Abstract
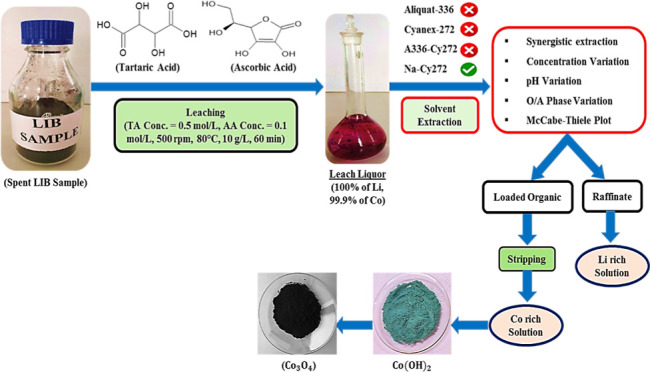
Recycling spent Li-ion batteries (LIBs) is paramount to pursuing resource efficiency and environmental sustainability. This study introduces a synergistic approach for selectively leaching and separating strategic metals from waste LIBs, representing a more efficient alternative to traditional single-acid-based leaching methods. The research also thoroughly analyzes diverse extraction parameters, aiming to achieve clean metal separation through synergistic concepts rather than single-phase extraction. The outcome of this study is developing a comprehensive downstream process, advancing the cause of sustainable waste management in the LIB industry. Under specific conditions with 0.6 mol/L total acid content (0.5 mol/L tartaric acid + 0.1 mol/L ascorbic acid), 99.9% cobalt and 100% lithium were effectively leached. The subsequent extraction process achieved a clean separation, with 48.3% of cobalt extracted using a mixture of 0.1 mol/L Alamine-336–Cyanex-272 (A-336–Cy-272) from the leach liquor with no coextraction of lithium, and this efficiency was improved to 67.3% by adjusting the pH from 2.44 to 7.5. However, it is worth noting that increasing the extractant concentration led to an antagonistic effect. To further enhance cobalt enrichment in the organic phase, the McCabe–Thiele plot method was recommended, employing saponified Cy-272. Moreover, the regeneration of saponified Cy-272 was investigated, and the stripped solution was processed with NaOH to form Co(OH)2, subsequently converting it into cobalt oxide (Co3O4) through calcination.
1. Introduction
In response to global energy challenges, recent technological advancements have driven the widespread adoption of Li-ion batteries (LIBs).1,2 The extended cycle life, reduced memory effect, lightweight design, exceptional stability, high energy density, and rechargeability of LIBs contribute to the escalating demand for the manufacturing and long-chain supply of these batteries rather than the conventional batteries like nickel–cadmium, nickel–metal hydride, and lead acid.3,4 This surge in LIB demand has spurred the growth of electric vehicles, the proliferation of portable electronics, and the functionalization of electrical tools.5,6 According to the statistical analysis of LIB lifecycles, 3 million metric tonnes of these batteries are anticipated to reach the end of their lifespan by 2030, thereby adding up the minerals present in LIBs as urban waste.7 Following these estimates, the focus has been directed toward mitigating the use of primary and secondary sources and deliberately adopting the circular economy concept for recovering and processing critical metals from spent batteries.8 It has been demonstrated that spent LIBs contain valuable components in concentrations significantly surpassing traditional ore grades, underscoring their immense recycling potential from urban mines and industrial scraps, which reduces carbon footprints and alleviates the accumulation of minerals in impending waste.9−11 Furthermore, these used LIBs pose environmental hazards, as they are flammable and may contain harmful substances like organic solvent electrolytes (which can release harmful gases upon volatilization and decomposition) and heavy metals such as Co, Mn, and Ni, capable of causing serious harm to groundwater.12 Effective recycling of spent LIBs prevents resource wastage and mitigates environmental pollution, making it a crucial endeavor for sustainability. Pyrometallurgical, hydrometallurgical, and direct recycling of preprocessed and mechanically treated spent LIBs have proven highly beneficial in recovering major essential elements.13,14 Hydrometallurgical processes offer distinct advantages, including the requirement for lower temperatures, reduced energy consumption, higher recovery rates, production of pure end-products, and utilization of straightforward techniques. These inherent benefits make hydrometallurgy a preferred and advantageous choice compared to pyrometallurgical and other conventional methods.15,16 The hydrometallurgical approach involves using different processes such as leaching, solvent extraction, precipitation, adsorption, and many more, owing to their differential solubility in different solvents to reinstate minerals in the desired form.17,18 Leaching, a pivotal step in the hydrometallurgical process involves the dissolution of essential metal ions from waste LIBs into a solution, typically utilizing acidic lixiviants, either organic or inorganic.19 While both types of acids can yield similar leaching efficiencies, organic acids are favored for their eco-friendly attributes.20 They contribute to a reduced environmental impact during leaching, acting as environmentally benign solvents that reduce exposure to toxic gases and address water salinity concerns.21 Notably, these biodegradable acids are preferred over highly acidic conventional inorganic acids due to their biogenic production, ease of processing, and recyclability.22 Consequently, the current leaching approach promotes the utilization of diverse organic acids, such as acetic acid, citric acid, succinic acid, and lactic acid, among others, as leaching agents. Multiple organic acid leaching systems have been developed to recycle metals from waste LIBs. Li et al. reported that 100% of Li and 90% of Ni were recovered from waste cathode materials using citric acid (1.25 mol/L) as the leachant.23 In a study by He et al., 2 mol/L l-tartaric acid (TA) was used to leach 99.1% Li, 99.3% Ni, 98.6% Co, and 99.4% Mn from the waste LIB sample.24 However, it is worth noting that a common challenge in the complete dissolution of waste cathodic materials when employing a single organic acid as the leachant is the requirement for higher acid concentrations.25 Several studies have suggested that the consumption of acid can be significantly reduced by combining different organic acids.26−29 Furthermore, when chelated with anion groups within the leaching solution, the stability of some metal ions, like Co2+ and Mn2+, can significantly enhance the leaching efficiency.30 To achieve synergism in leaching, pairing one acid acting as a chelating agent with another serving as a reducing agent is essential. While effective, traditional reductants like H2O231 and NaHSO332 pose environmental risks. For instance, hydrogen peroxide usage produces anthraquinone, a substance harmful to the environment.33 Therefore, seeking renewable and organic reductants, such as ascorbic acid (AA), emerges as a more environmentally friendly alternative.34 Consequently, proposing a synergistic leaching system characterized by lower total acid content, mild operating conditions, minimal environmental impact, and a commitment to ensure high leaching efficiencies is imperative. Many researchers have predominantly focused their investigations on the leaching phase,35−38 neglecting the significance of downstream processes essential for the retrieval of pure metals from the leach solution. The utilization of the solvent extraction method, characterized by its high separation factor, minimal energy consumption, operational convenience, and obtaining the desired form of final products, has gained widespread traction within the hydrometallurgical sector, surpassing traditional precipitation techniques.39−41 Through a comprehensive analysis of various research endeavors, it was ascertained that implementing acidic extractants could significantly enhance the separation of Li and Co.42−44 However, our prior study demonstrated that while acidic extractants are proficient in cobalt extraction, their use can lead to the undesired coextraction of lithium, consequently impeding the achievement of a clean separation process.45 Due to its improved efficiency, selectivity, and lower costs, synergistic extraction has distinct advantages over single-phase extraction. Synergistic extraction increases separation yields by more efficiently targeting particular metals by mixing numerous extractants. Furthermore, synergistic systems offer the potential for decreased extractant consumption, a lessened environmental footprint, and the capacity to diminish the coextraction of undesired components, resulting in the generation of purer product streams. In addressing the identified research gaps, this proposed work undertakes a synergistic approach for the selective leaching and separation of strategic metals from spent LIBs. Various parameters, including the influence of reductants, total acid content, and many more, have been methodically examined to optimize the leaching conditions. Likewise, a comprehensive analysis of various extraction factors has been conducted to achieve the clean separation of both metals, culminating in the development of a complete downstream process. Characterization of the initial LIB sample, the resulting residue, and the final product has been carried out with a qualitative assessment in mind, ensuring a thorough understanding of the process and its outcomes.
2. Experimental Section
2.1. Materials and Reagents
The waste LIB sample was sourced from an authorized waste material supplier, which was then subject to a grinding process to achieve a homogeneous particle size. Building on insights from our earlier research, which explored various size fractions of the LIB sample, a notable correlation was observed: smaller particle sizes corresponded to a higher leaching efficiency. Therefore, to align with these findings, all experiments were conducted using spent LIB samples with a particle size below 25 μ. AA, TA, glucose, oxalic acid, formic acid, and ethylene glycol were purchased from Merck Life Science Pvt. Ltd. Merck analytical-grade reagents were used for all other chemical components, and Millipore water was employed in the preparation of all solutions.
2.2. Analytical Methods
The total metal content in the waste LIB sample was examined through inductively coupled plasma optical emission spectroscopy (ICP–OES) (model: iCAP PRO, Thermo Scientific). This analysis was performed after treating the sample with a 3:1 solution of HCl and HNO3, known as aqua regia (AR). For digestion, 1 g of the sample was mixed with 40 mL of AR and brought to a boil for 1 h. After cooling, the mixture was filtered into a 100 mL volumetric flask and diluted to a final volume of 100 mL. Various characterizations of solid samples before and after leaching were performed through X-ray diffraction (XRD) using a Rigaku Ultima IV instrument, and scanning electron microscopy–energy-dispersive spectrometry (SEM–EDS) was conducted with an EVO-18 apparatus from Carl Zeiss. The total metal contents obtained from the waste LIB sample, as determined through both AR treatment and EDS analysis, are presented in Table 1.
Table 1. Total Metal Contents in the Waste LIB Sample.
| metals | Li | Co | Cu | Al |
|---|---|---|---|---|
| wt % (AR) | 3.9 ± 0.02 | 34.12 ± 0.14 | 0.31 ± 0.05 | 0.27 ± 0.06 |
| wt % (EDS) | – | 34.21 ± 0.09 | 0.41 ± 0.03 | 0.47 ± 0.02 |
2.3. Leaching Process
The leaching process for the waste LIB sample involved a 500 mL three-necked flat-bottomed flask equipped with a temperature sensor, a vapor condenser, and a magnetic stirrer. After leaching, filtration was employed to achieve solid–liquid separation, and the total content of metal ions in the leach solution was measured using ICP–OES. The metal ions’ leaching efficiency (% L) was determined by dividing the metal concentration in the leach solution by the total metal content in AR and multiplying the result by 100.
2.4. Extraction Process
In the extraction experiments, the aqueous and organic phases were vigorously shaken in a separating funnel of 125 mL capacity for 15 min at a controlled temperature of 30 ± 0.5 °C. Following phase separation, the resulting aqueous phase, known as raffinate, was collected, and the concentration of metal ions was quantified. Distribution ratios (D) were calculated using eq 1, while extraction efficiency (% E) was determined using eq 2.
| 1 |
where Vaq and Vorg signify the volume of the aqueous and organic phase solutions, respectively, and Ci and Cf represent the concentration of metal ions before and after extraction, respectively.
| 2 |
To develop the synergistic extractant, a combination of two extractants was examined. The degree of synergistic effect can be evaluated using the synergistic coefficient (SC), which is defined by eq 3.
| 3 |
where DA and DB represent the distribution ratios achieved when each extractant is used individually and DA+B signifies the distribution ratio when the mixture is employed. When the SC is positive, it signifies the presence of a synergistic effect, whereas if the SC is negative, it indicates the occurrence of an antagonistic effect.
3. Results and Discussion
3.1. Characterization of the LIB Sample
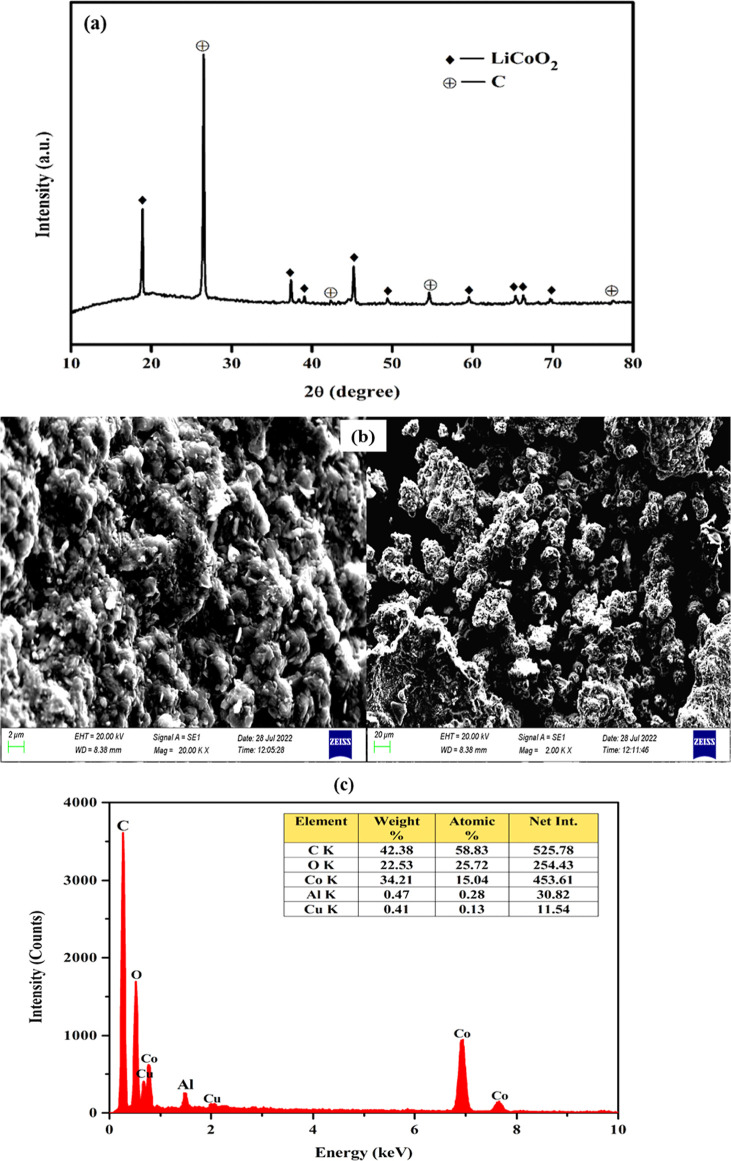
The waste LIB sample was characterized through XRD analysis, employing a Cu Kα radiation source with a wavelength (λ) of 1.5406 Å. The analysis was conducted at a temperature of 25 °C, scanning the LIB sample within the 2θ range spanning from 10 to 80°. The obtained diffraction patterns were meticulously compared against reference data (LiCoO2 = 00-050-0653 and C = 00-008-0415) from the JCPDS database to validate the results. As depicted in the XRD analysis (Figure 1a), the occurrence of lithium and cobalt in the form of LiCoO2 was unequivocally confirmed. The same original feed sample was also subjected to SEM analysis (Figure 1b) and EDS analysis (Figure 1c) at various magnifications. The SEM images revealed the existence of LiCoO2 particles characterized by irregular shapes and a relatively broad particle size distribution. Moreover, elemental analysis via EDS confirmed the presence of Co, Al, Cu, C, and O in the sample. It is worth noting that lithium was not detected during EDS elemental analysis. This can be attributed to its lightweight nature; lighter elements typically emit an Auger electron instead of a proton, contributing to their nondetection in EDS analysis.
Figure 1.
(a) XRD patterns, (b) SEM images, and (c) EDS analysis of the spent LIB sample.
3.2. Study of Leaching Parameters
3.2.1. Synergistic Leaching of Spent LIB Sample
TA, having pKa values of 2.98 and 4.34, is employed as the primary leaching agent for the leaching process. After an extensive literature review, it has been unveiled that TA and citric acid are inadequate for leaching metals from discarded LIBs unless a reducing agent is added.24,46−50 To analyze the synergistic effect on the efficacy of Li and Co leaching from the discarded LIB sample, various reductants, including H2O2, AA, glucose, oxalic acid, formic acid, and ethylene glycol, were used at a fixed concentration of 0.1 mol/L. The impact of TA on the leaching efficiencies of Li and Co with and without reductants is depicted in Figure 2. Based on the experimental findings, it was observed that among all the reductants AA was an effective reductant for enhancing the leaching efficiency of both the metal ions due to its redox properties, selective redox potentials, and chelating ability toward these metals. With 0.5 mol/L of TA and 0.1 mol/L of AA, 90.1% of Li and 75% of Co were leached out at ambient temperature, while without AA only 48.7% of Li and 13% of Co were recovered at a TA concentration of 0.5 mol/L. Our previous findings also reported that AA exhibited significantly higher leaching rates for Co and Li compared to other acids, and the detailed mechanism underlying the leaching process was also elucidated.51
Figure 2.
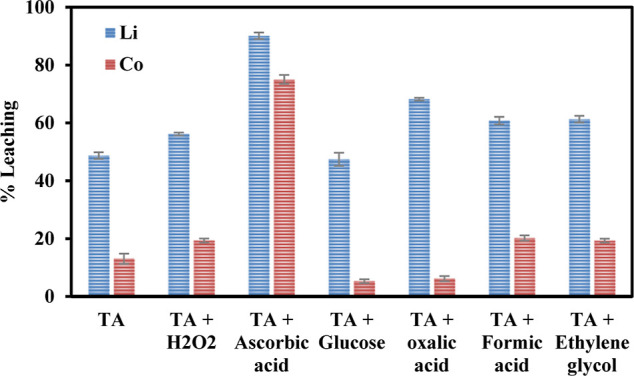
Impact of different reductants on the leaching efficiency of Co and Li (TA conc. = 0.5 mol/L, leaching time = 60 min, temperature = 30 °C, S/L = 10 g/L).
3.2.2. Impact of Total Acid Content
The total content of combined acid considerably impacts the leaching process. The concentrations of TA and AA have been varied to optimize the leaching efficacy. Based on the synergistic leaching study (Figure 2), about 90% of Li and 75% of Co were leached out at a total acid content of 0.6 mol/L. As indicated in Table 2, there is no discernible impact on the leaching efficacy of both metal ions by the increase in total acid content from 0.6 to 1.0 mol/L. By maintaining a constant AA concentration of 0.1 mol/L, the increase in TA concentration from 0.5 to 0.9 mol/L does not significantly improve the leaching efficiency. Likewise, when keeping the TA concentration constant at 0.5 mol/L, increasing the concentration of AA from 0.01 to 0.2 mol/L does not yield a substantial improvement in leaching efficiency. This lack of enhancement can be attributed to attaining the equilibrium point of H+ dissociation in the mixed acid leaching system.52 Therefore, it was determined that 0.6 mol/L is the ideal total acid content for conducting further experiments.
Table 2. Impact of Total Acid Content on the Leaching Efficiency of Co and Li (S/L = 10 g/L, Leaching Time = 60 min, Temperature = 30 °C, and Stirring Speed = 500 rpm).
| leaching agents + reductant | % leaching (Li) | % leaching (Co) |
|---|---|---|
| 0.5 mol/L TA + 0.01 mol/L AA | 61.57 ± 0.19 | 32.16 ± 0.14 |
| 0.5 mol/L TA + 0.05 mol/L AA | 78.36 ± 0.17 | 53.84 ± 0.11 |
| 0.5 mol/L TA + 0.1 mol/L AA | 90.10 ± 0.13 | 75.05 ± 0.08 |
| 0.7 mol/L TA + 0.1 mol/L AA | 90.76 ± 0.18 | 75.38 ± 0.12 |
| 0.9 mol/L TA + 0.1 mol/L AA | 91.53 ± 0.11 | 75.82 ± 0.09 |
| 0.5 mol/L TA + 0.15 mol/L AA | 90.89 ± 0.09 | 75.79 ± 0.07 |
| 0.5 mol/L TA + 0.2 mol/L AA | 92.05 ± 0.12 | 76.49 ± 0.08 |
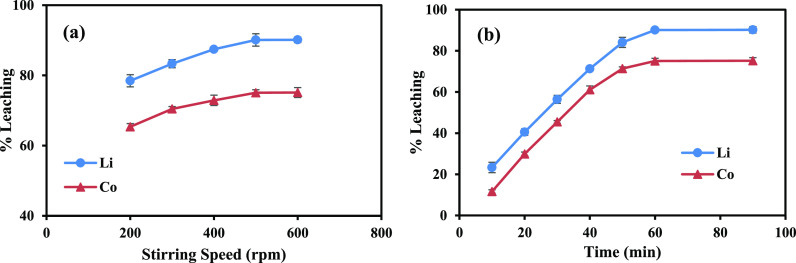
3.2.3. Impact of Stirring Speed and Leaching Time
The impact of leaching time and stirring speed was investigated to optimize the leaching conditions further. The objective was to identify the ideal combination of these parameters that would maximize the leaching efficiency while maintaining the desired concentration of 0.6 mol/L total acid content (0.1 mol/L AA and 0.5 mol/L TA). The effect of stirring speed on the leaching efficiency was studied while maintaining a constant leaching time (60 min). Different stirring speeds, such as 200 to 600 rpm, were tested (Figure 3a). When the stirring speed was increased from 200 to 500 rpm, the recovery percentages of Li and Co increased from 78.4 and 65.3 to 90.1 and 75%, respectively. Beyond 500 rpm, the leaching rate remained constant, indicating that the optimal stirring speed was fixed at 500 rpm. The outcomes showed that the stirring speed substantially impacted the leaching rate. Mass transfer limitations might occur at low stirring speeds, reducing the contact between the leaching solution and the LIB sample and leading to a lower leaching efficiency. The leaching experiments were conducted at a temperature of 30 °C with a stirring speed of 500 rpm, using 0.5 mol/L TA and 0.1 mol/L AA at a solid-to-liquid ratio (S/L) of 10 g/L. The leaching duration was varied from 10 to 90 min (Figure 3b). At a leaching period of 10 min, only 23.3% of Li and 11.6% of Co were leached. However, as the leaching time was extended to 60 min, the leaching efficiencies of Co and Li significantly improved to 75 and 90.1%, respectively. Based on these findings, the leaching time of 60 min was selected as the optimal duration for the subsequent studies due to its ability to achieve the highest leaching efficiencies for both Li and Co without any significant improvement beyond this time.
Figure 3.
Impact of the (a) stirring speed and (b) leaching time on the leaching efficiency of Co and Li (TA conc. = 0.5 mol/L, AA conc. = 0.1 mol/L, temperature = 30 °C, S/L = 10 g/L).
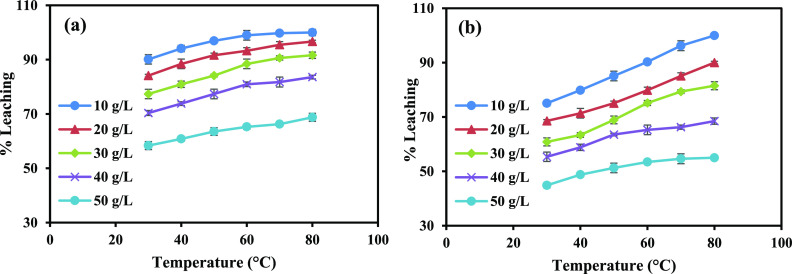
3.2.4. Impact of Temperature and S/L)
The influence of temperature and the S/L on the leaching of Li and Co was investigated by altering the temperature within the range of 30–80 °C and varying the S/L ratio from 10 to 50 g/L. As depicted in Figure 4a, the outcomes demonstrate that the leaching efficiency of Li exhibited an increment from 90.1 to 100% as the temperature was raised from 30 to 80 °C while keeping the S/L ratio at 10 g/L and maintaining all other conditions constant. However, as the S/L ratio was gradually elevated from 10 to 50 g/L, the efficiency of Li showcased a decline. Specifically, the leaching efficiency of Li reduced from 100 to 68.7% at a temperature of 80 °C. Likewise, analogous results were observed for Co, as shown in Figure 4b. The leaching efficiency of Co experienced a decrease from 99.9 to 55% when the S/L ratio was increased from 10 to 50 g/L while maintaining a temperature of 80 °C. Consequently, the ideal leaching parameters were established, encompassing the total acid content of 0.6 mol/L, temperature set at 80 °C, stirring speed of 500 rpm, leaching duration of 60 min, and S/L ratio maintained at 10 g/L.
Figure 4.
Impact of temperature and S/L leaching time on the leaching efficiency of (a) Li and (b) Co (TA conc. = 0.5 mol/L, AA conc. = 0.1 mol/L, leaching time = 60 min, stirring speed = 500 rpm).
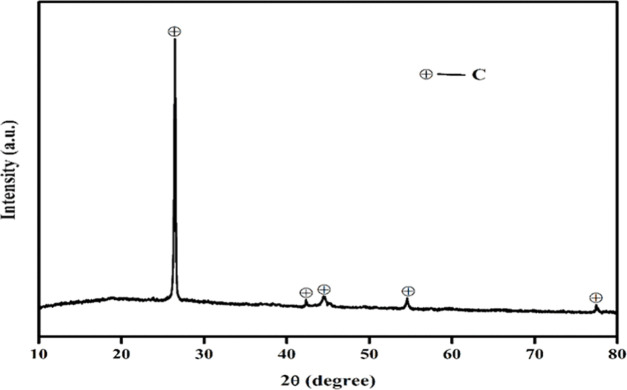
XRD analysis of the leached residue after leaching was carried out to verify the complete dissolution of Co and Li. The appearance of a carbon peak in the XRD patterns clearly demonstrated the complete dissolution of both metals (Figure 5). As the wt % of Al and Cu fall below 0.5%, their corresponding peaks remain undetectable through XRD analysis. To determine whether Al and Cu were coleached along with Co and Li, ICP–OES analysis was conducted. The results obtained from the ICP–OES analysis clearly confirmed that neither Cu nor Al underwent dissolution during the leaching process.
Figure 5.
XRD patterns of the leached residue after leaching.
3.3. Separation of Metals from Leach Liquor
After carefully establishing the optimal leaching conditions, 1 L of leach liquor was successfully produced. This solution exhibited a composition of 0.39 g/L of lithium and 3.411 g/L of cobalt. To facilitate further investigations, a deliberate dilution of this solution was performed, resulting in a 10-fold reduction in concentration ([Li] = 0.039 g/L and [Co] = 0.3411 g/L).
3.3.1. Synergistic Approach for Extraction of Co and Li
A comprehensive investigation was performed to analyze the individual and synergistic effects of the Alamine-336 (A-336) and Cyanex-272 (Cy-272) extractants. This study seeks to understand their impact on the extraction efficiency of both metals, ultimately influencing the efficacy of metal separation processes. In this study, kerosene was used as the organic solvent of choice for solvent extraction. Its preference arises from a well-balanced set of properties, with low viscosity aiding efficient phase separation, while its immiscibility with water ensures clear separation of phases. The practical advantages of kerosene, such as relatively low toxicity and cost-effectiveness, further endorse its suitability for this application. Conducting extraction studies at an initial pH of 2.44, the outcomes are compiled in Table 3. The results indicate that both A-336 and A-336-HCl exhibit limited selectivity in extracting one of the metals. Similarly, utilizing 0.1 mol/L Cy-272 led to the extraction of only 18.7% of Co, accompanied by the undesired coextraction of Li, thereby falling short of achieving effective clean separation. However, the binary mixtures of Cy-272 and A-336 showcased a pronounced synergistic impact on cobalt extraction, surpassing that of lithium, resulting in a markedly improved separation outcome. At a pH of 2.44, employing 0.1 mol/L A-336–Cy-272 resulted in an extraction yield of approximately 48.3% for cobalt with no involvement of lithium. The SC was calculated (Table 3), and it was noticed that it is positive. However, to further enhance the extraction efficiency, exploring pH variation as a means of optimization is imperative.
Table 3. Impact of Different Extractants on the Extraction Efficiency of Both Metals.
| sl no | extractants | initial pH | % ELi | % ECo | DCo | SC (Co) |
|---|---|---|---|---|---|---|
| 1 | 0.1 mol/L A-336 | 2.44 | 0.2 | 0.002 | ||
| 2 | 0.1 mol/L A-336-HCl | 2.44 | 16.6 | 0.2 | ||
| 3 | 0.1 mol/L Cy-272 | 2.44 | 8.56 | 18.7 | 0.23 | |
| 4 | 0.1 mol/L A-336–Cy-272 | 2.44 | 48.3 | 0.935 | 0.605 |
3.3.2. Impact of pH on Extraction of Co
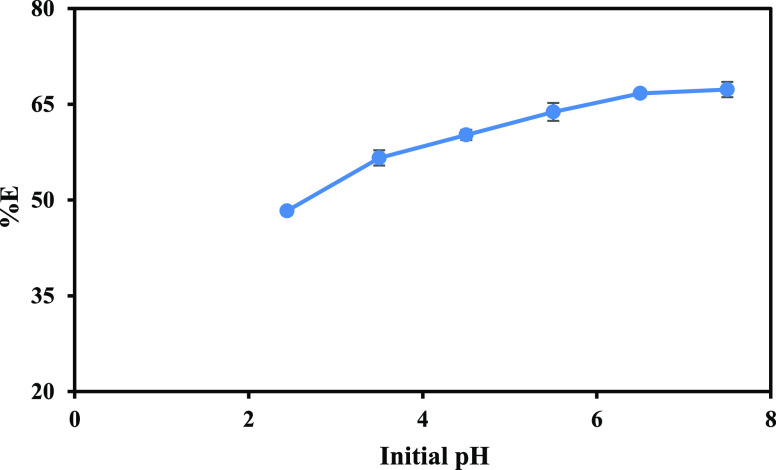
The variation of pH is crucial in the extraction of metals from LIBs due to its profound impact on the solubility and speciation of metal ions. Additionally, the pH can influence the formation of metal complexes with extractants, affecting the selectivity and coextraction of undesired elements. Optimizing pH allows for tailored separation processes, enhancing metal recovery while minimizing contamination. Therefore, by employing a mixture of 0.1 mol/L A-336–Cy-272, we systematically varied the initial pH of the leach liquor to assess its influence on the extraction efficiency of cobalt. Notably, we observed a progressive improvement in extraction efficiency as the pH was increased, increasing from 48.3% at a pH of 2.44 to a commendable 67.3% at pH 7.5 (Figure 6). Given the comparable efficiencies observed at pH 6.5 and 7.5, both pH levels were selected for further optimization in conjunction with variations in extractant concentration.
Figure 6.
Impact of pH on the extraction efficiency of Co.
3.3.3. Impact of Extractant Concentration
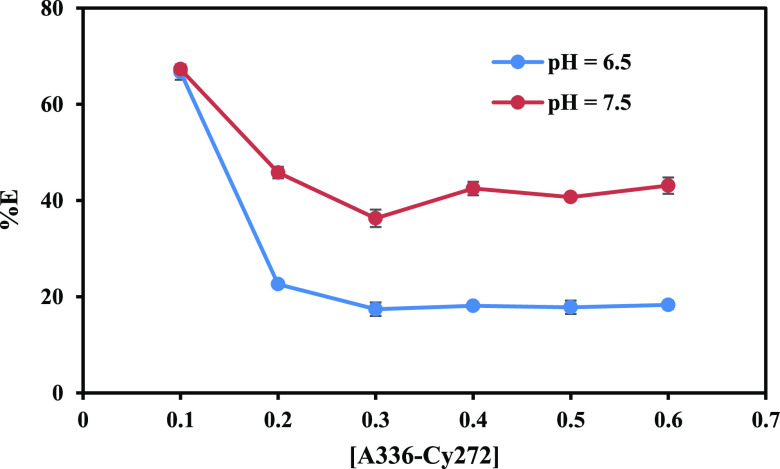
To enhance the extraction efficiency, we systematically varied the extractant concentration within the binary mixture, ranging from 0.1 to 0.6 mol/L. At a pH of 7.5, employing 0.1 mol/L A-336–Cy-272 resulted in the extraction of an impressive 67.3% of cobalt into the organic phase. However, as the concentration was increased to 0.6 mol/L, this efficiency notably decreased to 43.1% (Figure 7). This decline pointed to an antagonistic effect at higher concentrations, potentially attributed to increased viscosity and a reduced likelihood of forming complexes with metal ions for extraction. Significantly, both pH levels exhibited a decline in extraction efficiency with increased concentration, but it was observed that at pH 7.5 the efficiency remained higher compared to pH 6.5. Consequently, pH 7.5 was picked as the optimal pH for proceeding with further experiments.
Figure 7.
Plot of percentage extraction of cobalt versus [A-336–Cy-272] concentration.
3.3.4. Role of Saponified Cy-272
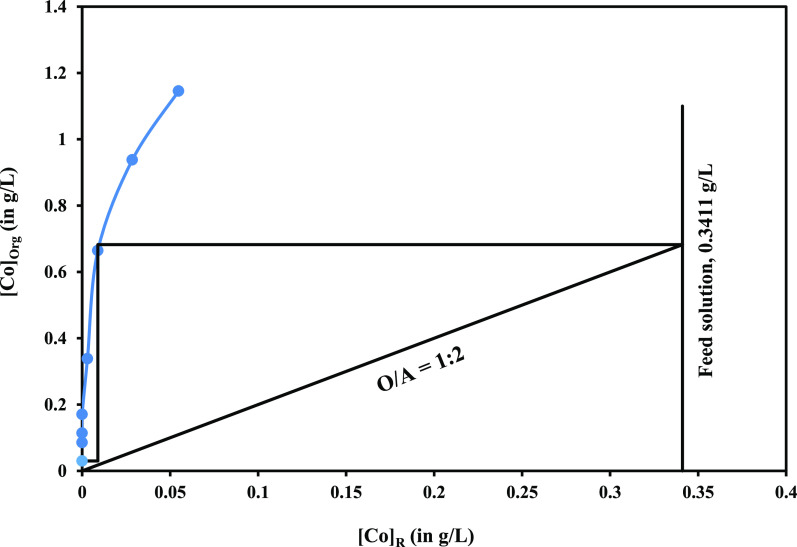
Recognizing that the binary mixture of A-336 and Cy-272, while proficient in selective extraction, fell short in enhancing cobalt extraction efficiency, another strategy for improved separation needs to be developed. Saponified Cy-272 is a viable alternative to the binary combination for achieving a cleaner separation of both metals. Remarkably, using 0.1 mol/L saponified Cy-272, an impressive 99.1% of cobalt was efficiently extracted into the organic phase with no involvement of lithium, signifying a substantial improvement in the separation process. While a remarkable 99.1% cobalt extraction was achieved at an organic-to-aqueous (O/A) ratio of 1:1, it is essential to further optimize the process for cobalt enrichment in the organic phase. To accomplish this, the utilization of a McCabe–Thiele plot becomes crucial for more systematic analysis and efficient separation. Maintaining the total volume of phases fixed, the leach solution was equilibrated with 0.1 mol/L saponified Cy-272 at an O/A ratio ranging from 4/1 to 1/4, and the results are displayed in Figure 8. It was recommended to implement a two-stage counter-current extraction with an O/A ratio of 1:2, and the same was performed. The analysis of R2 (second stage raffinate) revealed a cobalt concentration of 0 mg/L, indicating the complete removal of cobalt. The E1 (first stage of loaded organic) contained 0.66 g/L Co, showing the enrichment of Co in the loaded organic phase.
Figure 8.
McCabe–Thiele plot for the enrichment of Co.
3.3.5. Regeneration of Saponified Cy-272
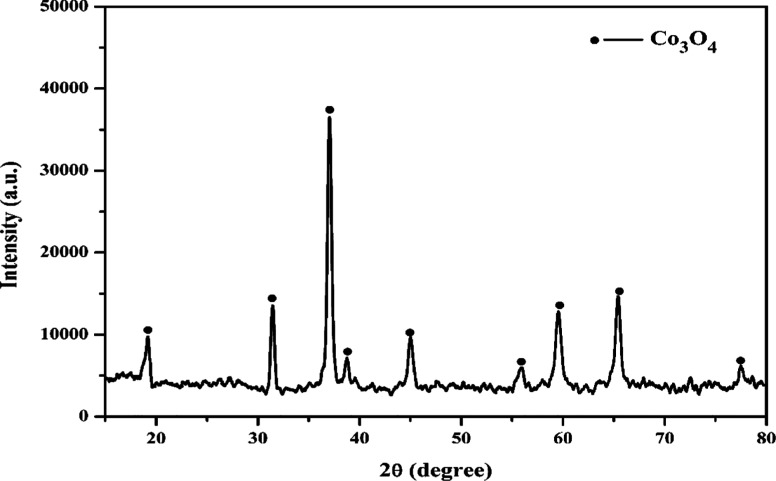
In the pursuit of obtaining pure cobalt oxide and ensuring the reusability of the extractant, a critical step involves the back-extraction of Co from the loaded organic phase. Sufficiently loaded saponified Cy-272 was generated through a two-stage counter-current extraction process, maintaining a 1:2 O/A phase ratio. To investigate the recovery of cobalt from the loaded organic phase, which initially contained 0.66 g/L Co, the H2SO4 concentration was varied within the range of 0.1 to 0.8 mol/L while keeping an O/A ratio of 1:1. It was observed that more than 93% of cobalt was successfully stripped from the organic phase when utilizing 0.8 mol/L H2SO4. Following the stripping process, cobalt precipitation was executed using sodium hydroxide as the precipitating agent. Co was precipitated in the form of cobalt hydroxide [Co(OH)2]. Subsequently, the obtained product underwent calcination at 550 °C for a duration of 2 h to transform it into cobalt oxide (Co3O4). Figure 9a,b illustrates the cobalt precipitates before and after the calcination process, showcasing the transformation. The final product’s identity was validated through XRD, and the analysis results are depicted in Figure 10.
Figure 9.

Image of cobalt precipitates before (a) and after (b) the calcination process.
Figure 10.
XRD patterns of final product after calcination
4. Conclusions
This study presents an innovative synergistic approach, offering a more efficient alternative to conventional single-acid-based leaching methods for selectively recovering strategic metals from waste LIBs. Utilizing a mixture of 0.5 mol/L TA and 0.1 mol/L AA, the leaching process achieved exceptional efficiency, with Co and Li being leached at rates of 99.9 and 100%, respectively. These results highlight the efficacy of a synergistic approach to leaching as a more efficient and cost-effective alternative compared to traditional single-acid-based leaching methods for selectively recovering strategic metals from spent LIBs. Utilizing the synergistic extractant comprising 0.1 mol/L A-336–Cy-272, an extraction efficiency of 48.3% was achieved, notable for its exclusive cobalt extraction without any lithium coextraction. This result underscores the advantages of employing a synergistic approach over traditional single-phase extraction methods. Despite attempts to enhance efficiency through pH and concentration adjustments, optimal results remained elusive. Consequently, the study delved into the role of saponified Cy-272 and explored the application of the McCabe–Thiele plot to enrich the cobalt content. The final product was obtained through precipitation process followed by calcination to achieve the desired cobalt oxide. This holistic approach contributes to a deeper comprehension of the process and its potential for efficient and sustainable metal recovery from waste LIBs.
Acknowledgments
The authors express their gratitude to S‘O’A (Deemed to be University) for providing the research facilities.
Author Contributions
Sibananda Sahu (First author): writing—original draft, visualization, data curation, formal analysis, investigation, and software. Mili Agrawala (Second author): formal analysis, investigation, and data curation. Smruti Rekha Patra (Third author): formal analysis, investigation, and data curation. Niharbala Devi (Corresponding author): conceptualization, methodology, supervision, writing—review and editing, and validation.
The authors declare no competing financial interest.
References
- Nishi Y. Lithium Ion Secondary Batteries; Past 10 Years and the Future. J. Power Sources 2001, 100 (1–2), 101–106. 10.1016/S0378-7753(01)00887-4. [DOI] [Google Scholar]
- Masias A.; Marcicki J.; Paxton W. A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6 (2), 621–630. 10.1021/acsenergylett.0c02584. [DOI] [Google Scholar]
- He L.-P.; Sun S.-Y.; Song X.-F.; Yu J.-G. Recovery of Cathode Materials and Al from Spent Lithium-Ion Batteries by Ultrasonic Cleaning. Waste Manage. 2015, 46, 523–528. 10.1016/j.wasman.2015.08.035. [DOI] [PubMed] [Google Scholar]
- Xiao J.; Niu B.; Xu Z. Highly Efficient Selective Recovery of Lithium from Spent Lithium-Ion Batteries by Thermal Reduction with Cheap Ammonia Reagent. J. Hazard. Mater. 2021, 418, 126319. 10.1016/j.jhazmat.2021.126319. [DOI] [PubMed] [Google Scholar]
- Goyal M.; Singh K.; Bhatnagar N. Circular Economy Conceptualization for Lithium-Ion Batteries- Material Procurement and Disposal Process. Chem. Eng. Sci. 2023, 281, 119080. 10.1016/j.ces.2023.119080. [DOI] [Google Scholar]
- National Minerals Information Center , Mineral Commodity Summaries; National Minerals Information Center, 2022. https://www.usgs.gov/centers/national-minerals-information-center/mineral-commodity-summaries.
- Grima-Carmena L.; Oyonarte-Andrés S.; Giner-Sanz J. J.; García-Gabaldón M.; Bosch-Mossi F.; Pérez-Herranz V. Statistical Analysis of the Effect of the Electrochemical Treatment and the Acid Concentration on the Leaching of NMC Cathodes from Spent Li-Ion Batteries. J. Environ. Chem. Eng. 2023, 11 (5), 110423. 10.1016/j.jece.2023.110423. [DOI] [Google Scholar]
- Boxall N. J.; King S.; Cheng K. Y.; Gumulya Y.; Bruckard W.; Kaksonen A. H. Urban Mining of Lithium-Ion Batteries in Australia: Current State and Future Trends. Miner. Eng. 2018, 128, 45–55. 10.1016/j.mineng.2018.08.030. [DOI] [Google Scholar]
- Wang S.; Yu J. A Comparative Life Cycle Assessment on Lithium-Ion Battery: Case Study on Electric Vehicle Battery in China Considering Battery Evolution. Waste Manag. Res. 2021, 39 (1), 156–164. 10.1177/0734242X20966637. [DOI] [PubMed] [Google Scholar]
- Velázquez-Martínez O.; Valio J.; Santasalo-Aarnio A.; Reuter M.; Serna-Guerrero R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5 (4), 68. 10.3390/batteries5040068. [DOI] [Google Scholar]
- Jin S.; Mu D.; Lu Z.; Li R.; Liu Z.; Wang Y.; Tian S.; Dai C. A Comprehensive Review on the Recycling of Spent Lithium-Ion Batteries: Urgent Status and Technology Advances. J. Clean. Prod. 2022, 340, 130535. 10.1016/j.jclepro.2022.130535. [DOI] [Google Scholar]
- Huang B.; Pan Z.; Su X.; An L. Recycling of Lithium-Ion Batteries: Recent Advances and Perspectives. J. Power Sources 2018, 399, 274–286. 10.1016/j.jpowsour.2018.07.116. [DOI] [Google Scholar]
- Fahimi A.; Alessandri I.; Cornelio A.; Frontera P.; Malara A.; Mousa E.; Ye G.; Valentim B.; Bontempi E. A Microwave-Enhanced Method Able to Substitute Traditional Pyrometallurgy for the Future of Metals Supply from Spent Lithium-Ion Batteries. Resour. Conserv. Recycl. 2023, 194, 106989. 10.1016/j.resconrec.2023.106989. [DOI] [Google Scholar]
- Gaines L.; Richa K.; Spangenberger J. Key Issues for Li-Ion Battery Recycling. MRS Energy Sustain. 2018, 5 (1), E14 10.1557/mre.2018.13. [DOI] [Google Scholar]
- Lerchbammer R.; Gerold E.; Antrekowitsch H. Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material. Metals 2023, 13 (8), 1330. 10.3390/met13081330. [DOI] [Google Scholar]
- Zhao Y.; Pohl O.; Bhatt A. I.; Collis G. E.; Mahon P. J.; Rüther T.; Hollenkamp A. F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2 (1), 167–205. 10.3390/suschem2010011. [DOI] [Google Scholar]
- Zhou L.-F.; Yang D.; Du T.; Gong H.; Luo W.-B. The Current Process for the Recycling of Spent Lithium Ion Batteries. Front. Chem. 2020, 8, 578044. 10.3389/fchem.2020.578044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu S.; Mohapatra M.; Devi N. Effective Hydrometallurgical Route for Recovery of Energy Critical Elements from E-Wastes and Future Aspects. Mater. Today: Proc. 2022, 67, 1016–1023. 10.1016/j.matpr.2022.05.491. [DOI] [Google Scholar]
- Mohanty A.; Sahu S.; Sukla L. B.; Devi N. Application of Various Processes to Recycle Lithium-Ion Batteries (LIBs): A Brief Review. Mater. Today: Proc. 2021, 47, 1203–1212. 10.1016/j.matpr.2021.03.645. [DOI] [Google Scholar]
- Horeh N. B.; Mousavi S. M.; Shojaosadati S. A. Bioleaching of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries Using Aspergillus Niger. J. Power Sources 2016, 320, 257–266. 10.1016/j.jpowsour.2016.04.104. [DOI] [Google Scholar]
- Gerold E.; Schinnerl C.; Antrekowitsch H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7 (1), 4. 10.3390/recycling7010004. [DOI] [Google Scholar]
- Jung J. C.-Y.; Sui P.-C.; Zhang J. A Review of Recycling Spent Lithium-Ion Battery Cathode Materials Using Hydrometallurgical Treatments. J. Energy Storage 2021, 35, 102217. 10.1016/j.est.2020.102217. [DOI] [Google Scholar]
- Li L.; Ge J.; Wu F.; Chen R.; Chen S.; Wu B. Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. J. Hazard. Mater. 2010, 176 (1–3), 288–293. 10.1016/j.jhazmat.2009.11.026. [DOI] [PubMed] [Google Scholar]
- He L.-P.; Sun S.-Y.; Mu Y.-Y.; Song X.-F.; Yu J.-G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5 (1), 714–721. 10.1021/acssuschemeng.6b02056. [DOI] [Google Scholar]
- Zhang X.; Li L.; Fan E.; Xue Q.; Bian Y.; Wu F.; Chen R. Toward Sustainable and Systematic Recycling of Spent Rechargeable Batteries. Chem. Soc. Rev. 2018, 47 (19), 7239–7302. 10.1039/C8CS00297E. [DOI] [PubMed] [Google Scholar]
- Nayaka G. P.; Zhang Y.; Dong P.; Wang D.; Pai K. V.; Manjanna J.; Santhosh G.; Duan J.; Zhou Z.; Xiao J. Effective and Environmentally Friendly Recycling Process Designed for LiCoO2 Cathode Powders of Spent Li-Ion Batteries Using Mixture of Mild Organic Acids. Waste Manage. 2018, 78, 51–57. 10.1016/j.wasman.2018.05.030. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Hu X.; He T.; Yuan X.; Li X.; Shi H.; Yang L.; Shao P.; Wang C.; Luo X. Rapid Extraction of Valuable Metals from Spent LiNixCoyMn1-x-yO2 Cathodes Based on Synergistic Effects between Organic Acids. Waste Manage. 2023, 165, 19–26. 10.1016/j.wasman.2023.04.020. [DOI] [PubMed] [Google Scholar]
- Santhosh G.; Nayaka G. P. Cobalt Recovery from Spent Li-Ion Batteries Using Lactic Acid as Dissolution Agent. Clean. Eng. Technol. 2021, 3, 100122. 10.1016/j.clet.2021.100122. [DOI] [Google Scholar]
- Xu M.; Kang S.; Jiang F.; Yan X.; Zhu Z.; Zhao Q.; Teng Y.; Wang Y. A Process of Leaching Recovery for Cobalt and Lithium from Spent Lithium-Ion Batteries by Citric Acid and Salicylic Acid. RSC Adv. 2021, 11 (44), 27689–27700. 10.1039/D1RA04979H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Li J.; Kang D.; Zhou T.; Ma H. A Novel Closed-Loop Process for the Simultaneous Recovery of Valuable Metals and Iron from a Mixed Type of Spent Lithium-Ion Batteries. Green Chem. 2019, 21 (23), 6342–6352. 10.1039/C9GC02844G. [DOI] [Google Scholar]
- Sahu S.; Pati S.; Devi N. A Detailed Kinetic Analysis of the Environmentally Friendly Leaching of Spent Lithium-Ion Batteries Using Monocarboxylic Acid. Metals 2023, 13 (5), 947. 10.3390/met13050947. [DOI] [Google Scholar]
- Meshram P.; Pandey B. D.; Mankhand T. R. Hydrometallurgical Processing of Spent Lithium Ion Batteries (LIBs) in the Presence of a Reducing Agent with Emphasis on Kinetics of Leaching. Chem. Eng. J. 2015, 281, 418–427. 10.1016/j.cej.2015.06.071. [DOI] [Google Scholar]
- Zhang Y.; Meng Q.; Dong P.; Duan J.; Lin Y. Use of Grape Seed as Reductant for Leaching of Cobalt from Spent Lithium-Ion Batteries. J. Ind. Eng. Chem. 2018, 66, 86–93. 10.1016/j.jiec.2018.05.004. [DOI] [Google Scholar]
- Sahu S.; Devi N. Hydrometallurgical Treatment of Spent Lithium Ion Batteries Using Environmentally Friendly Leachant and Extractant. J. Mater. Cycles Waste Manag. 2023, 25 (6), 3303–3315. 10.1007/s10163-023-01754-0. [DOI] [Google Scholar]
- Meng F.; Liu Q.; Kim R.; Wang J.; Liu G.; Ghahreman A. Selective Recovery of Valuable Metals from Industrial Waste Lithium-Ion Batteries Using Citric Acid under Reductive Conditions: Leaching Optimization and Kinetic Analysis. Hydrometallurgy 2020, 191, 105160. 10.1016/j.hydromet.2019.105160. [DOI] [Google Scholar]
- Yang J.; Jiang L.; Liu F.; Jia M.; Lai Y. Reductive Acid Leaching of Valuable Metals from Spent Lithium-Ion Batteries Using Hydrazine Sulfate as Reductant. Trans. Nonferrous Met. Soc. China 2020, 30 (8), 2256–2264. 10.1016/S1003-6326(20)65376-6. [DOI] [Google Scholar]
- Roshanfar M.; Golmohammadzadeh R.; Rashchi F. An Environmentally Friendly Method for Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Gluconic and Lactic Acids. J. Environ. Chem. Eng. 2019, 7 (1), 102794. 10.1016/j.jece.2018.11.039. [DOI] [Google Scholar]
- Meshram P.; Pandey B. D.; Mankhand T. R. Recovery of Valuable Metals from Cathodic Active Material of Spent Lithium Ion Batteries: Leaching and Kinetic Aspects. Waste Manage. 2015, 45, 306–313. 10.1016/j.wasman.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Lei S.; Song S.; Sun W.; Wang L. Stepwise Recycling of Valuable Metals from Ni-Rich Cathode Material of Spent Lithium-Ion Batteries. Waste Manage. 2020, 102, 131–138. 10.1016/j.wasman.2019.09.044. [DOI] [PubMed] [Google Scholar]
- Lei S.; Sun W.; Yang Y. Solvent Extraction for Recycling of Spent Lithium-Ion Batteries. J. Hazard. Mater. 2022, 424, 127654. 10.1016/j.jhazmat.2021.127654. [DOI] [PubMed] [Google Scholar]
- Liu T.; Chen J.; Li H.; Li K. An Integrated Process for the Separation and Recovery of Valuable Metals from the Spent LiNi0.5Co0.2Mn0.3O2 Cathode Materials. Sep. Purif. Technol. 2020, 245, 116869. 10.1016/j.seppur.2020.116869. [DOI] [Google Scholar]
- Liu T.; Chen J.; Shen X.; Li H. Regulating and Regenerating the Valuable Metals from the Cathode Materials in Lithium-Ion Batteries by Nickel-Cobalt-Manganese Co-Extraction. Sep. Purif. Technol. 2021, 259, 118088. 10.1016/j.seppur.2020.118088. [DOI] [Google Scholar]
- Wang F.; Sun R.; Xu J.; Chen Z.; Kang M. Recovery of Cobalt from Spent Lithium Ion Batteries Using Sulphuric Acid Leaching Followed by Solid-Liquid Separation and Solvent Extraction. RSC Adv. 2016, 6 (88), 85303–85311. 10.1039/C6RA16801A. [DOI] [Google Scholar]
- Jha A. K.; Jha M. K.; Kumari A.; Sahu S. K.; Kumar V.; Pandey B. D. Selective Separation and Recovery of Cobalt from Leach Liquor of Discarded Li-Ion Batteries Using Thiophosphinic Extractant. Sep. Purif. Technol. 2013, 104, 160–166. 10.1016/j.seppur.2012.11.024. [DOI] [Google Scholar]
- Sahu S.; Devi N. Effective Leaching of Spent Lithium-Ion Batteries Using DL-Lactic Acid as Lixiviant and Selective Separation of Metals through Precipitation and Solvent Extraction. Environ. Sci. Pollut. Res. Int. 2023, 30 (39), 90152–90167. 10.1007/s11356-022-24560-x. [DOI] [PubMed] [Google Scholar]
- Musariri B.; Akdogan G.; Dorfling C.; Bradshaw S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. 10.1016/j.mineng.2019.03.027. [DOI] [Google Scholar]
- Islam A.; Roy S.; Khan M. A.; Mondal P.; Teo S. H.; Taufiq-Yap Y. H.; Ahmed M. T.; Choudhury T. R.; Abdulkreem-Alsultan G.; Khandaker S.; Awual M. R. Improving Valuable Metal Ions Capturing from Spent Li-Ion Batteries with Novel Materials and Approaches. J. Mol. Liq. 2021, 338, 116703. 10.1016/j.molliq.2021.116703. [DOI] [Google Scholar]
- Yu M.; Zhang Z.; Xue F.; Yang B.; Guo G.; Qiu J. A More Simple and Efficient Process for Recovery of Cobalt and Lithium from Spent Lithium-Ion Batteries with Citric Acid. Sep. Purif. Technol. 2019, 215, 398–402. 10.1016/j.seppur.2019.01.027. [DOI] [Google Scholar]
- Nayaka G. P.; Pai K. V.; Santhosh G.; Manjanna J. Dissolution of Cathode Active Material of Spent Li-Ion Batteries Using Tartaric Acid and Ascorbic Acid Mixture to Recover Co. Hydrometallurgy 2016, 161, 54–57. 10.1016/j.hydromet.2016.01.026. [DOI] [Google Scholar]
- Li L.; Zhai L.; Zhang X.; Lu J.; Chen R.; Wu F.; Amine K. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Ultrasonic-Assisted Leaching Process. J. Power Sources 2014, 262, 380–385. 10.1016/j.jpowsour.2014.04.013. [DOI] [Google Scholar]
- Sahu S.; Devi N. Two-Step Leaching of Spent Lithium-Ion Batteries and Effective Regeneration of Critical Metals and Graphitic Carbon Employing Hexuronic Acid. RSC Adv. 2023, 13 (11), 7193–7205. 10.1039/D2RA07926G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S.; Sun C.; Zhou T.; Gao R.; Xie H. Ultrasonic-Assisted Leaching of Valuable Metals from Spent Lithium-Ion Batteries Using Organic Additives. Sep. Purif. Technol. 2021, 257, 117930. 10.1016/j.seppur.2020.117930. [DOI] [Google Scholar]