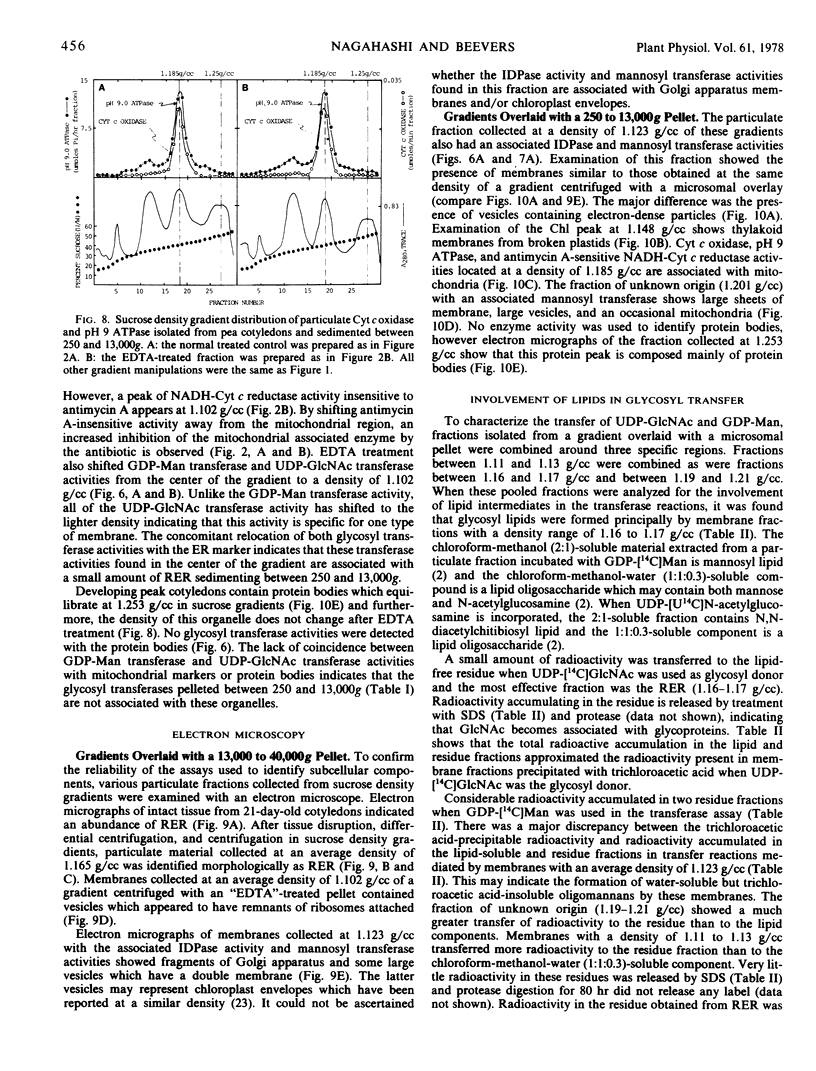
Abstract
Subcellular membrane fractions from 21-day-old pea (Pisum sativum) cotyledons that have associated UDP-N-acetylglucosamine N-acetylglucosaminyl transferase and GDP-mannose mannosyl transferase activities have been isolated and identified. The rough endoplasmic reticulum (RER) is the principal location of glycosyl transferases involved in the assembly of lipid-linked sugar intermediates and glycoproteins. Antimycin A-insensitive NADH-cytochrome c reductase activity was used to identify RER at a density of 1.165 g/cc in sucrose gradients. The high proportion of RER in this fraction was confirmed by electron microscopy.
Other mannosyl transferases are found at a density of 1.123 g/cc and 1.201 g/cc but these glycosyl transferases do not appear to be involved with the formation of lipid-linked sugar intermediates utilized in glycoprotein biosynthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Mense R. M. Glycoprotein Biosynthesis in Cotyledons of Pisum sativum L: Involvement of Lipid-linked Intermediates. Plant Physiol. 1977 Nov;60(5):703–708. doi: 10.1104/pp.60.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Martin S. S. Mitochondrial autonomy: incorporation of monosaccharides into glycoprotein by isolated mitochondria. Science. 1969 Apr 11;164(3876):190–192. doi: 10.1126/science.164.3876.190. [DOI] [PubMed] [Google Scholar]
- Brett C. T., Northcote D. H. The formation of oligoglucans linked to lipid during synthesis of beta-glucan by characterized membrane fractions isolated from peas. Biochem J. 1975 Apr;148(1):107–117. doi: 10.1042/bj1480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depierre J. W., Dallner G. Structural aspects of the membrane of the endoplasmic reticulum. Biochim Biophys Acta. 1975 Dec 29;415(4):411–472. doi: 10.1016/0304-4157(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein synthesis in plants: I. Role of lipid intermediates. Plant Physiol. 1977 Mar;59(3):341–347. doi: 10.1104/pp.59.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T., Valkovich G., Elbein A. D. Glycoprotein biosynthesis in plants. Formation of lipid-linked oligosaccharides of mannose and N-acetylglucosamine by mung bean seedlings. Arch Biochem Biophys. 1976 Jun;174(2):469–479. doi: 10.1016/0003-9861(76)90375-1. [DOI] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Koehler D. E., Leonard R. T., Vanderwoude W. J., Linkins A. E., Lewis L. N. Association of latent cellulase activity with plasma membranes from kidney bean abscission zones. Plant Physiol. 1976 Sep;58(3):324–330. doi: 10.1104/pp.58.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehle L., Fartaczek F., Tanner W., Kauss H. Formation of polyprenol-linked mono- and oligosaccharides in Phaseolus aureus. Arch Biochem Biophys. 1976 Aug;175(2):419–426. doi: 10.1016/0003-9861(76)90529-4. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. Membrane-bound mannosyl transferase in yeast glycoprotein biosynthesis. Biochim Biophys Acta. 1974 May 20;350(1):225–235. doi: 10.1016/0005-2744(74)90220-4. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J. Lipid linked sugars in glycoprotein synthesis. Science. 1975 Jun 6;188(4192):986–991. doi: 10.1126/science.167438. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J. A proposed pathway of plasma glycoprotein synthesis. Mol Cell Biochem. 1975 Jan 31;6(1):3–14. doi: 10.1007/BF01731862. [DOI] [PubMed] [Google Scholar]
- Philipp E. I., Franke W. W., Keenan T. W., Stadler J., Jarasch E. D. Characterization of nuclear membranes and endoplasmic reticulum isolated from plant tissue. J Cell Biol. 1976 Jan;68(1):11–29. doi: 10.1083/jcb.68.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P., Day P. R. An improved method for the isolation of spinach chloroplast envelope membranes. Plant Physiol. 1974 Nov;54(5):780–783. doi: 10.1104/pp.54.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Brew K. Glycosyltransferases in the Golgi membranes of onion stem. Biochem J. 1974 Aug;142(2):203–209. doi: 10.1042/bj1420203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Axelos M., Péaud-Lenoël C. Biosynthesis of mannan and mannolipids from GDP-Man by membrane fractions of sycamore cell cultures. Biochimie. 1976;58(10):1195–1211. doi: 10.1016/s0300-9084(76)80119-8. [DOI] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]