Abstract
Objectives:
Quality of surgery has recently become an essential topic in the prognosis of colon cancer. Complete mesocolic excision for colon cancer has recently gained popularity with high-quality surgery. Patient specimens after complete mesocolic excision with central vessel ligation procedures have an integrity of the mesocolon and the yield of three fields of lymph node harvest. We apply the glacial acid, absolute ethanol, water, and formaldehyde solution to each specimen based on the Japanese classification of lymph node groups and station numbers. We aim to identify the distribution and status of lymph node metastasis according to each tumor site and some pathological characteristics related to this disease.
Methods:
A prospective cohort study was performed on 45 laparoscopic complete mesocolic excision surgery patients.
Results:
2791 lymph nodes were harvested after complete mesocolic excision surgery. The average number was 62.0 ± 22.3 nodes. The mean tumor size (in the largest dimension) was 4.2 ± 1.8 cm. The average length of the resected bowel segments was 29.1 ± 7.7 cm. There are 63 (2.3%) node metastases in 2791 lymph nodes, in which 17/45 (37.8%) patients had pN(+). The minimum positive node size was 1 mm. The positive pericolic lymph nodes (station 1) accounted for the highest rate, with 53 nodes (1.9%). The number of lymph nodes in young age ⩽60 is more significant than in older. The results were similar, with a more significant node retrieval in the group with a tumor size >4.5 cm and specimen length >25 cm. The number of lymph nodes in lower tumor invasive (pT1,3) was smaller than pT4. Our research shows that the cecum, ascending, and descending colon had greater nodes than others, with a mean number of 78.6, 74.2, and 71.3, respectively.
Conclusions:
The metastasis and harvested lymph nodes accounted for the highest rate of colon cancer in station 1 and the lowest rate in station 3. The number of retrieved lymph nodes was significantly associated with tumor location, size, specimen length, and patient age.
Keywords: Lymph node, harvesting, retrieval, CME, colon cancer, GEWF
Introduction
Quality of surgery has recently become an essential topic in the prognosis of colon cancer (CC). 1 Complete mesocolic excision (CME) for CC has recently gained popularity with high-quality surgery. 2 This technique has become increasingly popular in recent years after evidence of increased disease-free survival (DFS) after CME in 2009. 3 Bertelsen et al. 4 report the 5-year outcomes with a significant reduction in recurrence to 9.7% in patients of the CME group versus 17.9% for those undergoing non-CME surgery in Union for International Cancer Control (UICC) 5th stage I–III for right-sided CC. And the mean nodal yield of the CME group was more than that of the control group (38 versus 21 nodes). The medical evidence on increased free disease survival after CME is mainly based on the results of some prominent authors such as Hohenberger, Bokey, and Bertelsen et al.3,5
En bloc resection of the colon and the mesocolon allows for precise CC staging and improves prognosis. 6 Patient specimens after CME with central vessel ligation (CVL) procedures have an integrity of the mesocolon and the yield of three fields of lymph node (LN) harvest.3,7 Especially, the extended longitudinal resection after CME with CVL significantly increased the lymph node yield (LNY). 8 Pathologic staging is the primary determinant of treatment and prognosis for patients with colorectal cancer, and LN status plays a significant role in the staging classification.9–12 International guidelines currently accept the concept that a minimum of 12 LNs is a quality measure. 12 However, the evidence is weak, and the debates are certainly still open on whether considering a limit of 12 nodes improves staging accuracy and prognosis. Despite this, more than one-quarter of patients are still incompletely resected by this standard. 13 The understanding of the lymphatic spread of CC is inconsistent. Hohenberger describes the spread as located in the pericolic LNs, but no more than 8 cm from the primary, and that it enters the LNs of central supply arteries, 3 while the range of regional LNs is divided according to feeding arteries about 10 cm from the tumor margin of the Japanese Society for Cancer of the Colon and Rectum (JSCCR). 14
The presence of metastatic LNs in the main group categorized as N3 using JSCCR classification was also not mentioned in the American Joint Committee on Cancer (AJCC) 8th and UICC 5th editions. And LN metastasis outside the regional LNs is classified as distant metastasis (M1) presenting in the JSCCR system but not in the AJCC and UICC classifications.14,15 CME colectomy has a more extended resected segment of the colon than traditional surgery and the complete mesentery with it, including regional LNs and those beyond regional LNs that belong to the tumor. We have, therefore, conducted a prospective analysis of our specimens to identify the distribution and status of LN metastasis according to each tumor site based on the Japanese classification of LN groups and station numbers. The assessment of LN metastasis in the main group is significant for CME with CVL procedure.
Methods
Patient cohort
A prospective cohort study was performed on patients undergoing laparoscopic CME surgery at the Digestive Surgery Departments between May 2021 and October 2022. This study was approved by the Institutional Review Board of our University (IRB Reference Number: H2021/443). Every patient gave written informed consent to take part in the study. Our cohort consisted of 45 patients, including 20 (46.7%) females and 25 (53.3%) males (ratio 1:1.2). Mean age was 60.9 years (median 60.9, range 28–88).
The database included patients with AJCC 8th stage I–III CC. Exclusion criteria were the presence of distant metastasis, multiprimary cancer in different locations, familial adenomatous polyposis or hereditary nonpolyposis, palliative resection, or an emergency operation.
These patients underwent laparoscopic colectomy with the CME and central vascular ligation principle at the feeding blood vessel branches that directly the tumor according to the Japanese Society of Colorectal Cancer guidelines for classification and treatment and Hohenberger’s principles of CME surgery.3,14,16 Fifty-one patients were included in this study, but three cases were converted to open surgery due to a large tumor, one case was peritoneal metastasis detected during surgery, one case did not perform CME technique with CVL, and one case changed the histopathological result after surgery.
The morphologic evaluation of the dissection plane in a surgical specimen is categorized based on the state of the dissection surface in the following ways 6 :
- Mesenteric plane: good-quality surgery, mesenteric surface intact, and smooth.
- Intramesocolic plane: the surgery is of moderate quality, with disruption not reaching the muscular layer in the mesocolon.
- Muscularis propria plane: poor-quality surgery exposing the muscularis propria.
Tumor histology: tumor grade was described as low grade: well differentiated, moderate grade: moderately differentiated, and high grade: poorly differentiated.
Database demographics and clinical data, such as the relationship between the number of LNs and age, sex, The American Society of Anesthesiologists (ASA) scoring system, 17 a nutrition risk score (NRS) based on quantitative subjective global assessment (Q-SGA), 18 tumor location, carcinoembryonic antigen (CEA), tumor size was calculated by the largest diameter of the tumor (cm), specimen length (cm), and AJCC 8th staging of patients, tumor histology were shown in Tables 1 and 2.
Table 1.
Associations of the number of retrieved lymph nodes with different clinicopathological parameters.
| Factors | n | % | Total | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|---|
| 45 | 2791 | 62.02 | 22.30 | 22 | 117 | ||
| Age | |||||||
| ⩽60 | 24 | 53.3% | 1628 | 67.83 | 24.26 | 35 | 117 |
| >60 | 21 | 46.7% | 1163 | 55.38 | 18.17 | 22 | 88 |
| Gender | |||||||
| Male | 25 | 55.6% | 1580 | 63.20 | 24.54 | 30 | 113 |
| Female | 20 | 44.4% | 1211 | 60.55 | 19.67 | 22 | 117 |
| ASA | |||||||
| 1 | 30 | 66.7% | 2016 | 67.20 | 21.78 | 36 | 117 |
| 2 | 12 | 26.7% | 612 | 51.0 | 18.74 | 22 | 85 |
| 3 | 3 | 6.7% | 163 | 54.33 | 30.11 | 30 | 88 |
| NRS/Q-SGA* | |||||||
| A | 24 | 53.3% | 1567 | 65.29 | 22.89 | 35 | 113 |
| B | 17 | 37.8% | 1013 | 59.59 | 21.33 | 22 | 117 |
| C | 4 | 8.9% | 211 | 52.75 | 24.78 | 30 | 88 |
| Tumor location | |||||||
| Cecum | 7 | 15.6% | 550 | 78.57 | 15.68 | 60 | 110 |
| Ascending | 6 | 13.3% | 445 | 74.17 | 21.50 | 58 | 113 |
| Hepatic flexure | 8 | 17.8% | 514 | 64.25 | 23.81 | 30 | 107 |
| Transverse | 2 | 4.4% | 107 | 53.50 | 6.36 | 49 | 58 |
| Splenic flexure | 2 | 4.4% | 86 | 43.00 | 9.90 | 36 | 50 |
| Descending | 3 | 6.7% | 214 | 71.33 | 39.70 | 45 | 117 |
| Sigmoid | 17 | 37.8% | 875 | 51.47 | 17.61 | 22 | 96 |
| CEA level | |||||||
| Non elevated ⩽ 5ng/ml | 26 | 57.8% | 1568 | 60.31 | 21.66 | 22 | 113 |
| Evaluated | 19 | 42.2% | 1223 | 64.37 | 23.53 | 33 | 117 |
| pT | |||||||
| 1 | 2 | 4.4% | 80 | 40.00 | 25.46 | 22 | 58 |
| 2 | 21 | 46.7% | 1396 | 66.48 | 27.02 | 30 | 117 |
| 3 | 18 | 40.0% | 1034 | 57.44 | 13.03 | 33 | 81 |
| 4 | 4 | 8.9% | 281 | 70.25 | 23.87 | 48 | 96 |
| pN | |||||||
| No | 28 | 62.2 | 1814 | 64.79 | 25.27 | 22 | 117 |
| N1–3 | 17 | 37.8 | 977 | 57.47 | 15.95 | 30 | 85 |
| AJCC 8th stage | |||||||
| I | 20 | 44.4 | 1342 | 67.10 | 27.90 | 22 | 117 |
| II | 8 | 17.8 | 472 | 59.00 | 17.19 | 43 | 96 |
| III | 17 | 37.8 | 977 | 57.47 | 15.95 | 30 | 85 |
| Tumor histology | |||||||
| High | 34 | 75.6% | 2196 | 64.59 | 24.78 | 22 | 117 |
| Moderate | 9 | 20.0% | 476 | 52.89 | 8.85 | 36 | 64 |
| Low | 2 | 4.4% | 119 | 59.50 | 0.71 | 59 | 60 |
| Tumor size, the largest dimension | |||||||
| ⩽4.5 | 26 | 57.8 | 1473 | 56.65 | 21.34 | 22 | 113 |
| >4.5 | 19 | 42.2 | 1318 | 69.37 | 22.00 | 37 | 117 |
| Specimen length | |||||||
| ⩽25 | 17 | 37.8 | 970 | 57.06 | 20.22 | 22 | 96 |
| >25 | 28 | 62.2 | 1821 | 65.04 | 23.30 | 30 | 117 |
Well nourished (SGA-A), moderately malnourished (SGA-B), or severely malnourished (SGA-C).
Table 2.
Factors affecting the number of retrieved lymph nodes.
| Factors | n | IRR (95 % CI) | p-Value a |
|---|---|---|---|
| Age | |||
| ⩽60 | 24 | 1.225 (1.136–1.321) | <0.01* |
| >60 | 21 | Ref | |
| Gender | |||
| Male | 25 | 1.044 (0.968–1.125) | 0.262 |
| Female | 20 | Ref | |
| ASA | |||
| 1 | 30 | Ref | |
| 2 | 12 | 0.76 (0.69–0.83) | <0.01* |
| 3 | 3 | 0.81 (0.70–0.95) | <0.01* |
| NRS/Q-SGA | |||
| A | 24 | Ref | |
| B | 17 | 0.91 (0.84–0.99) | 0.023* |
| C | 4 | 0.81 (0.70–0.93) | 0.004* |
| Tumor location | |||
| Cecum | 7 | Ref | |
| Ascending | 6 | 0.94 (0.83–1.07) | 0.366 |
| Hepatic flexure | 8 | 0.82 (0.72–0.92) | 0.001* |
| Transverse | 2 | 0.68 (0.55–0.84) | <0.001* |
| Splenic flexure | 2 | 0.55 (0.44–0.69) | <0.001* |
| Descending | 3 | 0.91 (0.78–1.06) | 0.230 |
| Sigmoid | 17 | 0.66 (0.59–0.73) | <0.001* |
| CEA level | |||
| Non elevated ⩽5 ng/ml | 26 | Ref | |
| Evaluated | 19 | 1.07 (0.99–1.15) | 0.088 |
| pT | |||
| 1 | 2 | 0.57 (0.44–0.73) | <0.001* |
| 2 | 21 | 0.95 (0.83–1.08) | 0.398 |
| 3 | 18 | 0.82 (0.72–0.93) | 0.003* |
| 4 | 4 | Ref | |
| pN | |||
| No | 28 | 1.13 (1.04–1.22) | 0.003* |
| N1–3 | 17 | Ref | |
| AJCC 8th stage | |||
| I | 20 | 1 | |
| II | 8 | 0.88 (0.79–0.98) | 0.016* |
| III | 17 | 0.86 (0.79–0.93) | <0.01* |
| Histology | |||
| High | 34 | Ref | |
| Moderate | 9 | 0.82 (0.74–0.90) | <0.01* |
| Low | 2 | 0.92 (0.77–1.11) | 0.383 |
| Tumor size | |||
| ⩽4.5 | 26 | Ref | |
| >4.5 | 19 | 1.22 (1.14–1.32) | <0.01* |
| Specimen length | |||
| ⩽25 | 17 | Ref | |
| >25 | 28 | 1.14 (1.05–1.23) | 0.001* |
Negative binomial with log link.
p < 0.05.
The study’s primary outcome was to evaluate different clinicopathological factors determining the number and distribution of LNs and the rate of LN metastasis by tumor location and LN groups.
This study has been reported in line with the STROBE criteria. 19
Pathological examination
After taking the specimen from the patient, the surgeon team quickly analyzed and measured parameters such as the integrity of the mesentery and some macroscopic results on the specimen. The length of the large bowel, the length of the ileum for the right side, the distance from the tumor to the high vessel tie, the closest bowel wall to the high vascular tie, and other parameters were measured on each patient’s colon specimen using morphometric quantitation. This specimen opened along the colon and measured tumor size in three dimensions. Then, this was resected one-fourth of the tumor at the suspected site of maximal invasion, usually at the thickest site in the middle of the tumor or the site of serosa or internal retraction. This part of the tumor was fixed in a 10% formalin solution. The remaining part was intact, with the colon mesentery fixed in a GEWF solution (Glacial acid, absolute ethanol, water, and formaldehyde) within 6–12 h. All mesenteric specimens after fixation were carefully dissected by one surgeon to fully record the LNs by groups with a size ⩾1 mm. We dissected the visceral peritoneum of the mesocolon. Based on the color of the ivory-white LNs, which is different from the surrounding yellow fatty tissue, to identify the LNs. When we take the LNs, we determine their position with the surrounding blood vessels to determine the location of the LNs by groups and station numbers. LN groups were coded according to the Colorectal Cancer Society. 14 For example, group #201 is a pericolic LN belonging to LN station 1 of the ascending ileocecal colon. The GEWF solution whitens the LNs and yellows the mesenteric fat tissue to facilitate the retrieval of these nodes. Each LN group was contained in a numbered tissue cassette to avoid confusion. The tumor was routinely cut by 5 mm around the suspected maximal invasion site to evaluate the tumor depth and morphologic classification of the dissection plane. These LNs are placed into individual cassettes marked with the patient code and station number with a pencil. If the LNs are too large >1 cm, the LNs are split in half but not separated and then placed separately for this large-sized group.
Two gastrointestinal pathologists independently reevaluated the original histopathological slides. We analyze the tumor stage according to the AJCC 8th TNM classification. Histologic type and grading were analyzed according to the WHO guidelines. 20
100-ml GEWF solution includes 85-ml ethanol absolute (CAS No. 64-17-5), 10-ml formaldehyde 37% (CAS No. 50-00-0), and 5-ml acetic acid (CAS No. 64-19-7).
Statistical analysis
A generalized linear model with a negative binomial and log link function was utilized to examine the association between the number of LNs and the predicted variables. The incidence rate ratios and 95% confidence intervals (CIs) were employed to determine the relationship between the predictors and outcome. Statistical significance was identified at a level of p < 0.05. Statistical analysis was performed using SPSS version 20.0.
Results
With 2791 LNs harvested after surgery in 45 patients, the average number was 62.0 ± 22.3 nodes (range: 22–117). The mean tumor size (in the largest dimension) was 4.2 ± 1.8 cm. The average length of the resected bowel segments was 29.1 ± 7.7 cm. 86.7% of patients had the mesocolic plane, and 13.3% had the intramesocolic plane.
Associations of the number of retrieved LNs and clinicopathological parameters are shown in Table 1. Most patients had an ASA score of 1 with 66.7%, significantly higher than ASA 2 and 3. Most of them were classified as well nourished (SGA-A), with 53.3%. Tumor locations were unevenly distributed in the study, with sigmoid colon tumors accounting for the most at 37.8%, while transverse colon and splenic flexure were only 4.4% each. Pathologically staged after microscopic examination of the resected specimen (pTNM), pT2 and pT3 were 86.7%, and pN0 was 62.2%.
Factors affecting the number of LNs harvested are shown in Table 2. The number of LNs was independent of the CEA level and gender. The number of LNs in young age ⩽60 is more significant than in older. The results were similar, with a more significant node retrieval in the group with a tumor size >4.5 cm and specimen length >25 cm (Figure 1). Amounts of the LNs in pTNM stage II and III were less significant than in stage I. However, the number of LNs in lower tumor invasive (pT1,3) was smaller than pT4 (Figure 2).
Figure 1.
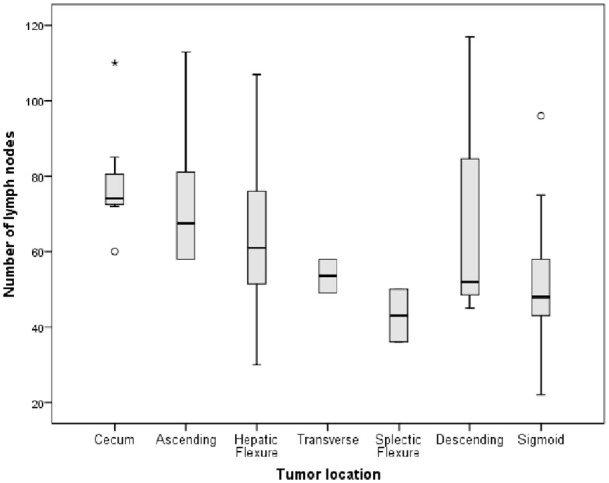
Relationship between the tumor sites and the number of lymph nodes.
Figure 2.
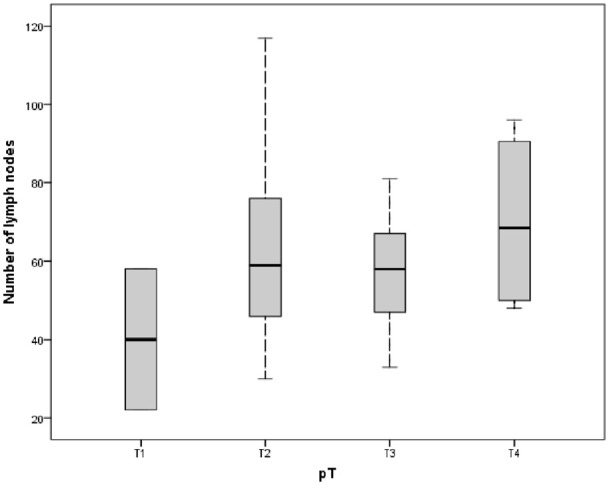
Relationship between the primary tumor (invasive carcinoma) (pT) and the number of lymph nodes.
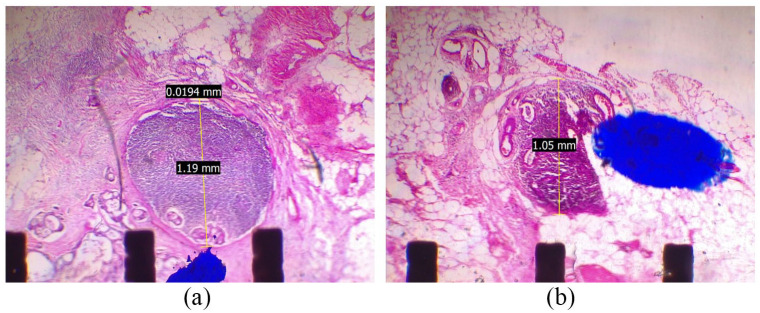
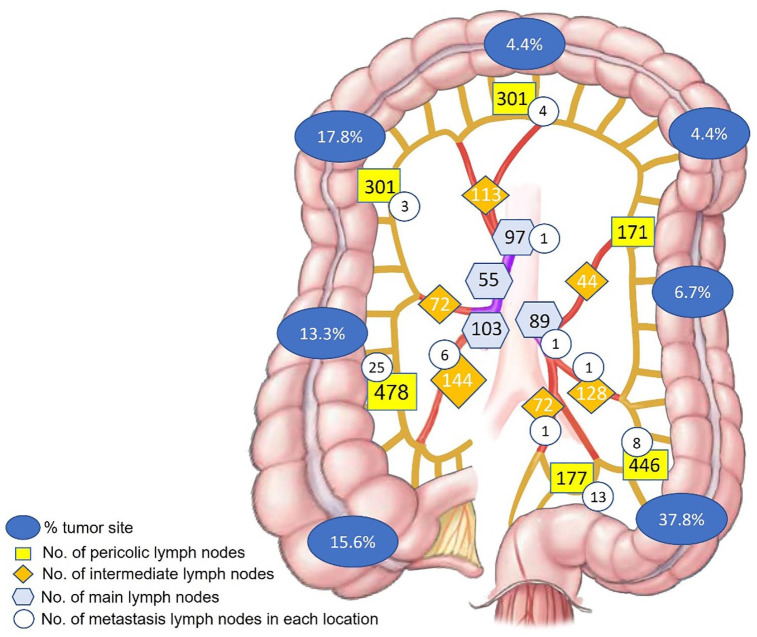
There are 63 (2.3%) node metastases in 2791 LNs, in which 17/45 (37.8%) patients had pN(+). The minimum positive node size was 1 mm (Figure 3). Our data showed that positive pericolic LNs (station 1) accounted for the highest rate, with 53 nodes (1.9%). Otherwise, only 8 (0.3%) positives in intermediate LNs (station 2) and 2 (0.07%) positives in main LNs (station 3) according to the JSCCR 14 (Figure 4), (Table 3).
Figure 3.
(a and b) The positive lymph nodes with 1 mm in size.
Figure 4.
The quantity and distribution of lymph nodes following up the tumor sites and the rate of lymph node metastasis in each regional LN.
Table 3.
Total number of lymph nodes in every station.
| Lymph node | Station 1 | Station 2 | Station 3 | Total |
|---|---|---|---|---|
| Pericolic lymph nodes | Intermediate lymph nodes | Main lymph nodes | ||
| Positive | 53 (1.9%) | 8 (0.3%) | 2 (0.07%) | 63 |
| No. nodes | 1874 (67.2%) | 573 (20.5%) | 344 (12.3%) | 2791 |
No. nodes: number of lymph nodes.
Discussion
CME surgery is increasingly popular because it is associated with better survival and prognosis outcomes.3–5 The results of extended lymphadenectomy in CME colectomy help pathological LN analysis much more efficiently, especially the number of LN retrieval. 21 Our data indicated that using the GEWF solution increases the number of LN harvests, especially in the case of CME. The specimen with CME surgery had an entire regional mesentery submitted to LN examination. It underwent GEWF solution and dissection to find all LNs, whether as small as 1 mm in size. According to Brown et al., 22 83% of additional LNs were under 2 mm because LNs are white, which makes detection easier when GEWF is employed. In extra nodes identified, 75% of all positive nodes were under 2.0 mm in size. Märkl et al. 23 stated that small LNs (less than 1 mm) play almost no role in the proper histopathological LN staging. However, they agreed that the finding of relatively small LNs (1–5 mm) was crucial for precise LN staging and was prognostically significant, with a link between a high LN harvesting rate and a favorable prognosis in CC.
The average number of LNs in our results was 62.0 ± 22.3, similar to Ahmadi et al.’s 24 study, which revealed 61 and 71 median LNs per cadaver in the ascending mesocolon and sigmoid, respectively. The research of Hida et al. 25 showed that the mean number of nodes examined per patient was 76.4 after the clearing method was performed. For adequate CC staging, guidelines advise regional lymphadenectomy with an LNY of at least 12 LNs. 15 Several recent studies have shown that the more LNs are harvested, the better the results. For example, Simões et al. 26 showed that LNY 22 LNs was related to prolonged DFS and overall survival (OS), especially for right-sided CC. 26 Guan et al. 27 revealed that 5-year cancer-specific survival was significantly improved for stage I–III right-sided CC patients with ⩾15 LNs.
Most patients were on ASA score of 1 with 66.7% and well nourished (SGA-A) with 53.3%. The impact of ASA score and NRS evaluated on short- and long-term morbidity and mortality rates of CC patients undergoing curative surgery.28,29 However, a normal healthy (ASA1) and well-nourished patient had significantly higher harvested LN numbers than the other group. Tekkis et al. 30 showed that increasing age and ASA grade significantly reduced the average number of LNs retrieved from the resection specimens. Cancer development is the progression from the primary tumor site or metastasis in cancer through lymphatics. This progression is consistent with developing LNs and systemic metastases from a localized cancer. Ferris believed that the roles of lymphatics, nodal metastasis, and antitumor immunity are related. 31 We have not found a way to explain how the number of LNs and the patient’s physical condition relate to this study’s results. However, patients with good immunity, general health, and nutritional status seem to have more LNs.
The gender and CEA level did not affect the LN retrieval. Although the CEA level is independent of LNs, the 18-node standard could be viewed as an alternative to the 12-node standard supported by the AJCC 8th guidelines to improve long-term survival and accurately determine the nodal stage for patients with CEA-elevated (⩾5 ng/ml) CC. 32 There are few studies on the correlation between the number of LNs and gender. Ichimasa et al. 33 suggest that the attribution of the female is correlated with LN metastasis in pT1 colorectal cancer.
Similar to the study of Shen et al., 34 the number of LN retrieval was significantly associated with the length of resected segments, patient age, and tumor location. 12 The LNs retrieved in older patients were fewer in number. 30 Shen recognized that reactive LNs are enlarged and easier to identify than normal LNs. Hence, Guan et al. 35 recommended that the LN examination for young CC patients be assessed differently, using a 22-node measure that may be more appropriate for CC patients under 40. And the other study suggested that a nine-node measure was available for patients aged ⩾80. 36 On the relationship between the length of the segment and the number of LNs, the length >25 cm had more LNs harvested than the group ⩽25 cm. One of the essential conclusions from Shen’s study was that the length of resected bowel segment was associated with the number of LNs recovered. Regarding tumor location, depending on the type of resection, the number of LNs decreases from the proximal to the distal location.12,30,34,37 Our research shows that the cecum, ascending, and descending colon had greater nodes than others, with a mean number of 78.6, 74.2, and 71.3, respectively.
The number of harvested LNs is associated with higher tumor stage and size. 12 Betge et al. 12 showed that tumor sizes >4.5 cm and higher AJCC stages were significantly associated with LN count. According to our data, more LNs were obtained in the group tumor size >4.5 cm. Our data show that a higher number of LNs harvested were associated with T-classification, but high LN count is unrelated to the tumor stage. In the SEER database searched for pN0 CCs, Ning et al. 38 showed that retrieved LNs were identified as an independent prognostic factor, and at least 18 LNs were associated with favorable prognosis in patients with pN0 CC. And this showed an alternative cut-off value for survival analysis for pN0 classification. Following this database, Cai et al. 39 revealed that a minimum of 19 LNs must be examined for optimal survival and adequate node staging in LN-negative right-sided CC. Individuals with 19 or more LN retrieval had a greater prevalence of LNs metastasis than those with fewer than 19 nodes. Although the number of LN retrieval is not proportional to the tumor stage, it still helps pN classification accurately. Some studies have suggested an association between survival and LN harvest.26,36
Although tumor sites were unevenly distributed in the colon, we plotted a correlation between the harvested LN sites and the metastatic nodes in a total of 2791 nodes. The total metastasis LN rate was 2.3%. However, the number of patients who had pN(+) (positive regional LNs) was 17 (37.8%), which corresponds to the study of Bertelsen et al., 5 with 35% in the CME group. The evaluation of small LNs is also essential. In the study of Schrembs et al., 40 up to 51% of metastatic LNs were 2–6 mm in size. 40 Although the positive LN rate at station 3 was 0.07% of the total LNs, up to 2/45 (4.4%) patients have positive LNs detected at this station. LN metastasis determines the advanced stage of cancer progression and requires adjuvant treatment after surgery. Although the rate of central LN metastasis is low, LNs can metastasize in theory, so all regional LNs should be removed during surgery.
Detection and evaluation of metastatic LNs by manual LN dissection is the standard of many treatment facilities, even major centers in our country. However, a methylene blue solution or fat clearance process can optimize LN retrieval. 23 These LN retrievals showed a significantly higher number of LNs, from an average of 20.8 with manual LN dissection increased to 68.8 LN harvesting when they tested the entire residual mesenteric fat in Brown et al.’s study. 22 More importantly, the pTNM disease stage of the patients was increased. That said, careful examination of mesenteric LNs may be necessary to ensure accurate pN status. The use of solutions after surgery helps to diagnose the stage of the disease more accurately, specifically increasing the patient’s stage after surgery, helping to identify patients who need adjuvant treatment more accurately after surgery. Another study by Hernanz et al. 41 showed that the additional LNs increased to about 10, and 4.4% of LNs revealed tumor metastasis when using a fat clearance solution.
There are many solutions to fix and detect LNs after surgery, for example, acetone, alcohol-xylene, methylene, and GEWF. However, GEWF is safe, cheap, and easy to prepare. 9
However, we need to proceed with more sample sizes and patient follow-ups to assess better the relationship between harvested LNs and OS. Our data are continuously collected, and we will publish the results of surgery, treatment, and 3-year OS time after surgery. Additional studies of solution use after the GEWF solution should be continued at our facility and others, comparing the results of using this solution and not using it, and comparing the results of using this solution and another solution (Carnoy) so that this solution can be used routinely on specimens and can help diagnose the postoperative staging more accurately.
Conclusions
LN harvesting is determined by many factors, such as the extent of lymphadenectomy during surgery, the length of the resected bowel segment, the use of fat clearance solution on the postoperative specimen, and many other factors associated with clinicopathological parameters with LN dissection. Adequate nodal staging is important in the pTNM stage. This process is performed by good-quality colectomy surgery and pathologists to maximize the number of LNs.
The metastasis nodes and harvested LNs accounted for the highest rate in station 1 and the lowest rate in station 3 of CC. The number of retrieved LNs was significantly associated with tumor location, size, specimen length, and patient age.
Acknowledgments
We would like to thank all members of the Digestive Surgery Department and the Pathology Department for their efforts and support.
Footnotes
Author contribution: MT Nguyen, CT Dang, and AV Pham identified the study concept and design. MT Nguyen and AV Pham performed a draft and synthesis of the data. MT Nguyen, DD Le, and AV Pham conducted the statistical analysis. MT Nguyen drafted the manuscript; CT Dang, TBS Nguyen, NC Pham, MD Pham, HT Nguyen, DTD Phan, DVP Nguyen, TP Nguyen, PV Doan, and DS Nguyen revised it. All authors participated in the approval of the final version.
CRediT authorship contribution statement: MT Nguyen: Concept, Method, Data collection, Data analysis. Data curation, Writing—original draft, Writing—review and editing. CT Dang: Conceptualization, Formal analysis, revision. TBS Nguyen: Data collection, revision. NC Pham: Data collection, revision. DD Le: Software, Resources, Data curation, Data analysis. MD Pham: Data collection, revision. HT Nguyen: Data collection, revision. DT Dung Phan: Data collection, revision. DVP Nguyen: Data collection, revision. TP Nguyen: Data collection, revision. PV Doan: Data collection, revision. DS Nguyen: Data collection, revision. AV Pham: Concept, Method, Data collection, Data analysis. Data curation, Writing—review and editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Minh Thao Nguyen was funded by the PhD Scholarship Program of Vingroup Innovation Foundation (VINIF), code VINIF.2023.TS.115.
Ethics approval: Ethical approval for this study was obtained from the Institutional Review Board of Hue University of Medicine and Pharmacy (Approval Number/ID: H2021/443).
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: Not applicable.
ORCID iD: Minh Thao Nguyen  https://orcid.org/0000-0003-0311-2019
https://orcid.org/0000-0003-0311-2019
References
- 1. den Dulk M, van de Velde CJ. Time to focus on the quality of colon-cancer surgery. Lancet Oncol 2008; 9: 815–817. [DOI] [PubMed] [Google Scholar]
- 2. West NP. Complete mesocolic excision for colon cancer: is now the time for a change in practice? Lancet Oncol 2019; 20: 1474–1476. [DOI] [PubMed] [Google Scholar]
- 3. Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 2009; 11: 354–364. [DOI] [PubMed] [Google Scholar]
- 4. Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015; 16: 161–168. [DOI] [PubMed] [Google Scholar]
- 5. Bertelsen CA, Neuenschwander AU, Jansen JE, et al. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 2019; 20: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 6. Hoshino N, Hida K, Sakurai T, et al. Pathologic assessment and specimen quality of surgery after CME. In: Liang J-T, Sugihara K, Kim NK. (eds.) Surgical treatment of colorectal cancer: Asian perspectives on optimization and standardization. Singapore: Springer, 2018, pp. 277–283. [Google Scholar]
- 7. West NP, Hohenberger W, Weber K, et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010; 28: 272–278. [DOI] [PubMed] [Google Scholar]
- 8. West NP, Kobayashi H, Takahashi K, et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 2012; 30: 1763–1769. [DOI] [PubMed] [Google Scholar]
- 9. Horne J, Bateman AC, Carr NJ, et al. Lymph node revealing solutions in colorectal cancer: should they be used routinely? J Clin Pathol 2014; 67: 383–388. [DOI] [PubMed] [Google Scholar]
- 10. Schumacher P, Dineen S, Barnett C, et al. The metastatic lymph node ratio predicts survival in colon cancer. Am J Surg 2007; 194: 827–832. [DOI] [PubMed] [Google Scholar]
- 11. Tsai HL, Huang CW, Yeh YS, et al. Factors affecting number of lymph nodes harvested and the impact of examining a minimum of 12 lymph nodes in stage I–III colorectal cancer patients: a retrospective single institution cohort study of 1167 consecutive patients. BMC Surg 2016; 16: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Betge J, Harbaum L, Pollheimer MJ, et al. Lymph node retrieval in colorectal cancer: determining factors and prognostic significance. Int J Colorectal Dis 2017; 32: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madoff RD. Defining quality in colon cancer surgery. J Clin Oncol 2012; 30: 1738–1740. [DOI] [PubMed] [Google Scholar]
- 14. Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon 2019; 3: 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jessup JM, Goldberg RM, Asare EA, et al. Colon and rectum. In: Greene FL, Fleming ID, Page DL, et al. (eds.) AJCC Cancer Staging Manual. New York: Springer, 2017, pp. 251–274. [Google Scholar]
- 16. Kessler H, Hohenberger W. Extended lymphadenectomy in colon cancer is crucial. World J Surg 2013; 37: 1789–1798. [DOI] [PubMed] [Google Scholar]
- 17. American Society of Anesthesiologists. ASA physical status classification system. Committee Econ 2020; 1–4. http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system
- 18. Nursal TZ, Noyan T, Tarim A, et al. A new weighted scoring system for Subjective Global Assessment. Nutrition 2005; 21: 666–671. [DOI] [PubMed] [Google Scholar]
- 19. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13: S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, et al. (eds.) WHO classification of tumors of the digestive system. Lyon: IARC Press, 2010, pp. 131–182. [Google Scholar]
- 21. Gao Z, Wang C, Cui Y, et al. Efficacy and safety of complete mesocolic excision in patients with colon cancer: three-year results from a prospective, nonrandomized, double-blind, controlled trial. Ann Surg 2020; 271: 519–526. [DOI] [PubMed] [Google Scholar]
- 22. Brown HG, Luckasevic TM, Medich DS, et al. Efficacy of manual dissection of lymph nodes in colon cancer resections. Mod Pathol 2004; 17: 402–406. [DOI] [PubMed] [Google Scholar]
- 23. Märkl B, Röle J, Arnholdt HM, et al. The clinical significance of lymph node size in colon cancer. Mod Pathol 2012; 25: 1413–1422. [DOI] [PubMed] [Google Scholar]
- 24. Ahmadi O, Mccall JL, Stringer MD. Mesocolic lymph node number, size, and density: an anatomical study. Dis Colon Rectum 2015; 58: 726–735. [DOI] [PubMed] [Google Scholar]
- 25. Hida J, Mori N, Kubo R, et al. Metastases from carcinoma of the colon and rectum detected in small lymph nodes by the clearing method. J Am Coll Surg 1994; 178: 223–228. [PubMed] [Google Scholar]
- 26. Simões P, Fernandes G, Costeira B, et al. Lymph node yield in the pathological staging of resected nonmetastatic colon cancer: the more the better? Surg Oncol 2022; 43: 101806. [DOI] [PubMed] [Google Scholar]
- 27. Guan X, Chen W, Liu Z, et al. Whether regional lymph nodes evaluation should be equally required for both right and left colon cancer. Oncotarget 2016; 7: 59945–59956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aro R, Ohtonen P, Rautio T, et al. Perioperative oral nutritional support for patients diagnosed with primary colon adenocarcinoma undergoing radical surgical procedures -Peri-Nutri Trial: study protocol for a randomized controlled trial. BMC Nutr 2022; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park JH, Kim DH, Kim BR, et al. The American Society of Anesthesiologists score influences on postoperative complications and total hospital charges after laparoscopic colorectal cancer surgery. Medicine (Baltimore) 2018; 97(18): e0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tekkis PP, Smith JJ, Heriot AG, et al. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis Colon Rectum 2006; 49: 1673–1683. [DOI] [PubMed] [Google Scholar]
- 31. Ferris RL, Lotze MT, Leong SPL, et al. Lymphatics, lymph nodes and the immune system: barriers and gateways for cancer spread. Clin Exp Metastasis 2012; 29(7): 729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H, Wang C, Liu Y, et al. The optimal minimum lymph node count for carcinoembryonic antigen elevated colon cancer: a population-based study in the SEER set and External set. BMC Cancer 2023; 23(1): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ichimasa K, Kudo S, Miyachi H, et al. Does gender predict lymph node metastasis in pT1 colorectal cancer? A systematic review and meta-analysis. Gastrointest Endosc 2016; 83: AB363–AB364. [Google Scholar]
- 34. Shen SS, Haupt BX, Ro JY, et al. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med 2009; 133: 781–786. [DOI] [PubMed] [Google Scholar]
- 35. Guan X, Wang Y, Hu H, et al. Reconsideration of the optimal minimum lymph node count for young colon cancer patients: a population-based study. BMC Cancer; 18(1): 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guan X, Chen W, Jiang Z, et al. Exploration of the optimal minimum lymph node count after colon cancer resection for patients aged 80 years and older. Sci Rep 2016; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lavy R, Madjar-Markovitz H, Hershkovitz Y, et al. Influence of colectomy type and resected specimen length on number of harvested lymph nodes. Int J Surg 2015; 24: 91–94. [DOI] [PubMed] [Google Scholar]
- 38. Ning FL, Pei JP, Zhang NN, et al. Harvest of at least 18 lymph nodes is associated with improved survival in patients with pN0 colon cancer: a retrospective cohort study. J Cancer Res Clin Oncol 2020; 146: 2117–2133. [DOI] [PubMed] [Google Scholar]
- 39. Cai Y, Cheng G, Lu X, et al. The re-evaluation of optimal lymph node yield in stage II right-sided colon cancer: is a minimum of 12 lymph nodes adequate? Int J Colorectal Dis 2020; 35: 623–631. [DOI] [PubMed] [Google Scholar]
- 40. Schrembs P, Martin B, Anthuber M, et al. The prognostic significance of lymph node size in node-positive colon cancer. PLoS One 2018; 13(8): e0201072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hernanz F, García-Somacarrera E, Fernández F. The assessment of lymph nodes missed in mesenteric tissue after standard dissection of colorectal cancer specimens. Colorectal Dis 2009; 12: e57–e60. [DOI] [PubMed] [Google Scholar]