Abstract
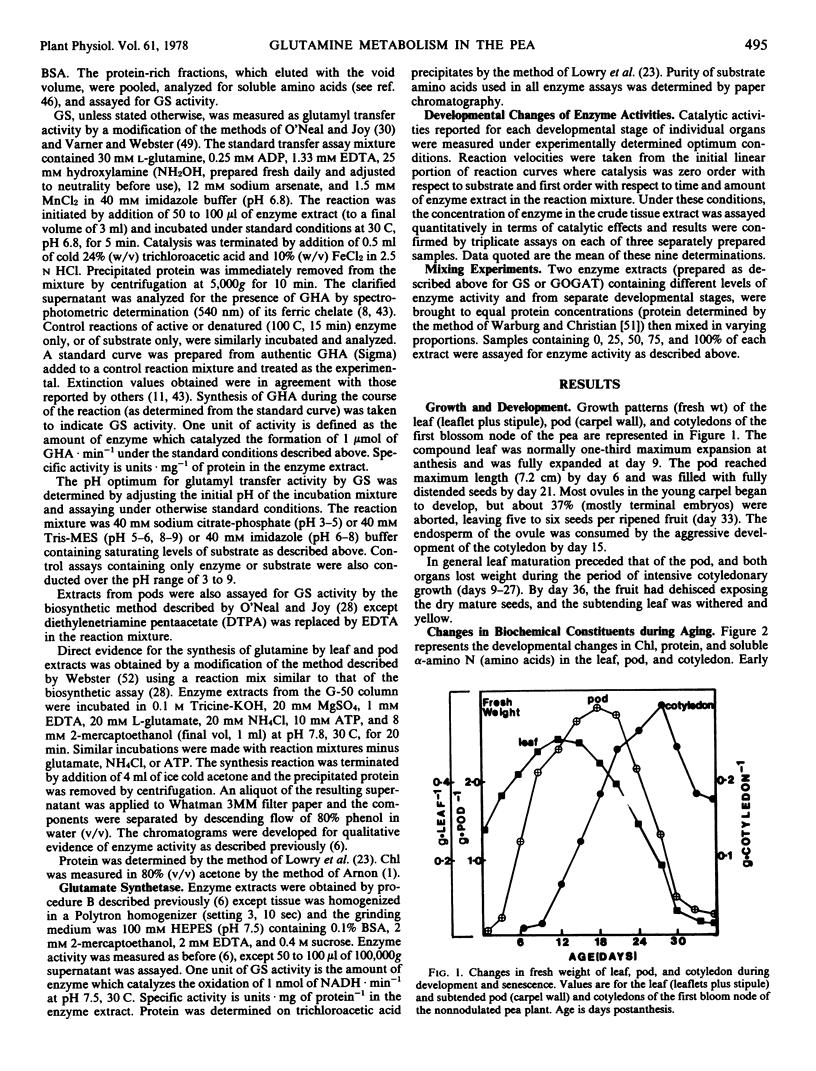
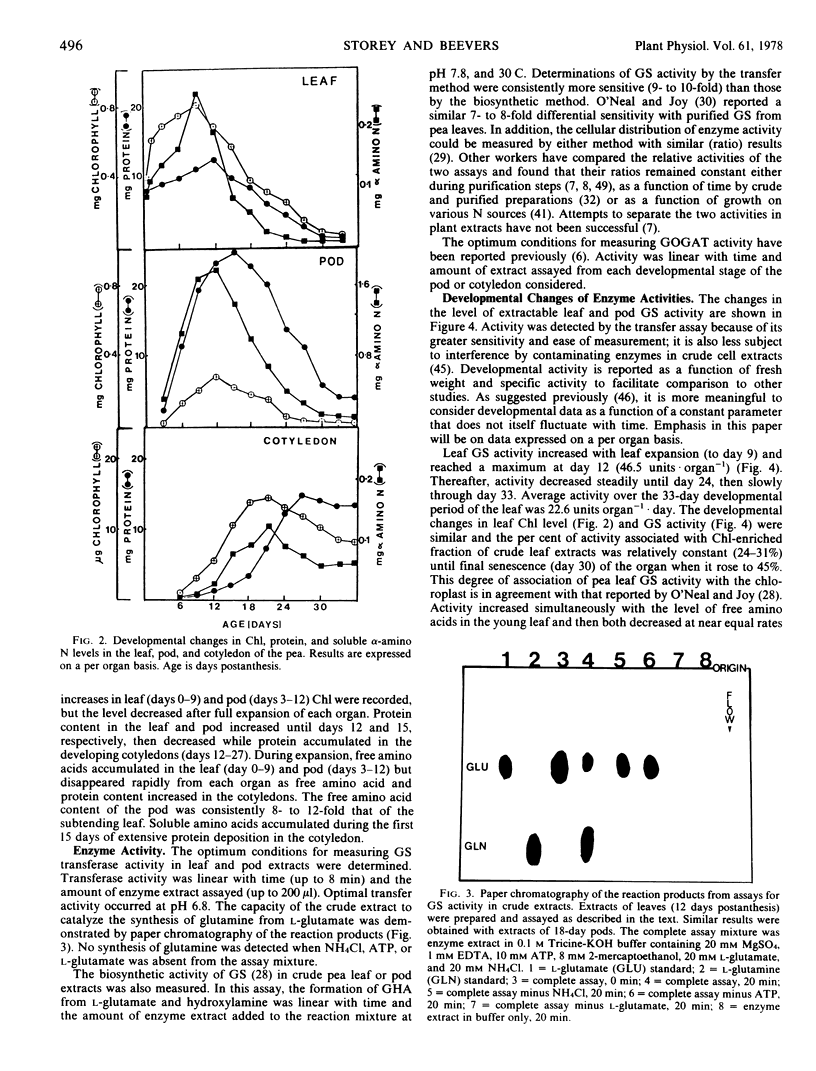
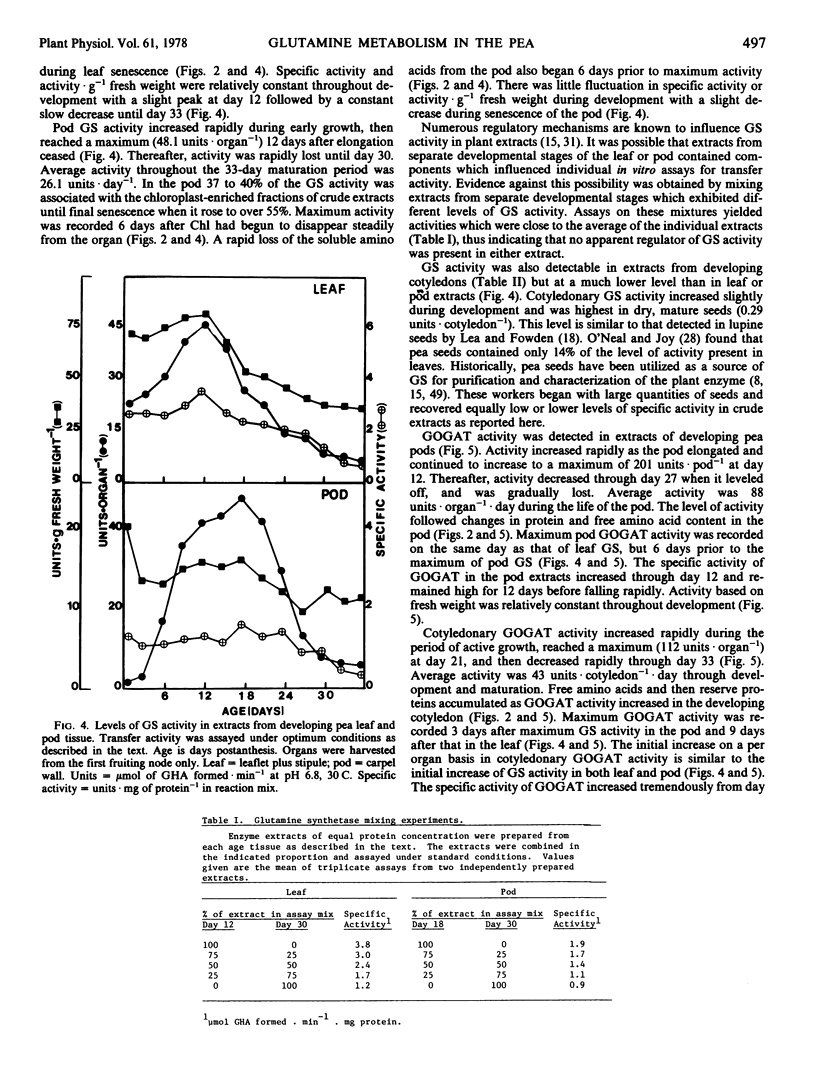
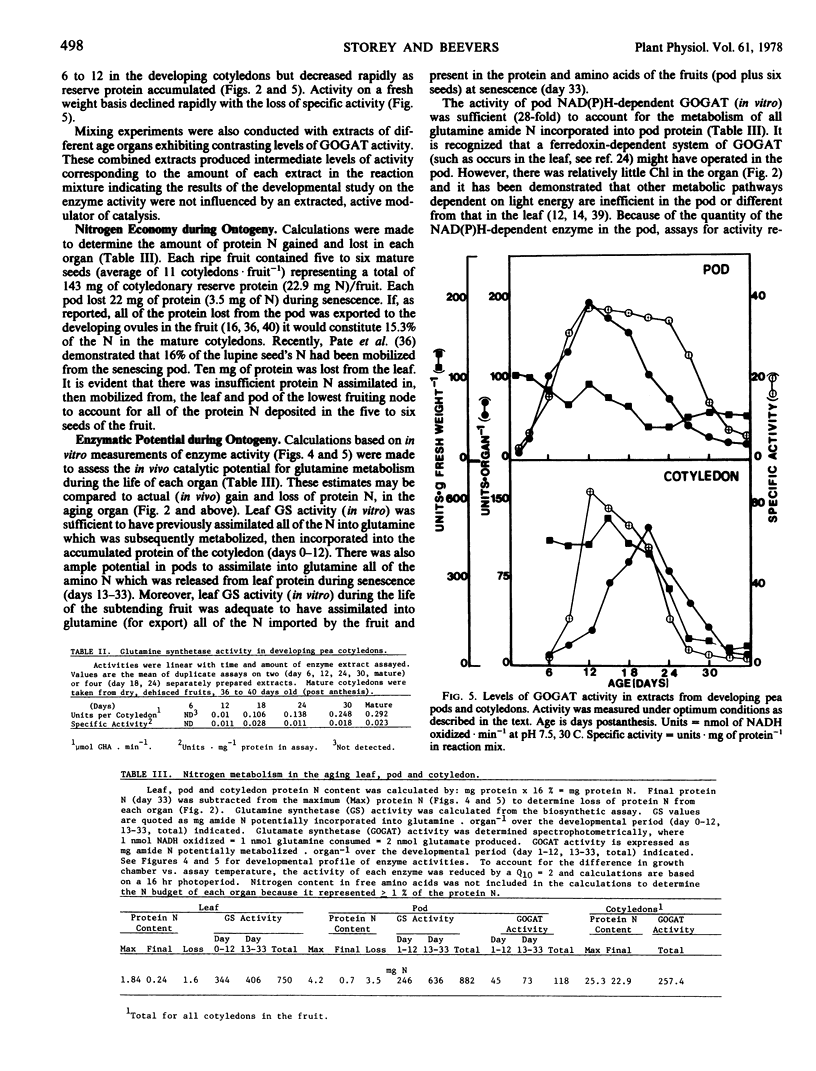
The metabolism of glutamine in the leaf and subtended fruit of the aging pea (Pisum sativum L. cv. Burpeeana) has been studied in relation to changes in the protein, chlorophyll, and free amino acid content of each organ during ontogenesis. Glutamine synthetase [EC 6.3.1.2] activity was measured during development and senescence in each organ. Glutamate synthetase [EC 2.6.1.53] activity was followed in the pod and cotyledon during development and maturation. Maximal glutamine synthetase activity and free amino acid accumulation occurred together in the young leaf. Glutamine synthetase (in vitro) in leaf extracts greatly exceeded the requirement (in vivo) for reduced N in the organ. Glutamine synthetase activity, although declining in the senescing leaf, was sufficient (in vitro) to produce glutamine from all of the N released during protein hydrolysis (in vivo). Maximal glutamine synthetase activity in the pod was recorded 6 days after the peak accumulation of the free amino acids in this organ.
In the young pod, free amino acids accumulated as glutamate synthetase activity increased. Maximal pod glutamate synthetase activity occurred simultaneously with maximal leaf glutamine synthetase activity, but 6 days prior to the corresponding maximum of glutamine synthetase in the pod. Cotyledonary glutamate synthetase activity increased during the assimilatory phase of embryo growth which coincided with the loss of protein and free amino acids from the leaf and pod; maximal activity was recorded simultaneously with maximal pod glutamine synthetase.
We suggest that the activity of glutamine synthetase in the supply organs (leaf, pod) furnishes the translocated amide necessary for the N nutrition of the cotyledon. The subsequent activity of glutamate synthetase could provide a mechanism for the transfer of imported amide N to alpha amino N subsequently used in protein synthesis. In vitro measurements of enzyme activity indicate there was sufficient catalytic potential in vivo to accomplish these proposed roles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Mense R. M. Glycoprotein Biosynthesis in Cotyledons of Pisum sativum L: Involvement of Lipid-linked Intermediates. Plant Physiol. 1977 Nov;60(5):703–708. doi: 10.1104/pp.60.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Storey R. Glutamate Synthetase in Developing Cotyledons of Pisum sativum. Plant Physiol. 1976 Jun;57(6):862–866. doi: 10.1104/pp.57.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. Isolation of glutamine synthetase and glutamotransferase from green peas. J Biol Chem. 1953 Apr;201(2):661–672. [PubMed] [Google Scholar]
- GROSSOWICZ N., WAINFAN E., BOREK E., WAELSCH H. The enzymatic formation of hydroxamic acids from glutamine and asparagine. J Biol Chem. 1950 Nov;187(1):111–125. [PubMed] [Google Scholar]
- Kingdon H. S. Feedback inhibition of glutamine synthetase from green pea seeds. Arch Biochem Biophys. 1974 Jul;163(1):429–431. doi: 10.1016/0003-9861(74)90496-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Localisation of glutamine synthetase in chloroplasts. Nat New Biol. 1973 Nov 14;246(150):61–62. doi: 10.1038/newbio246061a0. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal T. D., Joy K. W. Pea leaf glutamine synthetase: regulatory properties. Plant Physiol. 1975 Jun;55(6):968–974. doi: 10.1104/pp.55.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Growth and Development of Soybean (Glycine max [L.] Merr.) Pods: CO(2) Exchange and Enzyme Studies. Plant Physiol. 1975 Apr;55(4):745–748. doi: 10.1104/pp.55.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAACKE I. D. Protein synthesis in ripening pea seeds. III. Study of the pods. Biochem J. 1957 May;66(1):113–116. doi: 10.1042/bj0660113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys. 1973 Aug;157(2):613–624. doi: 10.1016/0003-9861(73)90681-4. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Webster G. C. Studies on the Enzymatic Synthesis of Glutamine. Plant Physiol. 1955 Sep;30(5):393–402. doi: 10.1104/pp.30.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]



