Abstract
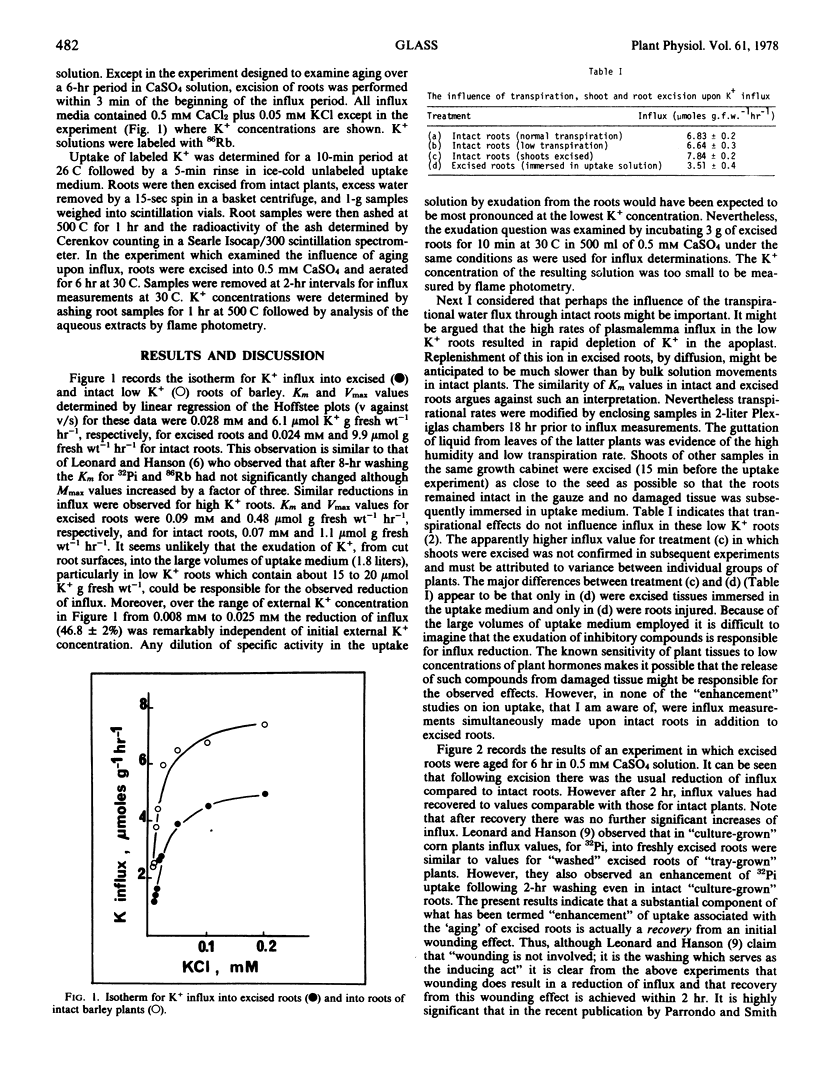
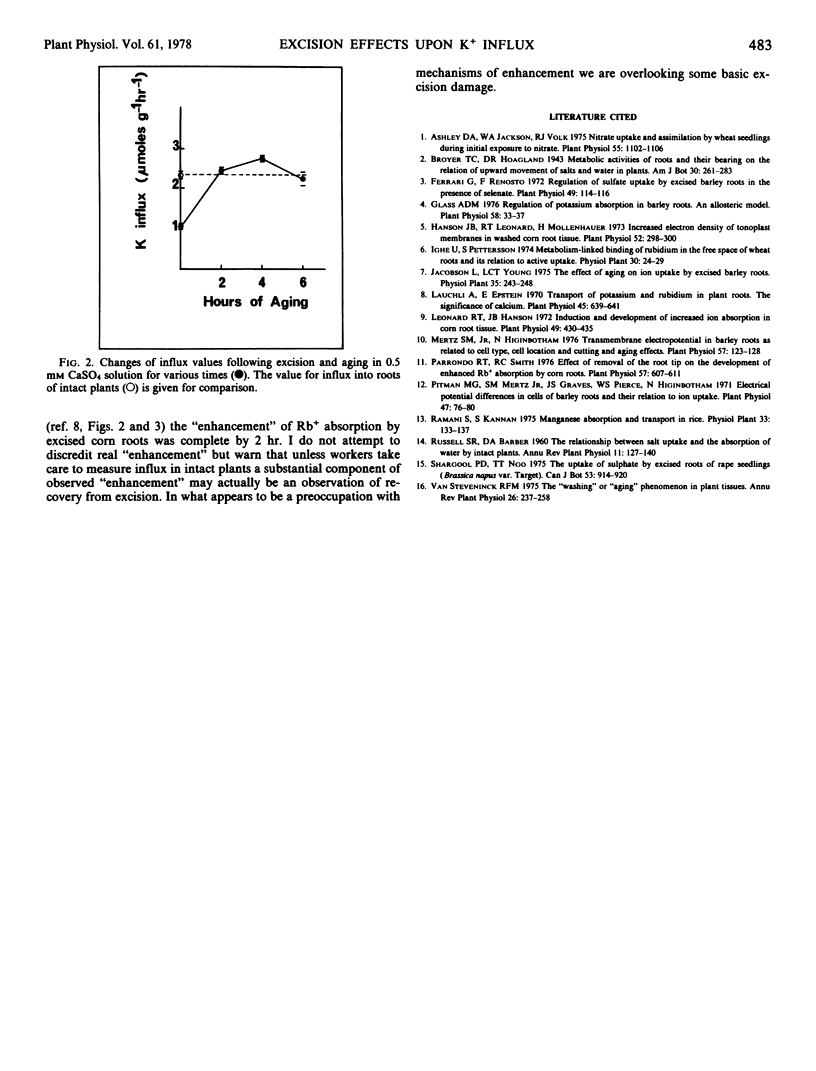
The influx of K+ from 86Rb-labeled solutions in the concentration range 0.008 to 0.2 mm into roots of intact plants and excised roots of barley plants (Hordeum vulgare [L.]) previously grown in 5 mm CaSO4 (low K+ roots) or 0.5 mm CaSO4 plus 5 mm KCl (high K+ roots) was measured. A consistent observation of these experiments was a substantial reduction of influx (usually by about 50%) following excision. The possible leakage of K+ into the medium and subsequent dilution of specific activity of labeled solutions was eliminated as an explanation for influx reduction in excised low K+ roots. Reduction of transpirational rates was also without effect upon influx into low K+ roots. Excision followed by 2 hours aging in 0.5 mm CaSO4 solution revealed that influx values recovered within the 2 hours to the values obtained in intact roots. It is concluded that much of the literature which describes the enhancement of ion uptake following excision actually describes excision damage followed by recovery.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley D. A., Jackson W. A., Volk R. J. Nitrate Uptake and Assimilation by Wheat Seedlings during Initial Exposure to Nitrate. Plant Physiol. 1975 Jun;55(6):1102–1106. doi: 10.1104/pp.55.6.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G., Renosto F. Regulation of sulfate uptake by excised barley roots in the presence of selenate. Plant Physiol. 1972 Feb;49(2):114–116. doi: 10.1104/pp.49.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol. 1976 Jul;58(1):33–37. doi: 10.1104/pp.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Leonard R. T., Mollenháuer H. H. Increased electron density of tonoplast membranes in washed corn root tissue. Plant Physiol. 1973 Sep;52(3):298–300. doi: 10.1104/pp.52.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A., Epstein E. Transport of potassium and rubidium in plant roots: the significance of calcium. Plant Physiol. 1970 May;45(5):639–641. doi: 10.1104/pp.45.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Higinbotham N. Transmembrane electropotential in barley roots as related to cell type, cell location, and cutting and aging effects. Plant Physiol. 1976 Feb;57(2):123–128. doi: 10.1104/pp.57.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrondo R. T., Smith R. C. Effect of removal of the root tip on the development of enhanced rb absorption by corn roots. Plant Physiol. 1976 Apr;57(4):607–611. doi: 10.1104/pp.57.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Mertz S. M., Graves J. S., Pierce W. S., Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971 Jan;47(1):76–80. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]



