Abstract
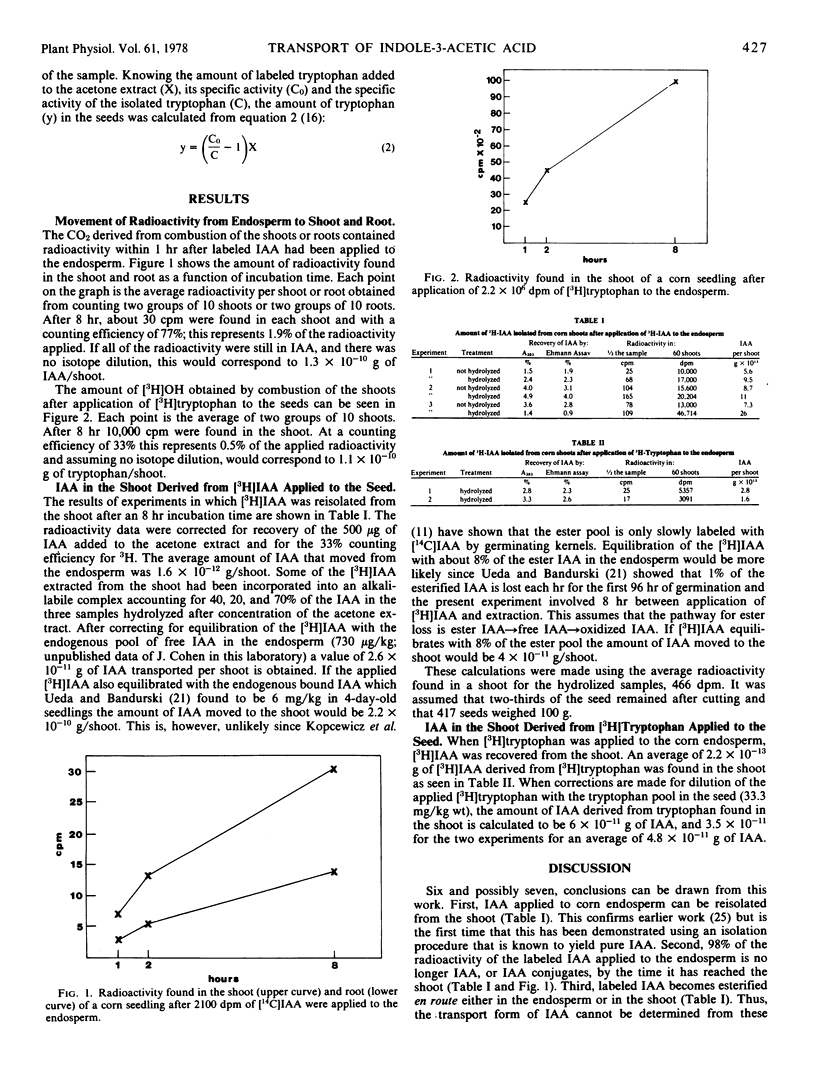
The structures and the concentrations of all of the indolylic compounds that occur in the endosperm of the seeds of corn (Zea mays L.) are known. Thus, it should be possible to determine which, if any, of the indolylic compounds of the endosperm can be transported to the seedling in significant amounts and thus help identify the seed-auxin precursor of Cholodny (1935. Planta 23:289-312) and Skoog (1937. J. Gen. Physiol. 20:311-334). Of interest is the transport of tryptophan, indole-3-acetic acid (IAA), and the esters of IAA, which comprise 95% of the IAA compounds of the seed. We have shown that: (a) IAA can move from the endosperm to the shoot; (b) the rate of movement of IAA from endosperm to shoot is that of simple diffusion; (c) 98% of the transported IAA is converted into compounds other than IAA, or IAA esters, en route; (d) some of the IAA that has moved into the shoot has been esterified; (e) labeled tryptophan applied to the endosperm can be found as labeled IAA in the shoot; and (f) with certain assumptions concerning IAA turnover, the rate of movement of IAA and tryptophan-derived IAA from the endosperm to shoot is inadequate for shoot growth or to maintain IAA levels in the shoot.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A. Concentrations of Indole-3-acetic Acid and Its Esters in Avena and Zea. Plant Physiol. 1974 Sep;54(3):257–262. doi: 10.1104/pp.54.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. H. Movement of indoleacetic acid in coleoptiles of Avena sativa L. II. Suspension of polarity by total inhibition of the basipetal transport. Plant Physiol. 1966 Jan;41(1):15–27. doi: 10.1104/pp.41.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H. Isolation of indole-3-acetic acid from corn kernels & etiolated corn seedlings. Plant Physiol. 1961 May;36(3):354–359. doi: 10.1104/pp.36.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcewicz J., Ehmann A., Bandurski R. S. Enzymatic Esterification of Indole-3-acetic Acid to myo-Inositol and Glucose. Plant Physiol. 1974 Dec;54(6):846–851. doi: 10.1104/pp.54.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival F. W., Bandurski R. S. Esters of indole-3-acetic Acid from Avena seeds. Plant Physiol. 1976 Jul;58(1):60–67. doi: 10.1104/pp.58.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. L. Diffusion of 133Xe through frog skins, toad bladders, and water boundary layers. Biophys J. 1977 Jul;19(1):49–62. doi: 10.1016/S0006-3495(77)85561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Bandurski R. S. A Quantitative Estimation of Alkali-labile Indole-3-Acetic Acid Compounds in Dormant and Germinating Maize Kernels. Plant Physiol. 1969 Aug;44(8):1175–1181. doi: 10.1104/pp.44.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse R. L., Zalik S. Translocation of Indole-3-acetic Acid-1'-C and Tryptophan-1-C in Seedlings of Phaseolus coccineus L. and Zea mays L. Plant Physiol. 1967 Oct;42(10):1363–1372. doi: 10.1104/pp.42.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]



