Sepsis remains a major cause of morbidity and mortality worldwide (1), but progress in identifying effective therapies has been slow (2), reflecting not only the variability of the clinical manifestations of sepsis (e.g., shock, renal failure, respiratory failure, coagulopathy) but also the patient-level biological heterogeneity and complexity of the pathogenic processes responsible for varied clinical manifestations. Although there is a widely accepted need for more research into the clinical heterogeneity of sepsis in both patients and animal models (https://www.nigms.nih.gov/News/Documents), the value of animal models to this research effort has been questioned by some investigators on the basis of differences in genomic responses between animals and humans (3), whereas others have stressed the importance of animal models and the need for further refinements (4, 5). This Viewpoint addresses the value of small animal models in both forward- and reverse-translational investigations of sepsis, with specific attention to their importance in advancing the understanding of disease pathogenesis and preclinical testing of new therapeutics, as well as efforts to improve the relevance of rodent models for the problem of human sepsis.
Many, but not all, biological responses to sepsis and severe infection are evolutionarily conserved in mice, rats, and humans. Although some genomic responses differ, there are similarities between proteomic, lipidomic, and glycomic signatures in mice and humans (3, 6, 7). Mice have been invaluable in dissecting human responses to infection, including the Nobel Prize–winning discovery of Toll-like receptors (8). Moreover, rodents with sepsis develop acute organ failures, such as respiratory failure and shock, and demonstrate heterogeneity in organ injury, as seen in humans.
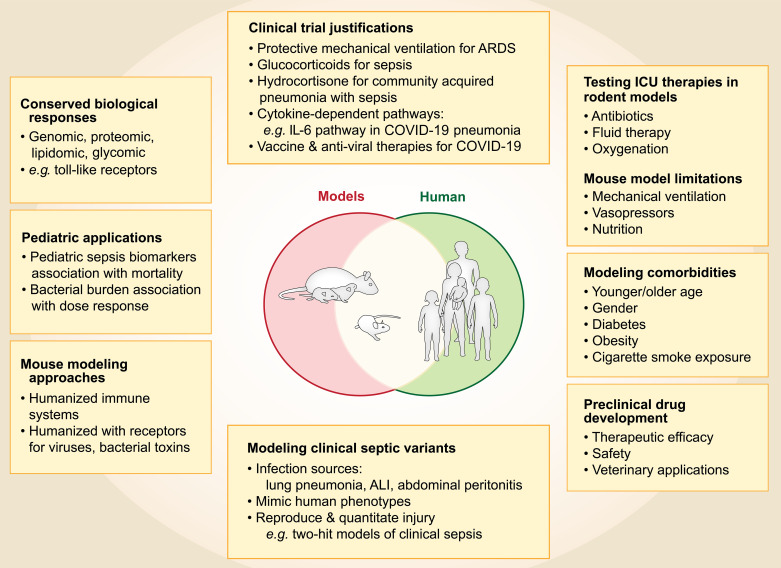
Given these important similarities, rodent models of sepsis have contributed to the scientific foundations of (and justification for) human studies that have led to fundamental advances in therapy of critically ill patients. Rat and mouse models provided the critical preclinical evidence (9, 10) that led to the landmark clinical trial of lung-protective mechanical ventilation for acute respiratory distress syndrome, a common sequela of sepsis, which identified a 9% absolute decrease in mortality (40–31%) (11). Mouse models of sepsis demonstrated the potential value of glucocorticoids (12), providing preclinical evidence supporting a recent clinical trial that reported the major morbidity and mortality benefit of hydrocortisone in severe community-acquired pneumonia with sepsis in critically ill patients (13). Mouse studies have provided important evidence about the importance of cytokine-dependent pathways in sepsis and organ injury, including the importance of the IL-6 pathway, the blockade of which has proven to benefit severe coronavirus disease (COVID-19) pneumonia (14). Furthermore, mouse models were critical for developing vaccines and antiviral therapies necessary to respond to COVID-19 (15).
Like all models, rodent models of sepsis have limitations (4, 5). Rodent models do not typically incorporate the many clinical comorbidities seen in patients or the treatments used in clinical care, including mechanical ventilation, vasopressors, and nutrition. However, efforts are being made to model comorbidities in mouse experiments, including using mice of younger and older ages; mice of different genders; and mice with diabetes, obesity, or cigarette smoke exposure, to more closely mimic the heterogeneity of human sepsis (16). In addition, the clinical value of rodent models has improved with the ability to monitor oxygenation and include antibiotics and fluid therapy (17). Animal models are sometimes criticized for lack of individual heterogeneity, but the ability to modify a single aspect of human heterogeneity in mice is actually a strength of the models, making them ideal for reverse-translation. For example, recent work in pediatrics illustrates how clinically identified biomarkers in children with sepsis can be reverse-translated to mice with experimental sepsis, with the results subsequently translated back to humans. Mice identified as having a high risk for mortality using the clinical biomarkers had a greater bacterial burden and could be rescued with higher doses of antibiotics, providing biological support for the hypothesis that high-risk critically ill children with septic shock may benefit from higher antibiotic doses (18).
Just as there is no stereotyped natural history of human sepsis, there is not a perfect animal model of human sepsis (3–5). Indeed, research in sepsis benefits from the complementary use of different animal models, varying the source of infection (lungs as in pneumonia and acute lung injury, the abdomen as in peritonitis) to reflect the different clinical disorders that cause human sepsis. Thus, rodent models can be selected to mimic the specific human sepsis phenotype being investigated and should include key quantitative and reproducible measures of injury (19).
“Humanized” mice that are genetically modified with a humanized immune system or human receptors for viruses and/or bacterial toxins augment relevance to human sepsis and syndromes such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections (20). Although there is a high degree of similarity between mouse and human genomes, the specific gene clusters in mice that best simulate the response to sepsis in humans are still uncertain, providing a rationale for this experimental approach.
In therapeutic drug development, rodent models provide valuable preclinical testing of new therapies and help to identify candidates for clinical testing in humans. Rodent models enable early critical evaluation of the safety and potential efficacy of new therapeutics for sepsis and other diseases, although predicting efficacy in humans with sepsis has been difficult. Nevertheless, without these preclinical models, direct human testing would be necessary for the initial development of novel therapeutics, which would increase the risk of toxicity in early-phase human studies that could have been prevented with the use of appropriate animal models. Additionally, veterinarians taking a “One Health” approach have translated data collected from rodent models of sepsis to the clinical management of severe infections and sepsis in cats, dogs, horses, and other animals (21). Thus, rodent models of sepsis have direct benefit to domestic animal species, in addition to humans.
Animal welfare in research is stringently protected by local and national animal research committees to minimize the distress and suffering of rodents and other animals used in research. Investigators must include a specific plan for vertebrate animal protection as part of the rigorous peer-review process for NIH and other federal grant applications. Once a research program is approved and funded, investigators must comply with three levels of oversight. The first is that of the local institutional animal care and use committee, which oversees and approves all animal research at an institution. The second is the NIH Office of Animal Welfare, which helps local institutional animal care and use committees interpret and enforce NIH Public Health Service policy, including the Guide for the Care and Use of Animals. The third is the international oversight provided by the Association for Assessment and Accreditation of Laboratory Animal Care International, which provides accreditation and oversight of animal research programs at most NIH-funded institutions.
In summary, rodent models have made important contributions to the understanding and treatment of sepsis and acute lung injury. In addition, improved rodent models using rapidly evolving genetic and molecular technologies, clinically relevant comorbidities, and appropriate safeguards for animal care will continue to be very important for future research in sepsis and other critical illnesses (Figure 1).
Figure 1.
The translational value of rodent models for human sepsis. ALI = acute lung injury; ARDS = acute respiratory distress syndrome.
Acknowledgments
Acknowledgment
The authors thank Diana Lim for the preparation of Figure 1.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202308-1489VP on December 13, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet . 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, et al. Redefining critical illness. Nat Med . 2022;28:1141–1148. doi: 10.1038/s41591-022-01843-x. [DOI] [PubMed] [Google Scholar]
- 3. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA . 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. Murine models of sepsis and trauma: can we bridge the gap? ILAR J . 2017;58:90–105. doi: 10.1093/ilar/ilx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai L, Rodgers E, Schoenmann N, Raju RP. Advances in rodent experimental models of sepsis. Int J Mol Sci . 2023;24:9578. doi: 10.3390/ijms24119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Arras L, Seng A, Lackford B, Keikhaee MR, Bowerman B, Freedman JH, et al. An evolutionarily conserved innate immunity protein interaction network. J Biol Chem . 2013;288:1967–1978. doi: 10.1074/jbc.M112.407205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hippensteel JA, LaRiviere WB, Colbert JF, Langouët-Astrié CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol . 2020;319:L211–L217. doi: 10.1152/ajplung.00199.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss HJ, O’Neill LAJ. Of flies and men—the discovery of TLRs. Cells . 2022;11:3127. doi: 10.3390/cells11193127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis . 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 10. Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med . 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 11. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med . 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12. Ghoneim HE, McCullers JA. Adjunctive corticosteroid therapy improves lung immunopathology and survival during severe secondary pneumococcal pneumonia in mice. J Infect Dis . 2014;209:1459–1468. doi: 10.1093/infdis/jit653. [DOI] [PubMed] [Google Scholar]
- 13. Dequin PF, Meziani F, Quenot JP, Kamel T, Ricard JD, Badie J, et al. CRICS-TriGGERSep Network Hydrocortisone in severe community-acquired pneumonia. N Engl J Med . 2023;388:1931–1941. doi: 10.1056/NEJMoa2215145. [DOI] [PubMed] [Google Scholar]
- 14. Gu T, Zhao S, Jin G, Song M, Zhi Y, Zhao R, et al. Cytokine signature induced by SARS-CoV-2 spike protein in a mouse model. Front Immunol . 2021;11:621441. doi: 10.3389/fimmu.2020.621441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bastarache JA. The future of sepsis research: time to think differently? Am J Physiol Lung Cell Mol Physiol . 2020;319:L523–L526. doi: 10.1152/ajplung.00368.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plataki M, Fan L, Sanchez E, Huang Z, Torres LK, Imamura M, et al. Fatty acid synthase downregulation contributes to acute lung injury in murine diet-induced obesity. JCI Insight . 2019;5:e127823. doi: 10.1172/jci.insight.127823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gotts JE, Bernard O, Chun L, Croze RH, Ross JT, Nesseler N, et al. Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice. Am J Physiol Lung Cell Mol Physiol . 2019;317:L717–L736. doi: 10.1152/ajplung.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong HR, Caldwell JT, Cvijanovich NZ, Weiss SL, Fitzgerald JC, Bigham MT, et al. Prospective clinical testing and experimental validation of the Pediatric Sepsis Biomarker Risk Model. Sci Transl Med . 2019;11:eaax9000. doi: 10.1126/scitranslmed.aax9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kulkarni HS, Lee JS, Bastarache JA, Kuebler WM, Downey GP, Albaiceta GM, et al. Update on the features and measurements of experimental acute lung injury in animals: an official American Thoracic Society workshop report. Am J Respir Cell Mol Biol . 2022;66:e1–e14. doi: 10.1165/rcmb.2021-0531ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boguslawski KM, McKeown AN, Day CJ, Lacey KA, Tam K, Vozhilla N, et al. Exploiting species specificity to understand the tropism of a human-specific toxin. Sci Adv . 2020;6:eaax7515. doi: 10.1126/sciadv.aax7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García A, Fox JG. A One Health perspective for defining and deciphering Escherichia coli pathogenic potential in multiple hosts. Comp Med . 2021;71:3–45. doi: 10.30802/AALAS-CM-20-000054. [DOI] [PMC free article] [PubMed] [Google Scholar]