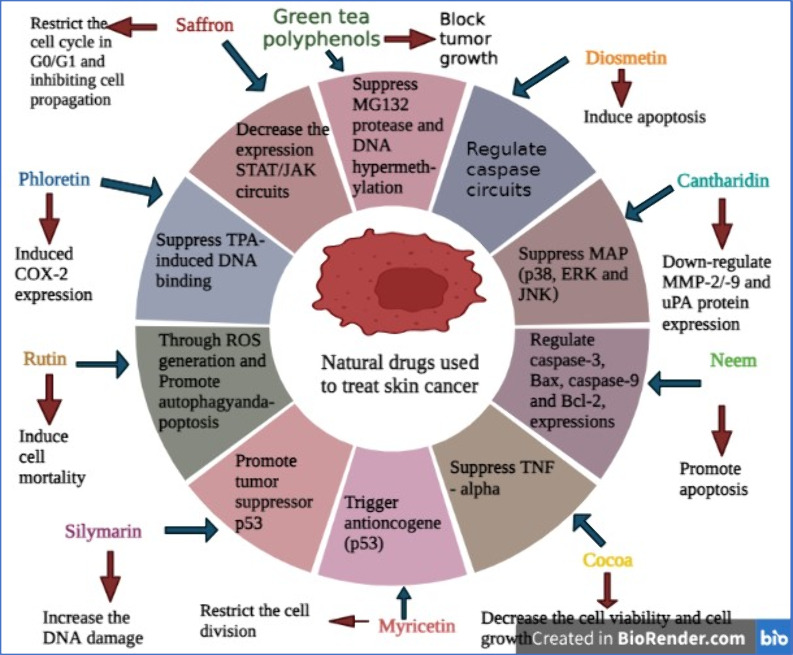
A pie illustration describes the main natural drugs and their mechanism for the treatment of skin cancer (created with BioRender.com).
7.6. Compounds with Their Ligand and Their Mechanism for the Treatment of Skin Cancer
7.6.1. Calcipotriol Combined with 5-Fluorouracil
Calcipotriol down-regulates SC. Calcipotriol combined with 5-FU treatment promotes HLA class II, TSLP, and natural killer cell group 2D (NKG2D) expression of ligands in the keratinocytes linked with a marked CD4+ T cell infiltration.104
7.6.2. Imiquimod
Imiquimod, which is also called R-837 or S-26308, was first approved by the US Food and Drug Administration (USFDA) for the treatment of anal and genital swellings or warts. Later, it was found that it possesses anticancer activity.171 Imiquimod is a Toll-like receptor ligand, used to treat different types of cutaneous malignancies.172 Recently, researchers proved that imiquimod promotes programmed cell death and autophagic cell mortality by inducing mitochondria-mediated apoptosis and ER-stress/PERK/PKR axis via an increasing ROS in SC cells.173
7.6.3. Cannabinoids
Cannabinoids are obtained from Cannabis sativa L., which is a traditional medical plant belonging to the family Cannabacea.174,175 Cannabinoids restrict the growth of breast cancer cells and down-regulate the oncogene (cyclooxygenase-2 (COX-2) and c-fos).176 Moreover, CB2 (CB2R) and GPR55 are the G-protein-coupled receptors overexpressed in tumors and cancer cells.178 Cannabinoids induce apoptosis and restrict angiogenesis during SC.177
7.6.4. Aminolevulinic Acid
Protoporphyrin IX (PphIX), a powerful photosensitizer used in photodynamic therapy, is produced from 5-aminolevulinic acid (5-ALA). Hollow mesoporous silica nanoparticles (HMSNPs) functionalized with folic acid (FA) were prepared to deliver 5-ALA. PphIX can produce reactive singlet oxygen (1O2), which is extremely toxic and eventually causes cell death or necrosis.178
7.6.5. Rosemary
Rosemary is a shrub obtained from Rosmarinus officinalis L. of the family Lamiaceae. Rosemary extracted from the leaf promotes intracellular ROS which induces necrosis. It exhibits high antagonist activity against multiple agonists including 6-formylindolo[3,2-b] carbazole (FICZ), indirubin (IND), pityriazepin (PZ), and the prototype ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in human keratinocyte cells and in vitro,179 which is briefly explained in Table 3.
Table 3. Compounds and Their Appropriate Ligands and Mechanisms.
| compound | ligand | mechanism | reference |
|---|---|---|---|
| calcipotriol combined with 5-fluorouracil | TSLP, HLA class II, and NKG2D | increases CD4+ T cell immune response | (104) |
| imiquimod | Toll-like receptor (TLR) ligand | promotes apoptosis and autophagic cell morbidity | (172) |
| cannabinoids | CB1r/CB2r, TVRPs, PPARs, and GPR55 | inhibits angiogenesis and promotes apoptosis | (177) |
| 5-aminolevulinic acid | folic acid (FA) | produces reactive singlet oxygen (1O2), which is highly toxic and leads to cell apoptosis or necrosis | (178) |
| rosemary | the prototypical ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), indirubin (IND), pityriazepin (PZ), and 6-formylindolo[3,2-b] carbazole (FICZ) | promotes intracellular ROS, which induces necrosis | (179,180) |
7.7. An Epigenetic Approach for the Management of Melanoma
7.7.1. Sulforaphane
Sulforaphane is an isothiocyanate obtained from cruciferous vegetables like broccoli, cauliflower, and cabbage.181 Researchers found that it inhibits breast cancer metastasis.182 However, when it is exposed to melanoma cells, it increases nuclear factor 2 (Nrf2) expression and restricts the transformation of cells, NQO-1 (NAD(P)H quinone dehydrogenase 1), and HO-1. The study revealed that its treatment resulted in a decrease in the CpG dinucleotide methylation ratio within the Nrf2 promoter region in melanoma cells. Additionally, sulforaphane inhibited the expression of DNA methyltransferases (DNMTs) and histone deacetylases (HDACs) including DNMT1, DNMT3a, DNMT3b, HDAC1, HDAC2, HDAC3, and HDAC4.182
7.7.2. Fucoxanthin
Fucoxanthin decreases ROS (reactive oxygen species) levels and promotes apoptosis. It also induces GSH (glutathione) levels via increasing GCLC (glutamate–cysteine ligase catalytic) subunit and GSS (glutathione synthetase) expression by the Akt/Nrf2 pathway in human keratinocyte cells.183
7.7.3. Curcumin
Curcumin is obtained from turmeric (Curcuma longa), family Zingiberaceae, which has been used for thousands of years for the prevention and treatment of various diseases.184,185 It can be used to treat several cancers like pancreatic cancer, gastric cancer, hepatic cancer, and colorectal cancer.186 Moreover, studies showed that curcumin promotes cell cycle arrest at the G1 phase in A375 cells by suppressing cyclin D and phosphorylated retinoblastoma.187
7.7.4. Epigallocatechin-3-gallate (EGCG)
EGCG is obtained from the leaf of Camellia sinensis, family Theaceae.188 It is a potential therapeutic candidate for the prevention and treatment of cervical and prostate cancer.189,190 However, EGCG acts as an epigenetic regulator of melanoma, which reduces the expression of DNMTs and inhibits DNA methylation in the SC cell lines SCC-13 and A431.191 In human SC cells, EGCG has been demonstrated to reactivate important tumor suppressor genes like cip1/p21 and p16INK4a by reducing DNA methylation and increasing histone acetylation.192
7.7.5. Mangiferin
Mangiferin is the main constituent obtained from the leaf of Mangifera indica L. and belongs to the family Anacardiaceae.193,194 It has protective effects against various types of cancer including neuronal, breast, colon, and lung cancers via inducing apoptosis.195,196 Researchers also found that mangiferin has the potential to treat melanoma.197 It inhibits several NF-kβ target genes during melanoma, like interleukin-6, tumor necrosis factor, interferon-γ, vascular endothelial growth factor receptor 2, matrix metalloprotease-19, and chemokine ligand 2, and inhibits angiogenesis of SC cells,198 as summarized in Table 4.
Table 4. Drugs, Type of Study, and Their Molecular Mechanism.
| compound | type of study | molecular mechanism | references |
|---|---|---|---|
| sulforaphane | in vitro | inhibits histone deacetylases and DNA methyltransferase expression | (199) |
| fucoxanthin | in vivo | induces GSH and GSS | (183) |
| curcumin | in vitro | suppresses cyclin D and phosphorylated retinoblastoma | (187) |
| epigallocatechin-3-gallate | in vitro | inhibits DNA methylation | (192) |
| mangiferin | - | down-regulates NFkB | (198) |
7.8. Photodynamic Therapy (PDT) Approach in the Management of Melanoma
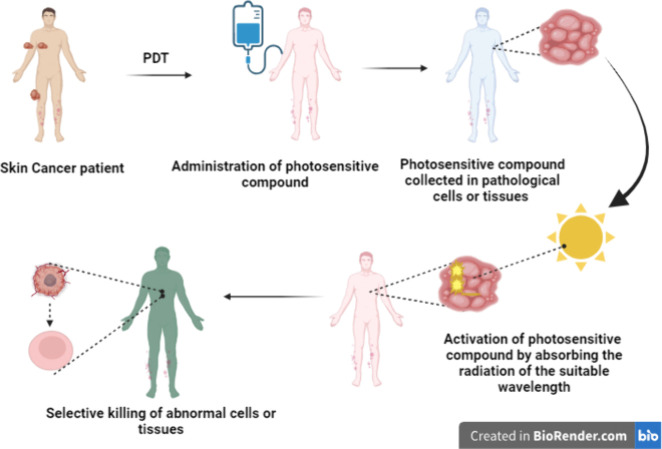
PDT is a noninvasive and modern type of therapy utilized for the treatment of various classifications, location of the tumor, and noncancerous ailments. It depends on the photosensitive compound (locally and/or systemic application) collected in pathological cells or tissues.200 This PS initiates the process of activation by absorbing the radiation of the suitable wavelength and leads to the selective killing of abnormal cells or tissues. Within the pathological tissues, photoirritative or photocytotoxic reactions take place in the location where the photosensitive compound is dispensed, which permits selective killing.201 Protoporphyrin-IX-induced PDT is usually utilized in dermatological practices like SC treatment.202 Treatment of various diseases by utilizing radiation began in ancient times and was used by the Indians, Egyptians, Chinese, and Greeks to treat dermal ailments. In India during the 15th century BC, treatment by utilizing an exogenous compound reacting with the radiation of the sunlight was observed. The mechanism of PDT enables cellular cytotoxicity, and the ROS that is produced promotes autophagy or a necrotic and/or apoptotic approach to cell mortality during the mechanism of PDT.203 Immunological responses, cellular morphology, enzymatic activity, light intensity and wavelength, oxygen concentration, PS subcellular location, and PS physiochemical features are the aspects that affect the mode and degree of cell mortality.204 Cell death programmed or nonprogrammed is determined based on these factors.205 The programmed cell mortality is apoptosis, which is generally characterized by nuclear and membrane destruction.206 When this type of cell mortality occurs, PSs typically localize in the cellular mitochondria, and it is usually a linked mechanism of cell mortality in PDT.207 Specific signals activate the apoptosis in target cells that, in response to these signals, activate various pathways leading to suicide.208 Protein caspases are triggered to destroy cellular contents like polypeptides and nucleic material as the pathways fail.209 Thus, apoptosis is a controlled and induced process.210 Nonprogrammed cell death is necrosis and inflammatory responses characterized by it, which are triggered by external stimuli like trauma or infections.211 The PS that causes necrosis is found in the plasma membranes of target cells. Events in necrotic cell mortality pathways include the motion of calcium ions over the endoplasmic reticulum, lysosomal rupture, cytoplasmic swelling, membrane permeability, breaking down of cell components, calcium-dependent calpain activation, and overall initiation of inflammatory responses.212 Sometimes, PDT-induced apoptotic cell mortality modes can change into necrosis. Different types of cell mortality in PDT tumor treatments are autophagy, apoptosis, and/or necrosis. During PDT, energy and electrons are transferred.207 The dose of PS given to target cells is used to excite the PS. This leads the cell to quickly degrade and die compared to apoptotic mortality.213 A review by Naidoo et al. reported that Dewaele and co-workers have noted in recent studies that later PDT irradiation of specific PSs and another type of cell mortality called autophagy can occur.207 When a cell tries to heal itself in response to photodamage, it experiences PDT-induced autophagy; though, if failure of response occurs then the cell is signaled for programmed apoptosis. The cell is signaled for programmed apoptosis if this response fails.214 PSs PDT for cutaneous indications frequently use a topical PS like methyl amino levulinate or 5-aminolevulinic acid, which are protoporphyrin IX precursors.215 Oral or intravenous PSs are required for visceral tumor treatment, and porfimer sodium is the frequently utilized PS for this indication.216 Numerous porfimer sodium derivatives have been developed, where the light at 630 nm (red) is absorbed by porfimer sodium and longer wavelengths are absorbed by second-generation agents, giving more energy for the killing of tumors and lowering the photosensitivity of skin.217 Third-generation agents are attached to carrier molecules like antibodies directed against tumor antigens, liposomes, and other biomolecules that allow the PS to enter the cell or receptor-positive surfaces on tumor cells and allow the PS to accumulate more specifically within the tumor cells, allowing for more precise targeting while sparing healthy tissue.218 Additionally, endocystic vesicles that release macromolecules into the cytosol through photochemical interfinalization and PSs have been proposed.219 Only a few PDT sensitizers have received approval from various nations like Japan, the Netherlands, and Canada, in contrast to the US Food and Drug Administration, which has only approved them for a small number of specific indications.220 PDT has been carried out by a variety of light sources like daylight, lasers, laser-emitting diodes, and incandescent light.221 The mode of delivery and light characteristics can be changed to target diverse tissue types. Blue light does not penetrate more deeply than red light.222 Light delivery mode, light exposure time, and the total light dose may be altered. In acute or traditional PDT within a small duration of time, high doses of light are administered; this is not selective and leads to apoptosis of normal neighboring cells or tissue. Acute PDT (aPDT) can lead to pain and a full-thickness skin ulcer that sometimes needs skin grafting or debridement. Additionally, aPDT causes a rapid loss of oxygen, which restricts the synthesis of ROS and consequently the possibility of tumor destruction.223 Fractionated therapy has similar side effects compared to aPDT but is more effective than single-dose treatments and has, in some instances, permitted clinical relapse and tumor resistance.222 When using metronomic PDT, light and PSs are administered for a longer duration at low rates or in fractions that are smaller than those of the aPDT fractions earlier mentioned.224 Although preclinical research is promising, the method has not been applied to human volunteers,216 as summarized in Figure 4.
Figure 4.
Illustration of the mechanism of photodynamic therapy in the management of skin cancer (created with BioRender.com).
7.9. Targeted Therapy for BRAF-Mutated Melanoma
A BRAF mutation is detected in early melanogenesis in a high percentage of melanocytic nevi, hence it cannot induce melanoma progression alone and needs additional genetic alterations at a later stage of progression, such as deletion of phosphatase with tensin homologue (PTEN), autophagy related 5 (ATG5), or cyclin-dependent kinase inhibitor 2A (CDKN2A), to give an advantage in the propagation of melanocytic cells to be transferred to melanoma cells.225
Furthermore, BRAF is one of the most frequently mutated oncogenes recognized in melanoma. The most frequent oncogenic BRAF mutations consist of a single point mutation at codon 600 (mostly V600E) that leads to the constitutive activation of the BRAF/MEK/ERK (MAPK) signaling pathway. Therefore, mutated BRAF has become a useful target for molecular therapy and the use of BRAF kinase inhibitors has shown promising results.226
However, recent advances in gene sequencing have shown that activated BRAF mutations are present in more than 50% of malignant melanomas and contribute to constitutive signals in the MAPK pathway. Besides the importance of its mutations in cell proliferation, BRAF is associated with lymph nodes and brain and liver metastasis along with the loss of PTEN expression and ATG5. Knowledge of this genetic alteration has led to the development of personalized and targeted therapy strategies which block different pathways, driving melanoma pathogenesis. Several targeted therapy agents such as vemurafenib, dabrafenib, and encorafenib have been approved by the FDA as BRAF inhibitors, as well as other immunotherapies such as anti-CTLA-4 (ipilimumab),227 which is summarized in Table 5.
Table 5. Targeted Therapy for Melanoma Using BRAF Inhibitors.
| BRAF inhibitor | targeted chemotherapy generation | current status | references |
|---|---|---|---|
| sorafenib | first-generation inhibitors | FDA approved | (225,228,229) |
| LY3009120, BGB659 | third-generation inhibitors | under phase I trial | (225,230) |
| dabrafenib | second-generation inhibitors | phase I trial | (225,231) |
| dabrafenib, trametinib, and spartalizumab (combination therapy) | third-generation inhibitors | under phase III trial | (232) |
| nivolumab and ipilimumab | third-generation inhibitors | phase III trial | (232,233) |
| vemurafenib | - | approved | (226,234) |
| temsirolimus | - | - | (5,235) |
7.10. Nanotechnology-Based Drug Delivery System for Melanoma
Recent decades have seen significant advancements in the field of nanotechnology, particularly in its use in medicine.236 Due to their distinct abilities to enhance drug delivery, nanoagents offer novel approaches for the treatment of many diseases. These obstacles can be overcome by using nanotechnology to achieve targeted drug delivery, enhance pharmacokinetics, and increase bioavailability.237 Various types of nanoagents have been utilized in clinical studies for drug delivery, immunotherapy, imaging diagnosis, and vaccine development. However, the full potential of nanotechnology in treating diseases is yet to be realized.238 Nanocarrier drug delivery systems (DDSs) are among the most important methods of utilizing nanomaterials. These DDSs utilize nanoparticles as carriers to transport active molecules, such as drugs, to the correct target in the body. DDSs are more precise than free drugs and can considerably augment the therapeutic effect of medications while decreasing potential side effects.239 Melanoma treatment has used many nanosystems such as lipid systems, natural nanosystems, polymeric systems, and inorganic nanoparticles.240 For example, lipid nanosystems, including liposomes, solid lipid nanoparticles, and nanoemulsions, have been created. Inorganic nanoparticle systems like gold nanoparticles, silica nanoparticles, nanotubes, and copper nanoparticles are also common. Exosomes, which are naturally occurring nanosystems, consist of polymeric micelles, dendritic macromolecules, nanospheres, hydrogels, and polymeric nanoparticles.240
7.10.1. Lipid Nanosystems
Lipid systems are highly effective in terms of physical stability, controlled release, and biocompatibility. These systems are also known for their low side effects, biodegradability, and excellent physical stability. They have been extensively researched for their potential in treating clinical diseases. Some examples of lipid systems are liposomes, solid lipid nanoparticles, and nanoemulsions. Among these, liposomes are a type of double nano DDS that has shown great promise in the treatment of cancer due to their favorable pharmacokinetic properties. Studies have also revealed that liposomes can enhance the effectiveness of medications in treating melanoma, especially those that target the cell cycle, like paclitaxel. Furthermore, liposomes can significantly prolong the half-life of drugs in circulation.241
7.10.2. Nanoparticles
Inorganic nanoparticles, like silver nanoparticles, gold nanoparticles, silica nanoparticles, and nanotubes, have good biocompatibility and enable simultaneous imaging and drug delivery.242 These nanoparticles, however, frequently need to be combined with other targeting ligands because they may not allow precise targeting of the affected region. One can create gold nanoparticles (1–150 nm) with a variety of geometries, including nanospheres, nanoshells, nanorods, and nanocages.243 Compared to other biomedical nanotechnologies, these particles possess a distinct set of physical, chemical, optical, and electronic properties. Additionally, they provide a versatile platform for various biochemical applications, including the delivery of genes, imaging agents, and drugs. By linking proteins, DNA, and smaller drug molecules to the surface chemistry of gold nanoparticles, a therapeutic effect has been observed in different types of tumors, including melanoma.244
7.10.3. Dendrimers
Dendrimers are unimolecular, monodisperse, synthetic polymers (15 nm) with layered architectures made up of a central core, an internal region made up of repeating units, and different terminal groups that determine the three-dimensional dendrimer characteristic structures.245 Due to their desirable characteristics, like well-defined size and molecular weight, mono dispersity, multivalency, the quantity of available internal cavities, the high degree of branching, and the abundance of surface functional groups, dendrimers can be prepared for the delivery of both hydrophobic and hydrophilic drugs, nucleic acids, and imaging agents. Numerous academic sources show that dendrimer-targeting ligands can cause the precise targeting and eradication of tumors.246
7.10.4. Hydrogels
Hydrogels are 3D (three-dimensional), hydrophilic polymeric networks that can absorb large amounts of water, biological fluids, or molecules. These systems have special qualities that increase the therapeutic agent’s effectiveness and reduce unfavorable side effects.242 Administering anticancer medications topically or through transdermal application using a drug delivery system can reduce side effects and improve the drug’s effectiveness. Compared to other methods of drug delivery and traditional therapies, using hydrogels to deliver antiproliferative agents in melanoma SC has numerous benefits.242 By controlling the structure on a molecular level, including cross-linking density and customizing properties such as biodegradation, degradation rate, pore size, mechanical strength, and chemical and biological responses to stimuli like pH, enzymes, and temperature, polymer engineers can design and create hydrogels. Additionally, the low cost of hydrogels compared to other polymeric formulations like nanoparticles, microparticles, and dendrimers is a significant advantage. Therefore, a hydrogel is a preferred option for melanoma cancer therapy due to its highly adaptable and modifiable characteristics.243
7.10.5. Exosomes
Exosomes are nanovesicles comprising various biomolecules. Target cells take up exosomes that are produced by cells during exocytosis. Exosomes can be utilized as natural nano DDSs because of their distinctive properties.244 Exosomes are double-membraned cellular vesicles that range in size from 30 to 150 nm. Additionally, these vesicles can target particular cells, pass through cell membranes, and carry a variety of biomolecules, such as proteins, lipids, and nucleic acids.245 Due to their unique pro-tumorigenic characteristic, exosomes may be used as a useful tool in the diagnosis and prognosis of cancer.246
8. Biological Approaches to Target Validation
The lack of selectivity for the population of regulatory T cells (Tregs) that infiltrate tumors (ti-Tregs) necessitates the need for a ti-Treg-specific biomarker, which causes the modulation and depletion strategies for these cells to have several undesirable side effects.247 Although antiprogrammed death (PD)-1 (aPD1) therapy is an effective treatment for metastatic melanoma, more than 50% of patients still progress. Researchers tested a first-of-its-kind immune-modulatory vaccine (IO102/IO103) against indoleamine-2,3-dioxygenase (IDO) and PD ligand 1 (PD-L1) to target immunosuppressive cells and tumor cells that express IDO and/or PD-L1. Nivolumab was also tested in amalgamation with the vaccine. The systemic toxicity profile was comparable to that of nivolumab monotherapy, which was the primary outcome, and the project was successful and safe. Efficacy and immunogenicity were secondary end points with 43% (CI, 27.4–60.8%) whole responses, and an objective response rate (ORR) of 80% (CI, 62.7–90.5%) was reached.248 To reduce toxicities during treatment, targeted therapy has replaced broad-based chemotherapy in current antitumor research initiatives, and new research demonstrates that changed cellular metabolism in tumor cells can be used as new targets for targeted intervention. The changed metabolic functions in cancerous cells and the changed glycosylation are because of their known effects on tumor tumorigenesis, metastasis, and drug resistance. Researchers believe that the enzymes necessary for the synthesis of UDP-hexoses, glycosyl donors for the synthesis of glycans, could be used as tumor therapeutic targets. A drug-like chemical fragment, GAL-012, was discovered via structure-based virtual screening and kinetic assays. It inhibits a small family of UDP-glucose pyrophosphorylase (UGP2), UDP-hexose pyrophosphorylases-galactose pyro-phosphorylase (GALT), and UDP-N-acetylglucosamine pyrophosphorylase (AGX1/UAP1) with an IC50 of 30 μM. The docking research confirmed that GAL-012 interacted with GALT binding sites at Ser192 and Trp190, UGP2 binding sites at Lys127 and Gly116, and UAP1/AGX1 at Lys407 and Asn327, respectively. GAL-012-2, one of the GAL-012 derivatives, also showed inhibitory activity against GALT and UGP2.249 Melanoma occurrence is still on the rise, and patients with metastatic melanoma still have poor prognoses. One of the main immune checkpoints is the cytotoxic T-lymphocyte antigen-4 (CTLA-4), which inhibits T cell activation pathways. A novel strategy to combat tumor-induced immune tolerance involves increasing T cell triggering by inhibiting CTLA-4 with an antibody. Early in 2011, the FDA approved ipilimumab after anti-CTLA-4 therapy recently demonstrated significant clinical benefits in patients with metastatic melanoma.250
9. Future Challenges of PDT
Compared to radiation therapy or surgery, PDT is less invasive and side effects are generally mild and do not last long. Based on the therapeutic protocol and PS, adverse effects related to PDT are respiratory insufficiency, anemia, constipation, pleural effusion, fever, edema, erythema, and photosensitivity.251 It is very challenging to use the photodynamic effect in disseminated metastases with the current technology because it only occurs selectively in the irradiated site.252 Oxygenation of tissue is important for the application of the photodynamic effect, therefore tumors surrounded by dense masses of tumor or necrotic tissue can result in inadequate PDT.253 When PDT is considered an option for diagnosis, the most crucial factor is the precision of target tissue irradiation. Because of the inadequate penetration of radiation into the tissue, it is difficult to treat deep tumors.252,254 PDT is a complex treatment that requires accurate planning, which can be done with the aid of computer programs or stimulation.255
10. Conclusion
SC is becoming a serious public health issue at present and its incidence is increasing worldwide. It occurs due to the involvement of various factors. UV exposure has the greatest impact on the risk of SC. UV rays (UVA and UVB) directly or indirectly damage the DNA and then produce oxidative stress, inflammation, mutation, and immunosuppression. The pathophysiology of SC is very diverse, and for its management, various ligands can be utilized for targeting, like antibodies, nanobodies, peptides, proteins, etc. According to various studies, natural drugs like galangin, phloretin, cantharidin, diosmetin, green tea polyphenols, and saffron are very effective for the treatment of SC at the early stages. Moreover, PDT is a noninvasive and modern type of therapy, utilized for the treatment of various types of cancer, tumor locations, and noncancerous ailments. However, there is a pressing need for effective therapy to cure SC, especially at the advanced stages.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Shaqra University, Saudi Arabia for supporting this work.
Author Contributions
Ø S.R.D. and M.K. contributed equally to this work. Participated in research design and supervision: N.M. and S.E.A. Data curation: N.M., M.K., S.E.A., M.A.M., S.D., and S.K. Writing–original draft preparation: M.A.M., M.K., S.D., and M.K.A. Writing–review and editing: A.F.A., D.M., M.A., and S.D.
The authors did not receive any funding.
The authors declare no competing financial interest.
References
- Kaleem M.; Perwaiz M.; Nur S. M.; Abdulrahman A. O.; Ahmad W.; Al-Abbasi F. A.; Kumar V.; Kamal M. A.; Anwar F. Epigenetics of Triple-Negative Breast Cancer via Natural Compounds. Curr. Med. Chem. 2022, 29 (8), 1436–1458. 10.2174/0929867328666210707165530. [DOI] [PubMed] [Google Scholar]
- Kaleem M.; Dalhat M. H.; Azmi L.; Asar T. O.; Ahmad W.; Alghanmi M.; Almostadi A.; Zughaibi T. A.; Tabrez S. An Insight into Molecular Targets of Breast Cancer Brain Metastasis. Int. J. Mol. Sci. 2022, 23 (19), 11687. 10.3390/ijms231911687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia D. N. Brief Review on COVID-19: The 2020 Pandemic Caused by SARS-CoV-2. Cureus. 2020, 12 (3), e7386 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbarzadeh Kaboli P.; Salimian F.; Aghapour S.; Xiang S.; Zhao Q.; Li M.; Wu X.; Du F.; Zhao Y.; Shen J.; Cho C. H.; Xiao Z. Akt-Targeted Therapy as a Promising Strategy to Overcome Drug Resistance in Breast Cancer – A Comprehensive Review from Chemotherapy to Immunotherapy. Pharmacol. Res. 2020, 156, 104806 10.1016/j.phrs.2020.104806. [DOI] [PubMed] [Google Scholar]
- Dhanyamraju P. K.; Patel T. N. Melanoma Therapeutics: A Literature Review. J. Biomed. Res. 2022, 36 (2), 77–97. 10.7555/JBR.36.20210163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan N.; Nadaf A.; Imran M.; Jiba U.; Sheikh A.; Almalki W. H.; Almujri S. S.; Mohammed Y. H.; Kesharwani P.; Ahmad F. J. Skin Cancer: Understanding the Journey of Transformation from Conventional to Advanced Treatment Approaches. Mol. Cancer 2023, 22 (1), 1–70. 10.1186/s12943-023-01854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller L.; Khammissa R. A. G.; Kramer B.; Altini M.; Lemmer J. Basal Cell Carcinoma, Squamous Cell Carcinoma and Melanoma of the Head and Face. Head Face Med. 2016, 12, 11. 10.1186/s13005-016-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M.; Lefevre J. G.; Baxter G.; Hamilton N. A. Non-Melanoma Skin Cancer Segmentation for Histopathology Dataset. Data Br. 2021, 39, 107587 10.1016/j.dib.2021.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijałkowska M.; Koziej M.; Antoszewski B. Detailed Head Localization and Incidence of Skin Cancers. Sci. Rep. 2021, 11 (1), 12391. 10.1038/s41598-021-91942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cives M.; Mannavola F.; Lospalluti L.; Sergi M. C.; Cazzato G.; Filoni E.; Cavallo F.; Giudice G.; Stucci L. S.; Porta C.; Tucci M. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21 (15), 5394. 10.3390/ijms21155394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.; Savas J.; Doerfler L. Nonsurgical Treatments for Nonmelanoma Skin Cancer. Dermatologic Clinics. 2019, 37 (4), 435–441. 10.1016/j.det.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Larese Filon F.; Buric M.; Fluehler C. UV Exposure, Preventive Habits, Risk Perception, and Occupation in NMSC Patients: A Case-Control Study in Trieste (NE Italy). Photodermatol. Photoimmunol. Photomed. 2019, 35 (1), 24–30. 10.1111/phpp.12417. [DOI] [PubMed] [Google Scholar]
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68 (6), 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cribier B.; Scrivener Y.; Grosshans E. Tumors arising in nevus sebaceus: A study of 596 cases. J. Am. Acad. Dermatol. 2000, 42 (2), 263–268. 10.1016/S0190-9622(00)90136-1. [DOI] [PubMed] [Google Scholar]
- Euvrard S.; Kanitakis J.; Claudy A. Skin Cancers after Organ Transplantation. N. Engl. J. Med. 2003, 348 (17), 1681–1691. 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- Silverberg M. J.; Leyden W.; Warton E. M.; Quesenberry C. P.; Engels E. A.; Asgari M. M. HIV Infection Status, Immunodeficiency, and the Incidence of Non-Melanoma Skin Cancer. J. Natl. Cancer Inst. 2013, 105 (5), 350–360. 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson L. J.; Borrowman T. A.; Vachon C. M.; Tollefson M. M.; Otley C. C.; Weaver A. L.; Roenigk R. K. Incidence of Basal Cell and Squamous Cell Carcinomas in a Population Younger than 40 Years. JAMA 2005, 294 (6), 681–690. 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- Okhovat J. P.; Beaulieu D.; Tsao H.; Halpern A. C.; Michaud D. S.; Shaykevich S.; Geller A. C. The First 30 Years of the American Academy of Dermatology Skin Cancer Screening Program: 1985–2014. J. Am. Acad. Dermatol. 2018, 79 (5), 884–891.e3. 10.1016/j.jaad.2018.05.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms P. W.; Harms K. L.; Moore P. S.; DeCaprio J. A.; Nghiem P.; Wong M. K. K.; Brownell I. The Biology and Treatment of Merkel Cell Carcinoma: Current Understanding and Research Priorities. Nat. Rev. Clin Oncol. 2018, 15 (12), 763–776. 10.1038/s41571-018-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuka A. G.; Book S. E. Basal Cell Carcinoma: Pathogenesis, Epidemiology, Clinical Features, Diagnosis, Histopathology, and Management. Yale J. Biol. Med. 2015, 88 (2), 167–179. [PMC free article] [PubMed] [Google Scholar]
- Moloney F. J.; Comber H.; Conlon P. J.; Murphy G. M. The Role of Immunosuppression in the Pathogenesis of Basal Cell Carcinoma. Br J. Dermatol. 2006, 154 (4), 790–791. 10.1111/j.1365-2133.2006.07156.x. [DOI] [PubMed] [Google Scholar]
- Wright C. Y.; du Preez D. J.; Millar D. A.; Norval M. The Epidemiology of Skin Cancer and Public Health Strategies for Its Prevention in Southern Africa. Int. J. Environ. Res. Public Health 2020, 17 (3), 1017. 10.3390/ijerph17031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román-Colón D.; Rabell-Bernal A.; Rodriguez-Franco G.; Santiago-Rivera L. S3661 A Blurry Surprise: A Case of Metastatic Melanoma. Am. J. Gastroenterol. 2021, 116 (1), e05879 10.14309/01.ajg.0000788176.61298.29. [DOI] [Google Scholar]
- Shetty A.; Janda M.; Fry K.; Brown S.; Yau B.; Schuckmann L.; Von; Thomas S.; Rayner J. E.; Spelman L.; Wagner G.; et al. Clinical Utility of Skin Cancer and Melanoma Risk Scores for Population Screening: TRoPICS Study. J. Eur. Acad. Dermatology Venereol. 2021, 35 (5), 1094–1098. 10.1111/jdv.17062. [DOI] [PubMed] [Google Scholar]
- Trager M. H.; Geskin L. J.; Samie F. H.; Liu L. Biomarkers in Melanoma and Non-Melanoma Skin Cancer Prevention and Risk Stratification. Exp. Dermatol. 2022, 31 (1), 4–12. 10.1111/exd.14114. [DOI] [PubMed] [Google Scholar]
- Thornton J.; Chhabra G.; Singh C. K.; Guzmán-Pérez G.; Shirley C. A.; Ahmad N. Mechanisms of Immunotherapy Resistance in Cutaneous Melanoma: Recognizing a Shapeshifter. Front. Oncol. 2022, 12, 880876 10.3389/fonc.2022.880876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seretis K.; Boptsi E.; Boptsi A.; Lykoudis E. G. The Impact of Treatment Delay on Skin Cancer in COVID-19 Era: A Case-Control Study. World J. Surg. Oncol. 2021, 19 (1), 350. 10.1186/s12957-021-02468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobre E. G.; Constantin C.; Neagu M. Skin Cancer Research Goes Digital: Looking for Biomarkers within the Droplets. J. Pers. Med. 2022, 12 (7), 1136. 10.3390/jpm12071136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kciuk M.; Marciniak B.; Mojzych M.; Kontek R. Focus on Uv-Induced Dna Damage and Repair—Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21 (19), 7264. 10.3390/ijms21197264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M.; Holman D. M.; Maguire-Eisen M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32 (3), 241–54. 10.1016/j.soncn.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bino S.; Duval C.; Bernerd F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19 (9), 2668. 10.3390/ijms19092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules. 2020, 25 (7), 1537. 10.3390/molecules25071537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppa M.; Gandini S. Sunbeds and Melanoma Risk: Time to Close the Debate. Curr. Opin. Oncol. 2019, 31 (2), 65–71. 10.1097/CCO.0000000000000507. [DOI] [PubMed] [Google Scholar]
- Gęgotek A.; Skrzydlewska E. The Role of ABC Transporters in Skin Cells Exposed to UV Radiation. Int. J. Mol. Sci. 2023, 24 (1), 115. 10.3390/ijms24010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehal K. S.; Bichakjian C. K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018, 379 (4), 363–374. 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]
- Huang J.; Zhang L.; Shi L.; Wu M.; Lv T.; Zhang Y.; Lai Y.; Tu Q.; Wang X.; Wang H. An Epidemiological Study on Skin Tumors of the Elderly in a Community in Shanghai, China. Sci. Rep. 2023, 13 (1), 4441. 10.1038/s41598-023-29012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milon A.; Bulliard J. L.; Vuilleumier L.; Danuser B.; Vernez D. Estimating the Contribution of Occupational Solar Ultraviolet Exposure to Skin Cancer. Br. J. Dermatol. 2014, 170 (1), 157–164. 10.1111/bjd.12604. [DOI] [PubMed] [Google Scholar]
- Dika E.; Scarfì F.; Ferracin M.; Broseghini E.; Marcelli E.; Bortolani B.; Campione E.; Riefolo M.; Ricci C.; Lambertini M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21 (15), 5572. 10.3390/ijms21155572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti A.; Sanna M.; Bataille V.; Falchi M. Genetics Plays a Role in Nevi Distribution in Women. Melanoma Manag. 2020, 7 (1), MMT35. 10.2217/mmt-2019-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S.; Fan W.; Rokohl A. C.; Guo Y.; Kakkassery V.; Heindl L. M. Genetic Factors Underlying Basal Cell Carcinoma Risk: A Narrative Review. Front. Oral Maxillofac. Med. 2023, 5, 1–12. 10.21037/fomm-21-70. [DOI] [Google Scholar]
- Parker E. R. The Influence of Climate Change on Skin Cancer Incidence – A Review of the Evidence. Int. J. Womens. Dermatol. 2021, 7 (1), 17–27. 10.1016/j.ijwd.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocholl M.; Ludewig M.; John S. M.; Bitzer E. M.; Wilke A. Outdoor Workers’ Perceptions of Skin Cancer Risk and Attitudes to Sun-Protective Measures: A Qualitative Study. J. Occup. Health 2020, 62 (1), e12083 10.1002/1348-9585.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keurentjes A. J.; Kezic S.; Rustemeyer T.; Hulshof C. T. J.; van der Molen H. F. Protection Against Solar Ultraviolet Radiation in Outdoor Construction Workers: Study Protocol for a Non-Randomized Controlled Intervention Study. Front. Public Heal. 2021, 9, 602933 10.3389/fpubh.2021.602933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu D.; Tsao H.; Michaud D. S.; Okhovat J. P.; Halpern A. C.; Geller A. C. Factors Associated with Suspected Nonmelanoma Skin Cancers, Dysplastic Nevus, and Cutaneous Melanoma among First-Time SpotMe Screening Program Participants during 2009–2010. J. Am. Acad. Dermatol. 2023, 88 (1), 60–70. 10.1016/j.jaad.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Wu S.; Cho E.; Li W. Q.; Weinstock M. A.; Han J.; Qureshi A. A. History of Severe Sunburn and Risk of Skin Cancer among Women and Men in 2 Prospective Cohort Studies. Am. J. Epidemiol. 2016, 183 (9), 824–833. 10.1093/aje/kwv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. H.; Mir M.; Qian L.; Baloch M.; Ali Khan M. F.; Rehman A. ur; Ngowi E. E.; Wu D. D.; Ji X. Y. Skin Cancer Biology and Barriers to Treatment: Recent Applications of Polymeric Micro/Nanostructures. J. Adv. Res. 2022, 36, 223–247. 10.1016/j.jare.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L.; Quinn A.; Stasko T. Skin Cancer and Immunosuppression. Dermatol. Clin. 2019, 37 (1), 83–94. 10.1016/j.det.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Yeung H.; Balakrishnan V.; Luk K. M. H.; Chen S. C. Risk of Skin Cancers in Older Persons Living with HIV: A Systematic Review. J. Assoc. Nurses. AIDS. Care. 2019, 30 (1), 80–86. 10.1097/JNC.0000000000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. Y.; Doiron P.; Maurer T. Cutaneous Malignancies in HIV. Curr. Opin HIV AIDS. 2017, 12 (1), 57–62. 10.1097/COH.0000000000000338. [DOI] [PubMed] [Google Scholar]
- Rollison D. E.; Viarisio D.; Amorrortu R. P.; Gheit T.; Tommasino M. An Emerging Issue in Oncogenic Virology: The Role of Beta Human Papillomavirus Types in the Development of Cutaneous Squamous Cell Carcinoma. J. Virol. 2019, 93 (7), e01003–18. 10.1128/JVI.01003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A. J.; Gonzalez A.; Clark E. S.; Khan W. N.; Rosen A. C.; Guzman W.; Rabinovitz H.; Badiavas E. V.; Kirsner R. S.; Ioannides T. Combined Systemic and Intratumoral Administration of Human Papillomavirus Vaccine to Treat Multiple Cutaneous Basaloid Squamous Cell Carcinomas. JAMA Dermatology 2018, 154 (8), 927–930. 10.1001/jamadermatol.2018.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekinis J.; Morley A. M. S. Ocular Surface Biopsies of Patients with Xeroderma Pigmentosum in the United Kingdom: A Retrospective Observational Case Series. Br. J. Ophthalmol. 2021, 105 (9), 1222–1230. 10.1136/bjophthalmol-2020-316125. [DOI] [PubMed] [Google Scholar]
- Lova Navarro M.; Vera Casaño Á.; Benito Lõpez C.; Fernández Ballesteros M. D.; Godoy Díaz D. J.; Crespo Erchiga A.; Romero Brufau S. Transient Neonatal Zinc Deficiency Due to a New Autosomal Dominant Mutation in Gene SLC30A2 (ZnT-2). Pediatr. Dermatol. 2014, 31 (2), 251–252. 10.1111/pde.12257. [DOI] [PubMed] [Google Scholar]
- Leachman S. A.; Lucero O. M.; Sampson J. E.; Cassidy P.; Bruno W.; Queirolo P.; Ghiorzo P. Identification, Genetic Testing, and Management of Hereditary Melanoma. Cancer Metastasis Rev. 2017, 36 (1), 77–90. 10.1007/s10555-017-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakumo Y.; Sakurai Y.; Kato T.; Hashimoto H.; Ichinoe M. REV7 in Cancer Biology and Management. Cancers (Basel). 2023, 15 (6), 1721. 10.3390/cancers15061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Conrad Waddington and the Origin of Epigenetics. J. Exp. Biol. 2015, 218 (6), 816–818. 10.1242/jeb.120071. [DOI] [PubMed] [Google Scholar]
- Wu S.; Zhang J.; Li F.; Du W.; Zhou X.; Wan M.; Fan Y.; Xu X.; Zhou X.; Zheng L.; Zhou Y. One-Carbon Metabolism Links Nutrition Intake to Embryonic Development via Epigenetic Mechanisms. Stem Cells Int. 2019, 2019, 3894101 10.1155/2019/3894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K.; Nakahara K.; Ito A.; Iijima Y.; Nomura R.; Kumar A.; Fujikawa K.; Adachi K.; Shimada Y.; Fujio S.; et al. Pivotal Role for S-Nitrosylation of DNA Methyltransferase 3B in Epigenetic Regulation of Tumorigenesis. Nat. Commun. 2023, 14 (1), 621. 10.1038/s41467-023-36232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadayifci F. Z.; Zheng S.; Pan Y. X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19 (12), 4055. 10.3390/ijms19124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J.; Kerr L.; Zhang Q.; Walker R. M.; Harris S. E.; Howard D. M.; Hawkins E. L.; Sandu A. L.; Steele J. D.; Waiter G. D.; et al. Local CpG Density Affects the Trajectory and Variance of Age-Associated DNA Methylation Changes. Genome Biol. 2022, 23 (1), 1–28. 10.1186/s13059-022-02787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soluri I. V; Zumerling L. M; Payan Parra O. A; Clark E. G; Blythe S. A Zygotic Pioneer Factor Activity of Odd-Paired/Zic Is Necessary for Late Function of the Drosophila Segmentation Network. Elife 2020, 9, e53916 10.7554/eLife.53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera A.; González-Avalos E.; Lio C. W. J.; Georges R. O.; Bellacosa A.; Nakayama T.; Rao A. Roles of TET and TDG in DNA Demethylation in Proliferating and Non-Proliferating Immune Cells. Genome Biol. 2021, 22 (1), 186. 10.1186/s13059-021-02384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan M. Pol β Gap Filling, DNA Ligation and Substrate-Product Channeling during Base Excision Repair Opposite Oxidized 5-Methylcytosine Modifications. DNA Repair (Amst). 2020, 95, 102945 10.1016/j.dnarep.2020.102945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. J.; Scheibe M.; Wongpalee S. P.; Liu W.; Cornett E. M.; Vaughan R. M.; Li X.; Chen W.; Xue Y.; Zhong Z.; et al. A DNA Methylation Reader Complex That Enhances Gene Transcription. Science. 2018, 362 (6419), 1182–1186. 10.1126/science.aar7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que S. K. T.; Zwald F. O.; Schmults C. D. Cutaneous Squamous Cell Carcinoma: Incidence, Risk Factors, Diagnosis, and Staging. J. Am. Acad. Dermatol. 2018, 78 (2), 237–247. 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- Azoulay E.; Russell L.; Van de Louw A.; Metaxa V.; Bauer P.; Povoa P.; Montero J. G.; Loeches I. M.; Mehta S.; Puxty K.; et al. Diagnosis of Severe Respiratory Infections in Immunocompromised Patients. Intensive Care Med. 2020, 46 (2), 298–314. 10.1007/s00134-019-05906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter O.; Voss V. B.; Fluss R.; Boyce L. M.; DeFazio J. L.; Halpern A. C.; Marghoob A. A. Skin Cancer Risk among Testicular Germ-Cell Cancer Survivors: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol Venereol. 2022, 36 (7), 1025–1033. 10.1111/jdv.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen K. B.; Pedersen S. A.; Schmidt S. A. J.; Pottegård A. Use of Antiepileptic Drugs and Risk of Skin Cancer: A Nationwide Case-Control Study. J. Am. Acad. Dermatol. 2020, 82 (2), 326–335. 10.1016/j.jaad.2019.05.055. [DOI] [PubMed] [Google Scholar]
- Bilski P. J.; Wolak M. A.; Zhang V.; Moore D. E.; Chignell C. F. Photochemical Reactions Involved in the Phototoxicity of the Anticonvulsant and Antidepressant Drug Lamotrigine (Lamictal®). Photochem. Photobiol. 2009, 85 (6), 1327–1335. 10.1111/j.1751-1097.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- Chen M. L.; Wang S. H.; Wei J. C. C.; Yip H. T.; Hung Y. M.; Chang R. The Impact of Human Papillomavirus Infection on Skin Cancer: A Population-Based Cohort Study. Oncologist 2021, 26 (3), e473–e483. 10.1002/onco.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Aldabagh B.; Yu J.; Arron S. T. Role of Human Papillomavirus in Cutaneous Squamous Cell Carcinoma: A Meta-Analysis. J. Am. Acad. Dermatol. 2014, 70 (4), 621–629. 10.1016/j.jaad.2014.01.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa A.; Mostafa A.; Navarini A. A.; Dong J. Y. The Association between Smoking and Risk of Skin Cancer: A Meta-Analysis of Cohort Studies. Cancer Causes Control 2020, 31 (8), 787–794. 10.1007/s10552-020-01319-8. [DOI] [PubMed] [Google Scholar]
- De Hertog S. A. E.; Wensveen C. A. H.; Bastiaens M. T.; Kielich C. J.; Berkhout M. J. P.; Westendorp R. G. J.; Vermeer B. J.; Bouwes Bavinck J. N. Relation between Smoking and Skin Cancer. J. Clin. Oncol. 2001, 19 (1), 231–238. 10.1200/JCO.2001.19.1.231. [DOI] [PubMed] [Google Scholar]
- Qiu F.; Liang C. L.; Liu H.; Zeng Y. Q.; Hou S.; Huang S.; Lai X.; Dai Z. Impacts of Cigarette Smoking on Immune Responsiveness: Up and down or Upside Down?. Oncotarget 2017, 8 (1), 268–284. 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong-Tieben L. M.; Berkhout R. J M; Vermeer B. J.; van der Woude F. J.; ter Schegget J.; Smits H. L.; Bouwes Bavinck J. N. High Frequency of Detection of Epidermodysplasia Verruciformis-Associated Human Papillomavirus DNA in Biopsies from Malignant and Premalignant Skin Lesions from Renal Transplant Recipients. J. Invest. Dermatol. 1995, 105 (3), 367–371. 10.1111/1523-1747.ep12320803. [DOI] [PubMed] [Google Scholar]
- Nieuwenburg S. A.; Adan F.; Ruijs M. W. G.; Sonke G. S.; van Leerdam M. E.; Crijns M. B. Cumulative Risk of Skin Cancer in Patients with Li-Fraumeni Syndrome. Fam. Cancer 2020, 19 (4), 347–351. 10.1007/s10689-020-00178-1. [DOI] [PubMed] [Google Scholar]
- D’Orazio J.; Jarrett S.; Amaro-Ortiz A.; Scott T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14 (6), 12222–12248. 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didona D.; Paolino G.; Bottoni U.; Cantisani C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines. 2018, 6 (1), 6. 10.3390/biomedicines6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailore N. N.; Sarojini B. K.; Harshitha K. R. Fabrication and Determination of the Sun Protection Factor and Ultraviolet Protection Factor for Piscean Collagen/Bischalcone Derivative (B1) Composite Films with Wide-Range UV Shielding. ACS Omega. 2022, 7 (32), 27876–27885. 10.1021/acsomega.2c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernerd F.; Passeron T.; Castiel I.; Marionnet C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23 (15), 8243. 10.3390/ijms23158243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand R. M.; Wipf P.; Durham A.; Epperly M. W.; Greenberger J. S.; Falo L. D. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front Pharmacol. 2018, 9, 920. 10.3389/fphar.2018.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. G.; Padron F.; Pfeifer G. P. UVA Radiation, DNA Damage, and Melanoma. ACS Omega. 2022, 7 (37), 32936–32948. 10.1021/acsomega.2c04424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emri G.; Paragh G.; Tósaki Á.; Janka E.; Kollár S.; Hegedűs C.; Gellén E.; Horkay I.; Koncz G.; Remenyik É. Ultraviolet Radiation-Mediated Development of Cutaneous Melanoma: An Update. J. Photochem. Photobiol. B 2018, 185, 169–175. 10.1016/j.jphotobiol.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Khan A. Q.; Travers J. B.; Kemp M. G. Roles of UVA Radiation and DNA Damage Responses in Melanoma Pathogenesis. Environ. Mol. Mutagen. 2018, 59 (5), 438–460. 10.1002/em.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Pons L.; Avila C.; Romano G.; Verde C.; Giordano D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Marine Drugs. 2018, 16 (9), 336. 10.3390/md16090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey B. J.; Kelly G. L.; Janic A.; Herold M. J.; Strasser A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression?. Cell Death Differ. 2018, 25 (1), 104–113. 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. L.; Zubair M.; John K.; Poirier M. C.; Martin F. L. Carcinogens and DNA Damage. Biochem. Soc. Trans. 2018, 46 (5), 1213–1224. 10.1042/BST20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. R.; Reindl K. M. Glutathione S-Transferases in Cancer. Antioxidants (Basel). 2021, 10 (5), 701. 10.3390/antiox10050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J.; Li G.; Yin J.; Li L.; Tan Y.; Wei H.; Liu B.; Deng L.; Tang J.; Chen Y.; Yi L. GSTP1 and Cancer: Expression, Methylation, Polymorphisms and Signaling (Review). Int. J. Oncol. 2020, 56 (4), 867–878. 10.3892/ijo.2020.4979. [DOI] [PubMed] [Google Scholar]
- Hagyousif Y. A.; Sharaf B. M.; Zenati R. A.; El-Huneidi W.; Bustanji Y.; Abu-Gharbieh E.; Alqudah M. A. Y.; Giddey A. D.; Abuhelwa A. Y.; Alzoubi K. H.; Soares N. C.; Semreen M. H. Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms. Int. J. Mol. Sci. 2023, 24 (2), 1604. 10.3390/ijms24021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. T.; Gray R. J.; Chen A. P.; Li S.; McShane L. M.; Patton D.; Hamilton S. R.; Williams P. M.; Iafrate A. J.; Sklar J.; et al. Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 2020, 38 (33), 3883–3894. 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman G.; Reed M. R.; Bielamowicz K.; Koss B.; Rodriguez A. CAR-T Therapies in Solid Tumors: Opportunities and Challenges. Curr. Oncol Rep. 2023, 25 (5), 479–489. 10.1007/s11912-023-01380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierlich P.; Mata A. I.; Donohoe C.; Brito R. M. M.; Senge M. O.; Gomes-da-Silva L. C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules. 2020, 25 (22), 5317. 10.3390/molecules25225317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L.; Li Y.; Xiong L.; Wang W.; Wu M.; Yuan T.; Yang W.; Tian C.; Miao Z.; Wang T.; Yang S. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct Target Ther. 2021, 6 (1), 201. 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J.; Całkosiński I.; Gamian A. Phage display--a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccin Immunother. 2012, 8 (12), 1817–1828. 10.4161/hv.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Z.; Sazgarnia A.; Rajabi O.; Seilanian Toosi M. Comparative Study of X-Ray Treatment and Photodynamic Therapy by Using 5-Aminolevulinic Acid Conjugated Gold Nanoparticles in a Melanoma Cell Line. Artif Cells Nanomed Biotechnol. 2017, 45 (3), 467–473. 10.3109/21691401.2016.1167697. [DOI] [PubMed] [Google Scholar]
- Gonçalves-Maia M.; Gache Y.; Basante M.; Cosson E.; Salavagione E.; Muller M.; Bernerd F.; Avril M. F.; Schaub S.; Sarasin A.; Braud V. M.; Magnaldo T. NK Cell and Fibroblast-Mediated Regulation of Skin Squamous Cell Carcinoma Invasion by CLEC2A Is Compromised in Xeroderma Pigmentosum. J. Invest. Dermatol. 2020, 140 (9), 1723–1732. 10.1016/j.jid.2020.01.021. [DOI] [PubMed] [Google Scholar]
- Pobłocki K.; Drzeżdżon J.; Kostrzewa T.; Jacewicz D. Coordination Complexes as a New Generation Photosensitizer for Photodynamic Anticancer Therapy. Int. J. Mol. Sci. 2021, 22 (15), 8052. 10.3390/ijms22158052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann F.; Karges J.; Gasser G. Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50 (11), 2727–2736. 10.1021/acs.accounts.7b00180. [DOI] [PubMed] [Google Scholar]
- Lou L.; Zhou S.; Tan S.; Xiang M.; Wang W.; Yuan C.; Gao L.; Xiao Q. Amplifying the Efficacy of ALA-Based Prodrugs for Photodynamic Therapy Using Nanotechnology. Front. Pharmacol. 2023, 14, 1137707 10.3389/fphar.2023.1137707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battah S.; Hider R. C.; MacRobert A. J.; Dobbin P. S.; Zhou T. Hydroxypyridinone and 5-Aminolaevulinic Acid Conjugates for Photodynamic Therapy. J. Med. Chem. 2017, 60 (8), 3498–3510. 10.1021/acs.jmedchem.7b00346. [DOI] [PubMed] [Google Scholar]
- Saha D.; Ryan K. R.; Lakkaniga N. R.; Acharya B.; Garcia N. G.; Smith E. L.; Frett B. Targeting Rearranged during Transfection in Cancer: A Perspective on Small-Molecule Inhibitors and Their Clinical Development. J. Med. Chem. 2021, 64 (16), 11747–11773. 10.1021/acs.jmedchem.0c02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C.; Huang S. C.; Wang Y. K.; Liu Y. T.; Wu T. K.; Chen T. M. Ligand-Functionalization of BPEI-Coated YVO4:Bi 3+,Eu3+ Nanophosphors for Tumor-Cell-Targeted Imaging Applications. Chem. - An Asian J. 2013, 8 (11), 2652–2659. 10.1002/asia.201300570. [DOI] [PubMed] [Google Scholar]
- Cunningham T. J.; Tabacchi M.; Eliane J. P.; Tuchayi S. M.; Manivasagam S.; Mirzaalian H.; Turkoz A.; Kopan R.; Schaffer A.; Saavedra A. P.; et al. Randomized Trial of Calcipotriol Combined with 5-Fluorouracil for Skin Cancer Precursor Immunotherapy. J. Clin. Invest. 2017, 127 (1), 106–116. 10.1172/JCI89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar D.; Raju R.; Kamal Y. T.; Salam S.; Kotta S.; Soman R. Porphyrinoid Photosensitizers for Targeted and Precise Photodynamic Therapy: Progress in Fabrication. Drug Formulation Design. 2023, 14–15. 10.5772/intechopen.109071. [DOI] [Google Scholar]
- Plekhova N.; Shevchenko O.; Korshunova O.; Stepanyugina A.; Tananaev I.; Apanasevich V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering. 2022, 9 (2), 82. 10.3390/bioengineering9020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz B.; Alexiou C.; Alvarez-Puebla R. A.; Alves F.; Andrews A. M.; Ashraf S.; Balogh L. P.; Ballerini L.; Bestetti A.; Brendel C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11 (3), 2313–2381. 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H.; Zhang T.; Qin S.; Huang Z.; Zhou L.; Shi J.; Nice E. C.; Xie N.; Huang C.; Shen Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15 (1), 132. 10.1186/s13045-022-01320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac-Lam M. F.; Hammonds D. M. Biotinylated Chlorin and Its Zinc and Indium Complexes: Synthesis and in Vitro Biological Evaluation for Photodynamic Therapy. Pharmaceuticals. 2017, 10 (2), 41. 10.3390/ph10020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woythe L.; Porciani D.; Harzing T.; van Veen S.; Burke D. H.; Albertazzi L. Valency and Affinity Control of Aptamer-Conjugated Nanoparticles for Selective Cancer Cell Targeting. J. Controlled Release 2023, 355, 228–237. 10.1016/j.jconrel.2023.01.008. [DOI] [PubMed] [Google Scholar]
- Manzari M. T.; Shamay Y.; Kiguchi H.; Rosen N.; Scaltriti M.; Heller D. A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6 (4), 351–370. 10.1038/s41578-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandland J.; Boyle R. W. Photosensitizer Antibody-Drug Conjugates: Past, Present, and Future. Bioconjugate Chem. 2019, 30 (4), 975–993. 10.1021/acs.bioconjchem.9b00055. [DOI] [PubMed] [Google Scholar]
- Yu L.; Wang Q.; Wong R. C. H.; Zhao S.; Ng D. K. P.; Lo P. C. Synthesis and Biological Evaluation of Phthalocyanine-Peptide Conjugate for EGFR-Targeted Photodynamic Therapy and Bioimaging. Dye. Pigment. 2019, 163, 197–203. 10.1016/j.dyepig.2018.11.055. [DOI] [Google Scholar]
- Díaz-Perlas C.; Varese M.; Guardiola S.; Sánchez-Navarro M.; García J.; Teixidó M.; Giralt E. Protein Chemical Synthesis Combined with Mirror-Image Phage Display Yields d-Peptide EGF Ligands That Block the EGF–EGFR Interaction. ChemBioChem. 2019, 20 (16), 2079–2084. 10.1002/cbic.201900355. [DOI] [PubMed] [Google Scholar]
- Mzabri I.; Addi M.; Berrichi A. Traditional and Modern Uses of Saffron (Crocus Sativus). Cosmetics. 2019, 6, 63. 10.3390/cosmetics6040063. [DOI] [Google Scholar]
- Wang C.-Z.; Ma Q.; Kim S.; Wang D. H.; Shoyama Y.; Yuan C.-S. Effects of Saffron and Its Active Constituent Crocin on Cancer Management: A Narrative Review. Longhua. Chin. Med. 2022, 5, 35–35. 10.21037/lcm-21-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Zhang B.; Wang Y.; Han S.; Wang C. Crocin Promotes Apoptosis of Human Skin Cancer Cells by Inhibiting the JAK/STAT Pathway. Exp. Ther. Med. 2018, 16 (6), 5079–5084. 10.3892/etm.2018.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas N Baba M. A. Nutraceutical Properties of the Green Tea Polyphenols. J. Food Process. Technol. 2014, 5 (11), 1. 10.4172/2157-7110.1000390. [DOI] [Google Scholar]
- Tewani K.; Monika M. Phytochemical Analysis, Anti-Oxidant Activity, Anti-Microbial Activity of Leaves of Camellia Sinensis (Theaceae Family) (White Tea). Int. J. Health Sci. (Qassim). 2022, 6 (S3), 5457–5469. 10.53730/ijhs.v6nS3.7147. [DOI] [Google Scholar]
- Miyata Y.; Shida Y.; Hakariya T.; Sakai H. Anti-Cancer Effects of Green Tea Polyphenols against Prostate Cancer. Molecules. 2019, 24 (1), 193. 10.3390/molecules24010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal V.; Agarwal A.; Harbour A.; Farooqi H.; Singh V. K.; Prasad R. Regular Intake of Green Tea Polyphenols Suppresses the Development of Nonmelanoma Skin Cancer through MiR-29-Mediated Epigenetic Modifications. J. Clin. Med. 2022, 11 (2), 398. 10.3390/jcm11020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca D.; Mandalari G.; Calderaro A.; Smeriglio A.; Trombetta D.; Felice M. R.; Gattuso G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants. 2020, 9 (3), 288. 10.3390/plants9030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.; Lee D. H.; Park S. Y.; Seol J. W. Diosmetin Inhibits Tumor Development and Block Tumor Angiogenesis in Skin Cancer. Biomed. Pharmacother. 2019, 117, 109091 10.1016/j.biopha.2019.109091. [DOI] [PubMed] [Google Scholar]
- Meephat S.; Prasatthong P.; Potue P.; Bunbupha S.; Pakdeechote P.; Maneesai P. Diosmetin Ameliorates Vascular Dysfunction and Remodeling by Modulation of Nrf2/Ho-1 and p-Jnk/p-Nf-Kb Expression in Hypertensive Rats. Antioxidants. 2021, 10 (9), 1487. 10.3390/antiox10091487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Ma S.; Li L.; Chen Y.; Yang Q.; Wang F.; Zheng M.; Miao S.; Shi X. Investigation into the in Vivo Mechanism of Diosmetin in Patients with Breast Cancer and COVID-19 Using Bioinformatics. Front. Pharmacol. 2022, 13, 983821 10.3389/fphar.2022.983821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman D. W.; Fe Andrés M.; Martínez-Díaz R. A.; Ibáñez-Escribano A.; Sonia Olmeda A.; González-Coloma A. Antiparasitic Properties of Cantharidin and the Blister Beetle Berberomeloe Majalis (Coleoptera: Meloidae). Toxins (Basel). 2019, 11 (4), 234. 10.3390/toxins11040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.; Huang N.; Wei J. Hepatotoxic Mechanism of Cantharidin : Insights and Strategies for Therapeutic Intervention. Front. Pharmacol. 2023, 14, 1201404 10.3389/fphar.2023.1201404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.-P.; Tsai C.-H.; Wu P.-P.; Hsu S.-C.; Liu H.-C.; Huang Y.-P.; Yang J.-H.; Chung J.-G. Cantharidin Impairs Cell Migration and Invasion of A375.S2 Human Melanoma Cells by Suppressing MMP-2 and −9 through PI3K/NF-KB Signaling Pathways. Anticancer Res. 2014, 35 (2), 729–738. [PubMed] [Google Scholar]
- Kumar V. S.; Navaratnam V. Neem (Azadirachta Indica): Prehistory to Contemporary Medicinal Uses to Humankind. Asian Pac. J. Trop. Biomed. 2013, 3 (7), 505–514. 10.1016/S2221-1691(13)60105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.; Gonzales C. B.; De La Chapa J. J.; Cabang A. B.; Fountzilas C.; Patel M.; Orozco S.; Wargovich M. J. The Highly Pure Neem Leaf Extract, SCNE, Inhibits Tumorigenesis in Oral Squamous Cell Carcinoma via Disruption of Pro-Tumor Inflammatory Cytokines and Cell Signaling. Front. Oncol. 2019, 9, 890. 10.3389/fonc.2019.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniqa A.; Kaur S.; Sadwal S. A Review on the Pathogenesis of Cutaneous Non-Melanoma Skin Cancer (NMSC) and Selected Herbs as Chemoprotective Agents. Int. J. Plant Based Pharm. 2023, 3 (3), 18–31. 10.29228/ijpbp.12. [DOI] [Google Scholar]
- Cerri M.; Reale L.; Zadra C. Metabolite Storage in Theobroma Cacao L. Seed: Cyto-Histological and Phytochemical Analyses. Front. Plant Sci. 2019, 10, n/a. 10.3389/fpls.2019.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osukoya O.; Fadaka A.; Adewale O.; Oluloye O.; Ojo O.; Ajiboye B.; Adewumi D.; Kuku A. In Vitro Anthelmintic and Antioxidant Activities of the Leaf Extracts of Theobroma Cacao L. AIMS Agric. Food. 2019, 4 (3), 568–577. 10.3934/agrfood.2019.3.568. [DOI] [Google Scholar]
- Mello G. H. d.; D'Avila C. M. d. S.; Viana A. R.; Krause L. M. F.; Cadona F. C. Cocoa Presents Cytotoxicity against Melanoma Cancer Cell Lines (A-375 e B16-F10) and Improves Chemotherapy Activity by Increasing Oxidative Stress. J. Food Biochem. 2022, 46 (12), e14512 10.1111/jfbc.14512. [DOI] [PubMed] [Google Scholar]
- Imran M.; Saeed F.; Hussain G.; Imran A.; Mehmood Z.; Gondal T. A.; El-Ghorab A.; Ahmad I.; Pezzani R.; Arshad M. U.; Bacha U.; Shariarti M. A.; Rauf A.; Muhammad N.; Shah Z. A.; Zengin G.; Islam S. Myricetin: A Comprehensive Review on Its Biological Potentials. Food. Sci. Nutr. 2021, 9 (10), 5854–5868. 10.1002/fsn3.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afroze N.; Pramodh S.; Hussain A.; Waleed M.; Vakharia K. A Review on Myricetin as a Potential Therapeutic Candidate for Cancer Prevention. 3 Biotechnol. 2020, 10 (5), 211. 10.1007/s13205-020-02207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Mendoza N.; Madrigal-Santillán E.; Morales-González Á.; Esquivel-Soto J.; Esquivel-Chirino C.; García-Luna y González-Rubio M. G.; Gayosso-de-Lucio J. A.; Morales-González J. A. Hepatoprotective Effect of Silymarin. World. Hepatol. 2014, 6 (3), 144–149. 10.4254/wjh.v6.i3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. Y.; Yen H.; Hsiao H. Y.; Su S. C. Phytochemicals in Skin Cancer Prevention and Treatment: An Updated Review. Int. J. Mol. Sci. 2018, 19 (4), 941. 10.3390/ijms19040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushki M.; Farrokhi Yekta R.; Amiri-Dashatan N. Critical Review of Therapeutic Potential of Silymarin in Cancer: A Bioactive Polyphenolic Flavonoid. J. Funct. Foods 2023, 104, 105502 10.1016/j.jff.2023.105502. [DOI] [Google Scholar]
- Prasad R. R.; Paudel S.; Raina K.; Agarwal R. Silibinin and Non-Melanoma Skin Cancers. J. Tradit. Complement. Med. 2020, 10 (3), 236–244. 10.1016/j.jtcme.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett D. L.; Alquraishi M.; Alani D.; Chahed S.; Donohoe D.; Voy B.; Whelan J.; Bettaieb A. Zyflamend Induces Apoptosis in Pancreatic Cancer Cells via Modulation of the JNK Pathway. Cell Commun. Signal. 2020, 18 (1), 126. 10.1186/s12964-020-00609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-caballero M.; Torres-vargas J. A.; Marrero A. D.; Martínez-poveda B.; Medina M. Á.; Quesada A. R. Angioprevention of Urologic Cancers by Plant-Derived Foods. Pharmaceutics. 2022, 14 (2), 256. 10.3390/pharmaceutics14020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekmekcioglu S.; Chattopadhyay C.; Akar U.; Gabisi A.; Newman R. A.; Grimm E. A. Zyflamend Mediates Therapeutic Induction of Autophagy to Apoptosis in Melanoma Cells. Nutr. Cancer. 2011, 63 (6), 940–949. 10.1080/01635581.2011.586488. [DOI] [PubMed] [Google Scholar]
- Prasad R.; Prasad S. B. A Review on the Chemistry and Biological Properties of Rutin, a Promising Nutraceutical Agent. Asian J. Pharm. Pharmacol. 2019, 5 (S1), 1–20. 10.31024/ajpp.2019.5.s1.1. [DOI] [Google Scholar]
- Pandey P.; Khan F.; Qari H. A.; Oves M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals. 2021, 14 (11), 1069. 10.3390/ph14111069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negahdari R.; Bohlouli S.; Sharifi S.; Maleki Dizaj S.; Rahbar Saadat Y.; Khezri K.; Jafari S.; Ahmadian E.; Gorbani Jahandizi N.; Raeesi S. Therapeutic Benefits of Rutin and Its Nanoformulations. Phytotherapy Research. 2021, 35 (4), 1719–38. 10.1002/ptr.6904. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Sikdar S.; Mukherjee A.; Khuda-Bukhsh A. R. Evaluation of Chemopreventive Potentials of Ethanolic Extract of Ruta Graveolens against A375 Skin Melanoma Cells in Vitro and Induced Skin Cancer in Mice in Vivo. J. Integr. Med. 2015, 13 (1), 34–44. 10.1016/S2095-4964(15)60156-X. [DOI] [PubMed] [Google Scholar]
- Ramalingam M.; Kim H.; Lee Y.; Lee Y. Il. Phytochemical and Pharmacological Role of Liquiritigenin and Isoliquiritigenin From Radix Glycyrrhizae in Human Health and Disease Models. Front. Aging. Neurosci. 2018, 10, 348. 10.3389/fnagi.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.; Yang L.; Deng X. N.; Sun Y. N. Effects of Isoliquiritigenin on Ovarian Cancer Cells. Onco. Targets. Ther. 2018, 11, 1633–1642. 10.2147/OTT.S149295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S.; Chen H.; Luo X.; An B.; Wu W.; Cao S.; Ruan S.; Wang Z.; Weng L.; Zhu H.; Liu Q. Isoliquiritigenin Suppresses Human Melanoma Growth by Targeting MiR-301b/LRIG1 Signaling. J. Exp. Clin. Cancer Res. 2018, 37 (1), 184. 10.1186/s13046-018-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli H. S.; Rath P.; Chauhan A.; Ramniwas S.; Vashishth K.; Varol M.; Jaswal V. S.; Haque S.; Sak K. Phloretin, as a Potent Anticancer Compound: From Chemistry to Cellular Interactions. Molecules 2022, 27 (24), 8819. 10.3390/molecules27248819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhate K. T.; Badwaik H.; Choudhary R.; Sakure K.; Agrawal Y. O.; Sharma C.; Ojha S.; Goyal S. N. Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients. 2022, 14 (17), 3638. 10.3390/nu14173638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B. Y. Biochemical Basis of Anti-Cancer-Effects of Phloretin—a Natural Dihydrochalcone. Molecules. 2019, 24 (2), 278. 10.3390/molecules24020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim A.; Hanrahan J. Biologically Active Compounds from Lamiaceae Family: Central Nervous System Effects. Studies in Natural Products Chemistry 2021, 68, 255–315. 10.1016/B978-0-12-819485-0.00017-7. [DOI] [Google Scholar]
- Xu Y.; Li X.; Wang H. Protective Roles of Apigenin Against Cardiometabolic Diseases: A Systematic Review. Front. Nutr. 2022, 9, 875826 10.3389/fnut.2022.875826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed Z.; Sadia H.; Iqbal M. J.; Shamas S.; Malik K.; Ahmed R.; Raza S.; Butnariu M.; Cruz-Martins N.; Sharifi-Rad J. Apigenin Role as Cell-Signaling Pathways Modulator: Implications in Cancer Prevention and Treatment. Cancer. Cell. Int. 2021, 21 (1), 189. 10.1186/s12935-021-01888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzoeva S.; Tong X.; Bridgeman B. B.; Plebanek M. P.; Volpert O. V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia (United States). 2018, 20 (9), 930–942. 10.1016/j.neo.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelić D.; Lower-Nedza A. D.; Brantner A. H.; Blažeković B.; Bian B.; Yang J.; Brajša K.; Vladimir-Knežević S. Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J. Chem. 2016, 2016, 2510621 10.1155/2016/2510621. [DOI] [Google Scholar]
- Mu J.; Liu T.; Jiang L.; Wu X.; Cao Y.; Li M.; Dong Q.; Liu Y.; Xu H. The Traditional Chinese Medicine Baicalein Potently Inhibits Gastric Cancer Cells. J. Cancer 2016, 7 (4), 453–461. 10.7150/jca.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E. O.; Cho E. J.; Jeong J. W.; Park C.; Hong S. H.; Hwang H. J.; Moon S. K.; Son C. G.; Kim W. J.; Choi Y. H. Baicalein Inhibits the Migration and Invasion of B16F10 Mouse Melanoma Cells through Inactivation of the Pi3K/Akt Signaling Pathway. Biomol. Ther. 2017, 25 (2), 213–221. 10.4062/biomolther.2016.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.; Hu X.; Xing Z.; Xing R.; Lv R.; Cheng X.; Su J.; Zhou Z.; Xu Z.; Nilsson S.; Liu Z. Baicalein Inhibits Prostate Cancer Cell Growth and Metastasis via the Caveolin-1/AKT/MTOR Pathway. Mol. Cell. Biochem. 2015, 406 (1–2), 111–119. 10.1007/s11010-015-2429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleem M.; Kayali A.; Sheikh R. A.; Kuerban A.; Hassan M. A.; Almalki N. A. R.; Al-abbasi F. A.; Anwar F.; Omran Z.; Alhosin M. In Vitro and In Vivo Preventive Effects of Thymoquinone against Breast Cancer : Role of DNMT1. Molecules. 2024, 29 (2), 434. 10.3390/molecules29020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A.; Alhosin M.; Papin C.; Ouararhni K.; Omran Z.; Zamzami M. A.; Al-Malki A. L.; Choudhry H.; Mély Y.; Hamiche A.; Mousli M.; Bronner C. Thymoquinone Challenges UHRF1 to Commit Auto-Ubiquitination: A Key Event for Apoptosis Induction in Cancer Cells. Oncotarget. 2018, 9 (47), 28599–28611. 10.18632/oncotarget.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah O.; Omran Z.; Hosawi S.; Hamiche A.; Bronner C.; Alhosin M. Thymoquinone Is a Multitarget Single Epidrug That Inhibits the UHRF1 Protein Complex. Genes. 2021, 12 (5), 622. 10.3390/genes12050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H.; Yu S. M.; Kim S. J. Inhibitory Effects on Melanogenesis by Thymoquinone Are Mediated through the β-Catenin Pathway in B16F10 Mouse Melanoma Cells. Int. J. Oncol. 2019, 56 (1), 379–389. 10.3892/ijo.2019.4930. [DOI] [PubMed] [Google Scholar]
- Kłos P.; Perużyńska M.; Baśkiewicz-Hałasa M.; Skupin-Mrugalska P.; Majcher M.; Sawczuk M.; Szostak B.; Droździk M.; Machaliński B.; Chlubek D. Response of Skin-Derived and Metastatic Human Malignant Melanoma Cell Lines to Thymoquinone and Thymoquinone-Loaded Liposomes. Pharmaceutics. 2022, 14 (11), 2309. 10.3390/pharmaceutics14112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S.; Matsuda H.; Oda Y.; Nakamura S.; Xu F.; Yoshikawa M. Melanogenesis Inhibitors from the Desert Plant Anastatica Hierochuntica in B16 Melanoma Cells. Bioorg. Med. Chem. 2010, 18 (6), 2337–2345. 10.1016/j.bmc.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Tang X.; Wang H.; Fan L.; Wu X.; Xin A.; Ren H.; Wang X. J. Luteolin Inhibits Nrf2 Leading to Negative Regulation of the Nrf2/ARE Pathway and Sensitization of Human Lung Carcinoma A549 Cells to Therapeutic Drugs. Free Radic. Biol. Med. 2011, 50 (11), 1599–1609. 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Lin Y. S.; Tsai P. H.; Kandaswami C. C.; Cheng C. H.; Ke F. C.; Lee P. P.; Hwang J. J.; Lee M. T. Effects of Dietary Flavonoids, Luteolin, and Quercetin on the Reversal of Epithelial-Mesenchymal Transition in A431 Epidermal Cancer Cells. Cancer Sci. 2011, 102 (10), 1829–1839. 10.1111/j.1349-7006.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- Ruan J. S.; Liu Y. P.; Zhang L.; Yan L. G.; Fan F. T.; Shen C. S.; Wang A. Y.; Zheng S. Z.; Wang S. M.; Lu Y. Luteolin Reduces the Invasive Potential of Malignant Melanoma Cells by Targeting B3 Integrin and the Epithelial-Mesenchymal Transition. Acta Pharmacol. Sin. 2012, 33 (10), 1325–1331. 10.1038/aps.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid J.; Shaito A.; El Hajj H.; Deleuze-masquefa C.; Bonnet P.-A.; El-Sabban M.; Saliba J. Biological Applications of Imiquimod Analogues: An Update (Review). World Acad. Sci. J. 2023, 5 (3), 1–11. 10.3892/wasj.2023.197. [DOI] [Google Scholar]
- Chuang K. C.; Chang C. R.; Chang S. H.; Huang S. W.; Chuang S. M.; Li Z. Y.; Wang S. T.; Kao J. K.; Chen Y. J.; Shieh J. J. Imiquimod-Induced ROS Production Disrupts the Balance of Mitochondrial Dynamics and Increases Mitophagy in Skin Cancer Cells. J. Dermatol. Sci. 2020, 98 (3), 152–162. 10.1016/j.jdermsci.2020.03.009. [DOI] [PubMed] [Google Scholar]
- Ding W. X.; Yin X. M. Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 2012, 393 (7), 547–564. 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellati F.; Borgonetti V.; Brighenti V.; Biagi M.; Benvenuti S.; Corsi L. Cannabis Sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed. Research International. 2018, 2018, 1691428 10.1155/2018/1691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specchio N.; Pietrafusa N.; Cross H. J. Source of Cannabinoids: What Is Available, What Is Used, and Where Does It Come From?. Epileptic Disorders. 2020, 22 (S1), 1–9. [DOI] [PubMed] [Google Scholar]
- Moreno E.; Andradas C.; Medrano M.; Caffarel M. M.; Pérez-Gómez E.; Blasco-Benito S.; Gómez-Cañas M.; Pazos M. R.; Irving A. J.; Lluís C.; et al. Targeting CB2-GPR55 Receptor Heteromers Modulates Cancer Cell Signaling. J. Biol. Chem. 2014, 289 (32), 21960–21972. 10.1074/jbc.M114.561761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milando R.; Friedman A. Cannabinoids: Potential Role in Inflammatory and Neoplastic Skin Diseases. Am. J. Clin. Dermatol. 2019, 20 (2), 167–180. 10.1007/s40257-018-0410-5. [DOI] [PubMed] [Google Scholar]
- Ma X.; Qu Q.; Zhao Y. Targeted Delivery of 5-Aminolevulinic Acid by Multifunctional Hollow Mesoporous Silica Nanoparticles for Photodynamic Skin Cancer Therapy. ACS Appl. Mater. Interfaces. 2015, 7 (20), 10671–10676. 10.1021/acsami.5b03087. [DOI] [PubMed] [Google Scholar]
- Pérez-Sánchez A.; Barrajón-Catalán E.; Ruiz-Torres V.; Agulló-Chazarra L.; Herranz-López M.; Valdés A.; Cifuentes A.; Micol V. Rosemary (Rosmarinus Officinalis) Extract Causes ROS-Induced Necrotic Cell Death and Inhibits Tumor Growth in Vivo. Sci. Rep. 2019, 9 (1), 808. 10.1038/s41598-018-37173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallimanis P.; Chinou I.; Panagiotopoulou A.; Soshilov A. A.; He G.; Denison M. S.; Magiatis P. Rosmarinus Officinalis L. Leaf Extracts and Their Metabolites Inhibit the Aryl Hydrocarbon Receptor (AhR) Activation In Vitro and in Human Keratinocytes: Potential Impact on Inflammatory Skin Diseases and Skin Cancer. Molecules. 2022, 27 (8), 1–19. 10.3390/molecules27082499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduchova A.; Anzenbacher P.; Anzenbacherova E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food. 2019, 22 (2), 121–126. 10.1089/jmf.2018.0024. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lu Q.; Li N.; Xu M.; Miyamoto T.; Liu J. Sulforaphane Suppresses Metastasis of Triple-Negative Breast Cancer Cells by Targeting the RAF/MEK/ERK Pathway. npj Breast Cancer 2022, 8 (1), 40. 10.1038/s41523-022-00402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Yang I.; Cao M.; Su Z.-y.; Wu R.; Guo Y.; Fang M.; Kong A.-N. Fucoxanthin Elicits Epigenetic Modifications, Nrf2 Activation and Blocking Transformation in Mouse Skin JB6 P+ Cells. AAPS J. 2018, 20 (2), 32. 10.1208/s12248-018-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S.; Aggarwal B. B.. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie I. F. F., Wachtel-Galor S, Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, 2011. https://www.ncbi.nlm.nih.gov/books/NBK92752/. [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Hegde M.; Parama D.; Girisa S.; Kumar A.; Daimary U. D.; Garodia P.; Yenisetti S. C.; Oommen O. V.; Aggarwal B. B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacology and Translational Science. 2023, 6 (4), 447–518. 10.1021/acsptsci.2c00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A.; Tommonaro G. Curcumin and Cancer. Nutrients. 2019, 11 (10), 2376. 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacescu E.; Chiroi P.; Zanoaga O.; Nutu A.; Budisan L.; Pirlog R.; Atanasov A. G.; Berindan-Neagoe I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants. 2023, 12 (2), 407. 10.3390/antiox12020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle D. G.; Ferreira D.; Zhou Y. D. Epigallocatechin-3-Gallate (EGCG): Chemical and Biomedical Perspectives. Phytochemistry. 2006, 67 (17), 1849–1855. 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alserihi R. F.; Mohammed M. R. S.; Kaleem M.; Imran Khan M.; Sechi M.; Zughaibi T. A.; Tabrez S. Comparative Efficacy of Epigallocatechin Gallate and Its Nano-Formulation in Prostate Cancer 3D Spheroids Model. J. King Saud Univ. - Sci. 2023, 35 (4), 102627 10.1016/j.jksus.2023.102627. [DOI] [Google Scholar]
- Alserihi R. F.; Mohammed M. R. S.; Kaleem M.; Khan M. I.; Sechi M.; Sanna V.; Zughaibi T. A.; Abuzenadah A. M.; Tabrez S. Development of (−)-Epigallocatechin-3-Gallate-Loaded Folate Receptor-Targeted Nanoparticles for Prostate Cancer Treatment. Nanotechnol. Rev. 2022, 11 (1), 298–311. 10.1515/ntrev-2022-0013. [DOI] [Google Scholar]
- Agarwal A.; Kansal V.; Farooqi H.; Prasad R.; Singh V. K. Epigallocatechin Gallate (EGCG), an Active Phenolic Compound of Green Tea, Inhibits Tumor Growth of Head and Neck Cancer Cells by Targeting DNA Hypermethylation. Biomedicines 2023, 11 (3), 789. 10.3390/biomedicines11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penta D.; Somashekar B. S.; Meeran S. M. Epigenetics of Skin Cancer: Interventions by Selected Bioactive Phytochemicals. Photodermatol. Photoimmunol. Photomed. 2018, 34 (1), 42–49. 10.1111/phpp.12353. [DOI] [PubMed] [Google Scholar]
- Ghosh B.; Majumder S.; Acharyya S.; Ghosh A.; Saha S.; Sarkar S.; Chakraborty S.; Bhattacharya M. Comparative Phytochemical Analysis of Mature Mango Leaves from Nineteen Cultivars of Murshidabad District, India. Asian J. Nat. Prod. Biochem. 2022, 20 (2), 48–55. 10.13057/biofar/f200202. [DOI] [Google Scholar]
- Garcia-Partida J. A.; Torres-Sanchez S.; MacDowell K.; Fernández-Ponce M. T.; Casas L.; Mantell C.; Soto-Montenegro M. L.; Romero-Miguel D.; Lamanna-Rama N.; Leza J. C.; Desco M.; Berrocoso E. The Effects of Mango Leaf Extract during Adolescence and Adulthood in a Rat Model of Schizophrenia. Front. Pharmacol. 2022, 13, 886514 10.3389/fphar.2022.886514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M.; Arshad M. S.; Butt M. S.; Kwon J. H.; Arshad M. U.; Sultan M. T. Mangiferin: A Natural Miracle Bioactive Compound against Lifestyle Related Disorders. Lipids in Health and Disease. 2017, 16 (1), 84. 10.1186/s12944-017-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccioloni M.; Bonfili L.; Mozzicafreddo M.; Cecarini V.; Scuri S.; Cocchioni M.; Nabissi M.; Santoni G.; Eleuteri A. M.; Angeletti M. Mangiferin Blocks Proliferation and Induces Apoptosis of Breast Cancer Cells: Via Suppression of the Mevalonate Pathway and by Proteasome Inhibition. Food Funct. 2016, 7 (10), 4299–4309. 10.1039/C6FO01037G. [DOI] [PubMed] [Google Scholar]
- Ayuni Lubakisar Y. Q.; Fauziah F.; Bellatasie R. Immunomodulator Activity Of Mangiferin From Mango (Mangifera Indica L.) In Cancer: A Systematic Review. Int. J. Pharm. Pharm. Sci. 2021, 13 (10), 7–11. 10.22159/ijpps.2021v13i10.42095. [DOI] [Google Scholar]
- Delgado-Hernandez R.; Hernandez-Balmaseda I.; Rodeiro-Guerra I.; Cesar Rodriguez Gonzalez J.; De Wever O.; Logie E.; Declerck K.; Perez-Novo C.; Vanden Berghe W. Anti-Angiogenic Effects of Mangiferin and Mechanism of Action in Metastatic Melanoma. Melanoma Res. 2020, 30 (1), 39–51. 10.1097/CMR.0000000000000647. [DOI] [PubMed] [Google Scholar]
- Shoaib S.; Ansari M. A.; Ghazwani M.; Hani U.; Jamous Y. F.; Alali Z.; Wahab S.; Ahmad W.; Weir S. A.; Alomary M. N.; Yusuf N.; Islam N. Prospective Epigenetic Actions of Organo-Sulfur Compounds against Cancer: Perspectives and Molecular Mechanisms. Cancers. 2023, 15 (3), 697. 10.3390/cancers15030697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A. Cancer Classification at the Crossroads. Cancers (Basel). 2020, 12 (4), 980. 10.3390/cancers12040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski S.; Knap B.; Przystupski D.; Saczko J.; Kędzierska E.; Knap-Czop K.; Kotlińska J.; Michel O.; Kotowski K.; Kulbacka J. Photodynamic Therapy – Mechanisms, Photosensitizers and Combinations. Biomed Pharmacother. 2018, 106, 1098–1107. 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- Curnow A.; AJ M. Enhancing Protoporphyrin IX Induced Photodynamic Therapy with a Topical Iron Chelating Agent in a Normal Skin Model. J. Heavy Met. Toxic. Dis. 2016, 1 (1), 1–9. 10.21767/2473-6457.100002. [DOI] [Google Scholar]
- Maiuri M. C.; Zalckvar E.; Kimchi A.; Kroemer G. Self-Eating and Self-Killing: Crosstalk between Autophagy and Apoptosis. Nat. Rev. Mol. Cell. Biol. 2007, 8 (9), 741–752. 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Robertson C. A.; Evans D. H.; Abrahamse H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol., B 2009, 96 (1), 1–8. 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kamal A.; Faazil S.; Malik M. S. Apoptosis-Inducing Agents: A Patent Review (2010–2013). Expert Opinion on Therapeutic Patents. 2014, 24 (3), 339–354. 10.1517/13543776.2014.877445. [DOI] [PubMed] [Google Scholar]
- Mroz P.; Yaroslavsky A.; Kharkwal G. B.; Hamblin M. R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers. 2011, 3 (2), 2516–2539. 10.3390/cancers3022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo C.; Kruger C. A.; Abrahamse H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. 10.1177/1533033818791795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Lima S.; Gajate C.; Mollinedo F. Triggers and Signaling Cross-Talk Controlling Cell Death Commitment. Cell Cycle. 2015, 14 (4), 465–466. 10.1080/15384101.2015.1006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redza-Dutordoir M.; Averill-Bates D. A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochimica et Biophysica Acta - Molecular Cell Research. 2016, 1863 (12), 2977–2992. 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Holohan C.; Van Schaeybroeck S.; Longley D. B.; Johnston P. G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer. 2013, 13 (10), 714–726. 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- Kono H.; Kimura Y.; Latz E. Inflammasome Activation in Response to Dead Cells and Their Metabolites. Current Opinion in Immunology. 2014, 30, 91–98. 10.1016/j.coi.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Belizário J.; Vieira-Cordeiro L.; Enns S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediators. Inflamm. 2015, 2015, 128076 10.1155/2015/128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y.; Chang T. W.; Hsieh W. H.; Hung M. C.; Lin I. H.; Lai S. C.; Tzeng Y. J. Simultaneous Induction of Apoptosis and Necroptosis by Tanshinone IIA in Human Hepatocellular Carcinoma HepG2 Cells. Cell. Death. Discovery 2016, 2, 16065. 10.1038/cddiscovery.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano A. P.; Demidova T. N.; Hamblin M. R. Mechanisms in Photodynamic Therapy: Part Two - Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagnosis and Photodynamic Therapy. 2005, 2 (1), 1–23. 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X.; Li Y.; Hamblin M. R. Photodynamic Therapy in Dermatology beyond Non-Melanoma Cancer: An Update. Photodiagnosis Photodyn Ther. 2017, 19, 140–152. 10.1016/j.pdpdt.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky R. L.; Bartenstein D. W.; Rogers G. S.; Isakoff S. J.; Chen S. T. Photodynamic Therapy for Solid Tumors: A Review of the Literature. Photodermatol. Photoimmunol. Photomed. 2019, 35 (5), 295–303. 10.1111/phpp.12489. [DOI] [PubMed] [Google Scholar]
- Golab J.; Agostinis P.; Berg K.; Cengel K. A.; Foster T. H.; Girotti A. W.; Gollnick S. O.; Hahn S. M.; Hamblin M. R.; Juzeniene A.; et al. Photodynamic Therapy of Cancer: An Update. Ca-a Cancer J. Clin. 2011, 61 (4), 250–281. 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juzeniene A.; Peng Q.; Moan J. Milestones in the Development of Photodynamic Therapy and Fluorescence Diagnosis. Photochemical and Photobiological Sciences. 2007, 6, 1234–1245. 10.1039/b705461k. [DOI] [PubMed] [Google Scholar]
- Volgger V.; Betz C. S. Photodynamic Therapy in the Upper Aerodigestive Tract. Overview and Outlook. J. Biophotonics. 2016, 9 (11–12), 1302–1313. 10.1002/jbio.201600036. [DOI] [PubMed] [Google Scholar]
- Rogers G. S. Continuous Low-Irradiance Photodynamic Therapy: A New Therapeutic Paradigm. JNCCN J. Natl. Compr. Cancer Netw. 2012, 10 (Suppl. 2), 14–17. 10.6004/jnccn.2012.0166. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice S.; Eisen D. B. Daylight Photodynamic Therapy: What Is Known and What Is yet to Be Determined. Dermatologic Surg. 2016, 42 (3), 286–295. 10.1097/DSS.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Bisland S. K.; Lilge L.; Lin A.; Rusnov R.; Wilson B. C. Metronomic Photodynamic Therapy as a New Paradigm for Photodynamic Therapy: Rationale and Preclinical Evaluation of Technical Feasibility for Treating Malignant Brain Tumors. Photochem. Photobiol. 2004, 80 (1), 22–30. 10.1111/j.1751-1097.2004.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Song J.; Nie L.; Chen X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chemical Society Reviews. 2016, 45 (23), 6597–6626. 10.1039/C6CS00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H.; Sørensen D. R.; Angell-Petersen E.; Peng Q.; Tromberg B.; Sun C. H.; Spetalen S.; Madsen S. Repetitive Photodynamic Therapy of Malignant Brain Tumors. Journal of Environmental Pathology, Toxicology and Oncology. 2006, 25 (1–2), 261–279. 10.1615/JEnvironPatholToxicolOncol.v25.i1-2.170. [DOI] [PubMed] [Google Scholar]
- Alqathama A. BRAF in Malignant Melanoma Progression and Metastasis: Potentials and Challenges. Am. J. Cancer Res. 2020, 10 (4), 1103–1114. [PMC free article] [PubMed] [Google Scholar]
- Castellani G.; Buccarelli M.; Arasi M. B.; Rossi S.; Pisanu M. E.; Bellenghi M.; Lintas C.; Tabolacci C. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers. 2023, 15 (16), 4026. 10.3390/cancers15164026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H.; Chin L.; Garraway L. A.; Fisher D. E. Melanoma: From Mutations to Medicine. Genes Dev. 2012, 26 (11), 1131–1155. 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko I. V.; Paraiso K. H. T.; Smalley K. S. M. Acquired and Intrinsic BRAF Inhibitor Resistance in BRAF V600E Mutant Melanoma. Biochem. Pharmacol. 2011, 82 (3), 201–209. 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.; Tian H. Current Development Status of MEK Inhibitors. Molecules. 2017, 22 (10), 1551. 10.3390/molecules22101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A.; Arita T.; Tsuchiya S.; Donelan J.; Chouitar J.; Carideo E.; Galvin K.; Okaniwa M.; Ishikawa T.; Yoshida S. Antitumor Activity of the Selective Pan-RAF Inhibitor TAK-632 in BRAF Inhibitor-Resistant Melanoma. Cancer Res. 2013, 73 (23), 7043–7055. 10.1158/0008-5472.CAN-13-1825. [DOI] [PubMed] [Google Scholar]
- Chapman P. B.; Hauschild A.; Robert C.; Haanen J. B.; Ascierto P.; Larkin J.; Dummer R.; Garbe C.; Testori A.; Maio M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364 (26), 2507–2516. 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelster M. S.; Amaria R. N. Combined Targeted Therapy and Immunotherapy in Melanoma: A Review of the Impact on the Tumor Microenvironment and Outcomes of Early Clinical Trials. Therapeutic Advances in Medical Oncology. 2019, 11, 1758835919830826. 10.1177/1758835919830826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J.; Chiarion-Sileni V.; Gonzalez R.; Grob J. J.; Cowey C. L.; Lao C. D.; Schadendorf D.; Dummer R.; Smylie M.; Rutkowski P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373 (1), 23–34. 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C. J. H.; Wong J. H.; El Farran C.; Tan B. X.; Coffill C. R; Loh Y.-H.; Lane D.; Arumugam P. Identification of Pathways Modulating Vemurafenib Resistance in Melanoma Cells via a Genome-Wide CRISPR/Cas9 Screen. G3 Genes, Genomes, Genet. 2021, 11 (2), jkaa069 10.1093/g3journal/jkaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotwals P.; Cameron S.; Cipolletta D.; Cremasco V.; Crystal A.; Hewes B.; Mueller B.; Quaratino S.; Sabatos-Peyton C.; Petruzzelli L.; Engelman J. A.; Dranoff G. Prospects for Combining Targeted and Conventional Cancer Therapy with Immunotherapy. Nat. Rev. Cancer. 2017, 17 (5), 286–301. 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- Green A. C.; Olsen C. M. Cutaneous Squamous Cell Carcinoma: An Epidemiological Review. Br J. Dermatol. 2017, 177 (2), 373–381. 10.1111/bjd.15324. [DOI] [PubMed] [Google Scholar]
- Borgheti-Cardoso L. N.; Viegas J. S. R.; Silvestrini A. V. P.; Caron A. L.; Praça F. G.; Kravicz M.; Bentley M. V. L. B. Nanotechnology Approaches in the Current Therapy of Skin Cancer. Adv. Drug. Delivery Rev. 2020, 153, 109–136. 10.1016/j.addr.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Das M.; Liu Y.; Huang L. Targeted Drug Delivery to Melanoma. Adv. Drug. Delivery Rev. 2018, 127, 208–221. 10.1016/j.addr.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Ko W. C.; Wang S. J.; Hsiao C. Y.; Hung C. T.; Hsu Y. J.; Chang D. C.; Hung C. F. Pharmacological Role of Functionalized Gold Nanoparticles in Disease Applications. Molecules. 2022, 27 (5), 1551. 10.3390/molecules27051551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Chen Y.; Zhu D.; Shi K.; Ma C.; Zhang W.; Rocchi P.; Jiang L.; Liu X. Self-Assembly of Amphiphilic Phospholipid Peptide Dendrimer-Based Nanovectors for Effective Delivery of SiRNA Therapeutics in Prostate Cancer Therapy. J. Controlled Release 2020, 322, 416–425. 10.1016/j.jconrel.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Yang C.; Lin Z. I.; Chen J. A.; Xu Z.; Gu J.; Law W. C.; Yang J. H. C.; Chen C. K. Organic/Inorganic Self-Assembled Hybrid Nano-Architectures for Cancer Therapy Applications. Macromolecular Bioscience. 2022, 22 (2), e2100349 10.1002/mabi.202100349. [DOI] [PubMed] [Google Scholar]
- Vishnubhakthula S.; Elupula R.; Durán-Lara E. F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Delivery 2017, 2017, 7275985 10.1155/2017/7275985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa S.; Meany E. L.; Gale E. C.; Klich J. H.; Saouaf O. M.; Mayer A. T.; Xiao Z.; Liong C. S.; Brown R. A.; Maikawa C. L.; Grosskopf A. K.; Mann J. L.; Idoyaga J.; Appel E. A. Injectable Nanoparticle-Based Hydrogels Enable the Safe and Effective Deployment of Immunostimulatory CD40 Agonist Antibodies. Adv. Sci. 2022, 9 (28), e2103677 10.1002/advs.202103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Ferrer J. L.; García-Ortega M. B.; Gallardo-Gómez M.; García M. Á.; Díaz C.; Boulaiz H.; Valdivia J.; Jurado J. M.; Almazan-Fernandez F. M.; Arias-Santiago S.; et al. Metabolomic Profile of Cancer Stem Cell-Derived Exosomes from Patients with Malignant Melanoma. Mol. Oncol. 2021, 15 (2), 407–428. 10.1002/1878-0261.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Pick H.; Gasilova N.; Li X.; Lin T. E.; Laeubli H. P.; Zippelius A.; Ho P. C.; Girault H. H. MALDI Detection of Exosomes: A Potential Tool for Cancer Studies. Chem. 2019, 5 (5), 1318–1336. 10.1016/j.chempr.2019.04.007. [DOI] [Google Scholar]
- Isola A. L.; Eddy K.; Chen S. Biology, Therapy and Implications of Tumor Exosomes in the Progression of Melanoma. Cancers. 2016, 8 (12), 110. 10.3390/cancers8120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme H.; Dombrecht B.; Kiss M.; Roose H.; Allen E.; Van Overmeire E.; Kancheva D.; Martens L.; Murgaski A.; Bardet P. M. R.; et al. Therapeutic Depletion of CCR8 + Tumor-Infiltrating Regulatory T Cells Elicits Antitumor Immunity and Synergizes with Anti-PD-1 Therapy. J. Immunother. Cancer 2021, 9 (2), e001749 10.1136/jitc-2020-001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen J. W.; Lorentzen C. L.; Martinenaite E.; Ellebaek E.; Donia M.; Holmstroem R. B.; Klausen T. W.; Madsen C. O.; Ahmed S. M.; Weis-Banke S. E.; et al. A Phase 1/2 Trial of an Immune-Modulatory Vaccine against IDO/PD-L1 in Combination with Nivolumab in Metastatic Melanoma. Nat. Med. 2021, 27 (12), 2212–2223. 10.1038/s41591-021-01544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Vankayalapati H.; Tang M.; Zheng Y.; Li Y.; Ma C.; Lai K. Discovery of Novel Inhibitors Targeting Multi-UDP-Hexose Pyrophosphorylases as Anticancer Agents. Molecules. 2020, 25 (3), 645. 10.3390/molecules25030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Y.; Zuo D.; Sarkar D.; Fisher P. B. Blockade of Cytotoxic T-Lymphocyte Antigen-4 as a New Therapeutic Approach for Advanced Melanoma. Expert Opinion on Pharmacotherapy. 2011, 12 (17), 2695–2706. 10.1517/14656566.2011.629187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straten D.; Mashayekhi V.; de Bruijn H. S.; Oliveira S.; Robinson D. J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers. 2017, 9 (2), 19. 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto G.; Bernegossi J.; de Freitas L.; Fontana C.; Chorilli M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21 (3), 342. 10.3390/molecules21030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia J. H.; Rodrigues J. A.; Pimenta S.; Dong T.; Yang Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics. 2021, 13 (9), 1332. 10.3390/pharmaceutics13091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N.; Szlasa W.; Saczko J.; Chwiłkowska A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics. 2021, 13 (6), 818. 10.3390/pharmaceutics13060818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankun J. A Thousand Words about the Challenges of Photodynamic Therapy. J. Med. Sci. 2019, 88 (3), 195–199. 10.20883/medical.391. [DOI] [Google Scholar]