Abstract
Lung cancer poses a global health challenge and stands as the leading cause of cancer-related deaths worldwide. However, its incidence, mortality, and characteristics are not uniform across all regions worldwide. Understanding the factors contributing to this diversity is crucial in a prevalent disease where most cases are diagnosed in advanced stages. Hence, prevention and early diagnosis emerge as the most efficient strategies to enhance outcomes. In Western societies, tobacco consumption constitutes the primary risk factor for lung cancer, accounting for up to 90% of cases. In other geographic locations, different significant factors play a fundamental role in disease development, such as individual genetic predisposition, or exposure to other carcinogens such as radon gas, environmental pollution, occupational exposures, or specific infectious diseases. Comprehensive clinical and molecular characterization of lung cancer in recent decades has enabled us to distinguish different subtypes of lung cancer with distinct phenotypes, genotypes, immunogenicity, treatment responses, and survival rates. The ultimate goal is to prevent and individualize lung cancer management in each community and improve patient outcomes.
Keywords: geographic differences, molecular diagnosis, molecular epidemiology, NSCLC
Introduction
Lung cancer is the second most diagnosed tumor worldwide, accounting for 2.2 million new cases globally across both genders. It is also the leading cause of cancer-related deaths, responsible for up to 1.8 million deaths in 2020. 1 While the distribution of lung cancer is relatively consistent, some regions have significantly higher incidence rates than others when adjusting by age. For example, Hungary and Serbia in Europe or French Polynesia in Oceania have high incidence rates. Conversely, Western or Middle Africa has very low lung cancer incidence rates. 1 These disparities can be attributed to variations in the distribution of risk factors, in addition to differences in reporting.
Among the risk factors for lung cancer, up to 80–90% of cases are attributed to tobacco consumption. 2 However, the World Health Organization (WHO) declares many other carcinogens including indoor radon gas, environmental pollution, asbestos, occupational exposure, or radiation. 3 Recent evidence shows that beyond external carcinogens, ethnicity 4 and genetic predisposition (associated with germline pathogenic variants in well-known high/moderate-penetrance cancer predisposition genes) 5 may also play a crucial role in lung cancer risk and the molecular profile of the lung tumor.
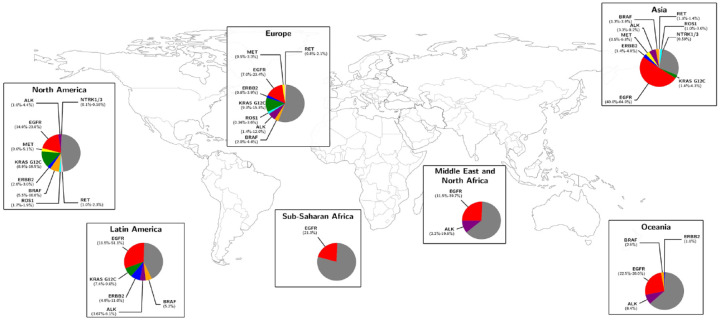
From a pathological perspective, lung cancer can be classified into two main subtypes: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), with NSCLC representing around 85% of cases. 6 Molecular characterization of NSCLC has revolutionized the treatment and management of these patients by identifying specific driver oncogenic alterations, for which targeted therapies have been developed. Thus, testing for oncogenic drivers – such mutations in EGFR, KRAS, BRAF, MET, ERBB2, fusions, or rearrangements in ALK, ROS1, RET, NTRK1/3, among others – is essential in NSCLC management. 7 Emerging evidence suggests that the prevalence of these genetic alterations in NSCLC varies significantly across different regions, influenced by epidemiological factors such as geography, ethnicity, smoking, or gender. 8 For instance, mutations in EGFR are more frequent in women with lung adenocarcinoma, especially among Asian descent and non-smokers, 9 while KRAS G12C mutations in NSCLC are associated with smoking history. 10 Distribution of main actionable genomic alterations across continents is represented in Figure 1.
Figure 1.
Distribution of the main actionable genomic alterations in non-small-cell lung cancer across the five continents. The gray proportion of each sector graphic represents ‘non-reported’ molecular information. Studies used for collecting the prevalence of each alteration are represented in Supplemental Table 1S.
Hence, the geographic distribution of lung carcinogens and their interaction with the specific particularities of each individual may impact the different patterns of lung cancer phenotypes and genotypes, immunogenicity, response to therapies, and even survival in different populations. Understanding the basis for different susceptibilities is essential to develop proper preventive measures and personalize lung cancer management for each population. In this review, we aim to examine the worldwide differences in lung cancer, with a particular focus on molecular alterations.
Lung cancer in America
Lung cancer in North America
Lung cancer incidence and mortality
Lung cancer remains a major health challenge in the United States (US) and Canada, with high incidence and mortality rates. In 2020, there were 227,875 new diagnoses of lung cancer in the US, comprising 10% of all tumor cases and ranking as the second most prevalent cancer type, following breast cancer. Lower incidence was observed in Canada, with 25,574 new lung cancer cases accounting for 9.3% of all cancer cases and ranking as the third leading cause of cancer in the country. 1 While historically lung cancer primarily affected men, the American Cancer Society projects that its incidence is rising among women. By 2023, it is estimated that out of 238,340 cases in the United States, 51% will be diagnosed in women. 11 In addition, lung cancer is the leading cause of cancer death in North America, accounting for approximately 160,000 deaths in the US and 23,000 deaths in Canada annually.11,12
Lung cancer carcinogens in North America
Tobacco smoking is the main cause of lung cancer in North America and is responsible for the high incidence rates. 11 Pan American Health Association published in their last report that 23% of adults in the US and 13% in Canada smoke tobacco, with higher rates among men. 13 Throughout the Americas, tobacco consumption is overall declining but mainly among men, while it has increased among women. This leads to a relatively similar male-to-female ratio in tobacco use compared to the global average (1.9 in the Americas versus 4.7 worldwide).
Beyond tobacco, other environmental factors contribute to the increased risk of developing lung cancer in both Canada and the US. 14 Radon, a radioactive gas that arises naturally as a decay product of uranium-238, stands as the second leading cause of lung cancer in the US. 15 Because there is no known safe level of radon exposure, the United States Environmental Protection Agency (EPA) recommends fixing homes with radon levels at or above 4 pCi/L (equivalent to 148 Bq/m3). 16 Despite this recommendation, some US territories such as Colorado, Idaho, Montana, Alaska, South Dakota, etc. are well above this limit. 17 Conversely, the median residential radon in Canada is 82 Bq/m3. However, high uranium concentrations in glacial tills and derived soils in the Western Prairies make the regions of Alberta and Saskatchewan in Canada the second-highest worldwide for indoor radon levels.18,19
Air pollution, specifically environmental particles measuring ⩽ 2.5 μm (PM2.5), has been associated with an increased lung cancer risk. 20 It is recommended that annual exposure to PM 2.5 should not exceed 5 µg/m3. 21 Both Canada and the US consistently record average levels lower than 10 μg/m3 but regions such as California, New York, and Pennsylvania in the US22,23 are significantly higher.
In addition, industries such as mining, construction, manufacturing, and transportation often entail exposure to hazardous agents such as asbestos, silica, diesel exhaust, and various chemicals. 14 Doll and Peto attributed up to 4% of US cancer deaths to occupational factors, with lung cancer being the major contributor. 24 Steenland et al. later estimated a similar range of 2.4–4.8% for such contributors. 25 The Occupational Cancer Research Centre reported that up to 8% of the lung cancer cases in Canada were caused by occupational asbestos and 2.5% of the cases by diesel engine exhaust. 26
Intrinsic factors related to lung cancer in North America
Differences in lung cancer incidence based on ethnicity or race have been described in the US. Data from the Surveillance, Epidemiology, and End Results Program show the highest rates of lung cancer (76.1 per 100,000) among African Americans compared to other ethnic groups, despite a lower prevalence of smoking. This suggests the existence of other factors that may make these populations more susceptible to lung cancer. 27 The Multiethnic Cohort study has investigated disease rates and genetic variations among five different ethnic groups in the US revealing disparities in genes involved in metabolism and carcinogen exposure, which could play a role in cancer predisposition. 28 Interestingly, when considering individuals who smoked less than 30 cigarettes daily, African Americans and Native Hawaiians had a notably higher risk of developing lung cancer compared to other populations.
These data further support ethnic and racial differences in lung cancer risk and carcinogenesis, which could be explained by a genetic background not completely understood. Mukheree et al. studied 5118 patients with NSCLC and SCLC at the Memorial Sloan Kettering Cancer Center in New York, finding pathogenic germline variants (PGV) in high/moderate penetrance genes in 4.3% of the patients, mainly in DNA damage repair (DDR) pathway genes. Other studies reported the prevalence of PGV across ancestry in the US, showing that individuals of European ancestry have a higher prevalence of genetic variants in DDR genes including BRCA2, CHEK2, ATM, and BRCA1.29,30
Molecular characterization of lung cancer in North America
The prevalence of oncogenic driver alterations in NSCLC, including EGFR, ALK, MET, and BRAF V600E, has been extensively studied in North America. A study conducted by Kris et al. in 2014 analyzed the prevalence of EGFR mutations in patients with NSCLC from the Lung Cancer Mutation Consortium database, which included a large number of patients from North America. The study reported that EGFR mutations were present in approximately 17% of NSCLC patients in North America, with a higher prevalence observed in female patients, non-smokers, and adenocarcinoma histology. 31 Another report analyzed data from The Cancer Genome Atlas and found a similar prevalence of EGFR mutations in North American patients with NSCLC. 32 The prevalence of ALK fusions in patients with NSCLC in North America has also been investigated in several studies. For example, a study by Shaw et al. reported a prevalence of ALK fusions of approximately 4% in patients with NSCLC in North America. 33 MET aberrations, including amplifications and mutations, are less frequent compared to EGFR and ALK alterations. A study by Frampton et al. in 2015 analyzed data from a commercial next-generation sequencing panel and reported a prevalence of MET alterations of ~2% in NSCLC in North America. 34 More recently, data from a nationwide real-world database 35 updated the prevalence of oncogenic drivers in NSCLC in the US, reporting rates of 35.5%, 17.8%, 2.8%, 2.3% of KRAS, EGFR, ERRB2, and BRAF V600E mutations; and 4.3%, 1.2%, 1.1%, and 0.1% in ALK, RET, ROS1, and NTRK fusions, respectively.
The influence of genetic ancestry on molecular profile has also been assessed in the US. EGFR mutations were more associated with Asian ancestry while MET dysregulations were more frequently observed in patients of Ashkenazi Jewish ethnicity. A rare variant in the ATM gene was linked to an increased risk of lung adenocarcinoma in the latter population. 36
Lung cancer in Latin America
Lung cancer incidence and mortality
Lung cancer is one of the leading causes of cancer-related deaths in Central America, South America, and the Caribbean accounting for about 86,627 deaths in 2020. 37 Specifically, it is the fourth cancer in incidence and first cause of mortality in South America and the Caribbean. However, in Central America, lung cancer is being surpassed by other cancer types as the main cause of death like liver, stomach, colorectal, breast, and prostate cancer. 37
Lung cancer carcinogens in Latin America
Tobacco usage rates vary considerably among the adult population in Latin America. In Central America, the estimated prevalence of tobacco use is generally less than 10% in most countries, including Panama, El Salvador, and Costa Rica. By contrast, South America exhibits notably higher prevalence rates, with countries such as Chile (29.2%), Argentina (24.5%), and Uruguay (21.5%) at the forefront. 13
According to WHO and the World Air Quality, Chile, Peru, Colombia, and Mexico are among the Latin American countries with the highest air pollution rates in the region. 38 A recent study in Chile showed a significant association between the level of PM2.5 across different boroughs and higher lung adenocarcinoma incidence. 39 Interestingly, these countries also have the highest incidence of EGFR mutant lung cancers. While the link between air pollution and EGFR mutant lung cancers in this region remains under investigation, the correlation becomes particularly intriguing in light of the preclinical and epidemiological studies conducted by the TRACERX consortium. 20 Exposure to indoor air pollution from wood smoke during cooking and heating has also been associated with lung cancer and studied in Mexico particularly. In a cohort of 914 patients with lung cancer, approximately 35% had been exposed to wood smoke. This subgroup exhibited higher rates of lung adenocarcinoma histology, with around 50% showing EGFR mutations and a lower incidence of KRAS mutations (6.7%) compared to smokers. 40
Arsenic water intake is a major carcinogen, associated with an increased risk of lung cancer. By the year 2012, it was estimated that 4.5 million Latin Americans were exposed to high levels of arsenic concentrations across multiple countries. The main areas of high levels of drinking water arsenic exposure are the Antofagasta region in Chile, central Argentina and Andes region, Bolivia, Peru, Ecuador, southeastern Brazil, Nicaragua, and Mexico. 41 In Antofagasta (Chile), the relative risk for mortality due to lung cancer is 3.61 higher in men and 3.26 in women compared to regions with no arsenic water contamination. 42 Arsenic is predominantly associated with squamous cell carcinoma histology, and in this Chilean region, it accounts for about two-thirds of lung cancers.43,44 A study performed using comparative genomic hybridization showed differential copy number alterations to non-arsenic exposed tumors; however, the mutational profile of arsenic-associated lung cancers remains to be elucidated. 43
Other carcinogens, such as residential radon gas exposure, have been scantly studied in Latin America. A recent systematic review analyzing 31 studies 45 was conducted in Brazil, Argentina, and Peru and less in Costa Rica, Chile, Colombia, Ecuador, Paraguay, and Venezuela. The range of radon concentration in these studies was 0–3.723 Bq/m3, with the highest concentrations reported in Lages Pintadas, Belo Horizonte, and Poços de Caldas in Brazil. However, there are no studies on the molecular biology of lung cancer in areas with high residential radon exposure in Latin America.
Intrinsic factors related to lung cancer in Latin America
Cranford et al. studied lung cancer incidence by ethnicity of people living in Florida, including the Hispanic population. 46 They found that, among the Hispanic population, individuals of South American origin had a higher lung cancer incidence adjusted by age compared to those from Central America. In this study, Cubans had the greatest incidence among Hispanics.
Regarding genetic cancer predisposition syndromes, germline TP53 mutations (Li-Fraumeni Syndrome) are a rare condition characterized by a predisposition to multiple tumors, including lung cancer. 47 In Southeastern Brazilian populations, it is estimated that up to 0.3% of the population carries germline TP53 mutations. 48 This high frequency is attributed to a founder mutation, the TP53 p.R337H. Of particular interest is the established association between germline TP53 alterations and the development of lung cancer with somatic EGFR mutations. In one study, patients with germline TP53 mutations in codon 337 had a rate of EGFR mutations as high as 89%. 49 This, among other factors, may contribute to the high EGFR mutation rates observed in Brazil.50,51
Molecular characterization of lung cancer in Latin America
In this vast region, which includes 33 countries, multiple factors affect lung cancer prevention and patient care. Socioeconomic barriers and inequity in access to fragmented and under-financed healthcare systems directly impact biomarker testing and access to targeted therapies in the region.52,53 Consequently, there is limited information about the prevalence of molecular drivers of lung cancer beyond EGFR mutations, ALK and ROS1 fusions, and PD-L1 expression, which are the minimum biomarkers to be tested.
The prevalence of EGFR somatic mutations in lung adenocarcinomas varies significantly across countries: from 14% in Argentina and 18% in Uruguay, 22% in Chile, 25% in Brazil and Colombia, 27% in Panama, 31% in Costa Rica or 34% in Mexico to 51% in Peru.51,54–56 Notably, the prevalence of EGFR mutations in Argentina and Uruguay is like in Spain, potentially linked to the predominance of Spanish immigration and European ancestry. On the other hand, in other countries where the preponderance of Native and African American ancestry predominates, the higher prevalence of EGFR mutations might be related to distinct ethnic and ancestry patterns. In a study including 601 lung cancer cases from Mexico and 552 from Colombia, ancestry was assessed using single nucleotide polymorphism (SNP) in tumor samples. Native American ancestry was positively correlated with EGFR mutations and negatively correlated with KRAS and STK11 mutations, as observed in Asian populations. 57
Regarding other molecular drivers, the distribution of KRAS mutations in the region does not vary significantly between countries such as Mexico, Colombia, and Peru, where rates have been reported at 12.9% and 16.8%, which is lower than rates reported in the United States and Europe. In Brazil, the reported prevalence of KRAS mutations is 24.2% and 23% in Argentina.50,58 In the case of ALK fusions, there are no significant differences in the reported prevalence in the region, ranging from 3.7% in Chile to 9.5% in Costa Rica.50,59,60
Overall, testing for oncogenic drivers in clinical practice remains an unmet need in most countries in the region and unfortunately, most patients are managed without this critical information.
Lung cancer in Asia
Lung cancer incidence and mortality
Lung cancer is the most diagnosed cancer among men in Asia, and the second most common cancer among women, after breast cancer. In 2020, 1.3 million people were diagnosed with lung cancer and it was the most common cause of death from cancer (slightly over 1.1 million deaths) in the region. 61 Incidence and mortality rates of lung cancer in East Asia are particularly concerning, with 34.4 and 28.1 cases per 100,000, respectively, which is higher than in Europe and the United States. 62 Sex disparities have been reported, with higher incidence (ratio 2.46) and mortality (ratio 2.5) for males compared to females. 63
Lung cancer carcinogens in Asia
About half of the world’s smokers live in Asia. 64 Male smoking rates remain high; indeed, half of the world’s male smokers live in three Asian countries: China, India, and Indonesia. 65 Tobacco use among children is also concerning in this area; about 34% of the world’s children aged 13–15 years using various forms of tobacco belong to the South-East Asia region. 66
Although tobacco smoking continues to be the leading global contributor to lung cancer, the incidence of lung cancer in non-smokers is increasing, particularly among non-smoking Asian women. Epidemiological studies conducted in East Asian countries such as the People’s Republic of China, Japan, Mongolia, North Korea, and the Republic of Korea showed that approximately one-third of all lung cancer patients in East Asia have never smoked 62 which is significantly higher than in Western countries.
Regarding residential radon, similarly to the published residential radon studies in North America and Europe, the China pooling study observed that long-term indoor radon exposure increases lung cancer risk, with an OR of 1.33 (1.01–1.36) at 100 Bq/m3. 67 National radon risk registries and communication strategies, as opposed to Europe or North America, are lacking in Asia. According to 2019 WHO data, China, Turkey, and Syria are the only Asian countries conducting national radon surveys, 68 and additionally, some large-scale radon studies are ongoing in several countries, including India, Israel, Japan, Korea, and the Philippines. 69 Data from national surveys show low median radon concentrations in China (37 Bq/m3), India (32 Bq/m3), or Japan (18 Bq/m3) and higher rates in Vietnam (79 Bq/m3) and Korea (91 Bq/m3). 70
Outdoor air pollution and particulate PM2.5 are a concerning health issue in Asia. China has the highest attributable death rate for lung cancer caused by PM2.5, and there is an increasing trend in all of East Asia except for South Korea and Japan. 71 Industry, traffic, and household biomass combustion for heating and cooking, prevalent in Asia, have become major sources of air pollutant emissions and have a larger impact on premature mortality. 72 However, the implementation of national environmental protection policies in Asian countries is still pending.
Diesel exhaust and other occupational expositions play a relevant role in lung cancer risk, in Asia with millions of Asian workers exposed, particularly for squamous cell and small cell carcinoma. 73 The risk of occupational exposure to crystalline silica and lung cancer is also well known. There is an increased risk of lung cancer with cumulative occupational exposure to silica in workers both with and without silicosis, regardless of smoking status, and also with a multiplicative risk with smoking and silica exposure for overall lung cancer risk. This is especially relevant in Asia since there are 11.5 million workers in India, and millions in China, of workers exposed to crystalline silica while manufacturing and installing stone countertop materials for household use and sandblasting denim for fashionwear.74,75
Intrinsic factors related to lung cancer in Asia
There are significant differences in the clinical and molecular profile of lung cancer between Asian and Caucasian patients. In addition to exposure history, differences in ethnicity and ancestry background are thought to be the major reason for the observed differences in germline and somatic alterations between Asian and Western patients with lung cancer.
In China, germline mutation landscape has been assessed in 1794 patients with NSCLC 76 reporting a prevalence of PGV in genes related to cancer risk, mainly in DNA repair pathways, of 5.9%. Interestingly, among PGV carriers, somatic oncogenic drivers were significantly prevalent especially MET dysregulation (7.6% in PGV carriers versus 3% in non-carriers) and KRAS mutations (16.8% versus 8.3%).
Specific germline alterations have also been studied in the Asian population. Hu et al. 77 reported a prevalence of 1.03% of germinal BRCA alterations among 6220 Asian patients with NSCLC with a high prevalence of somatic oncogene drivers, EGFR the most frequently mutated gene (53% of patients) among BRCA carriers. Germline EGFR mutations in East Asian and ERBB2 in Japanese patients with NSCLC have been described targeting never-smokers. 78
Molecular characterization of lung cancer in Asia
The proportions of actionable genetic alterations in Asia vary considerably across published studies and by histological subtype. Compared with Caucasian patients with NSCLC, Asian patients have a much higher prevalence of EGFR mutations (about 30–40% versus 7–15%, respectively), mainly among patients with adenocarcinoma and never-smokers. Around 5% of Asian and White patients with NSCLC have ALK-positive tumors. 79 On the other hand, KRAS mutations are less frequent in Asian patients (8–10% versus 20–30%). For KRAS G12C mutations, different studies reported a prevalence of 1.5–4.3% in NSCLC in Asia, which is significantly lower than the 10–15% described in Caucasian patients. 80
Data from a large lung cancer genomic screening project including more than 206 institutions in Japan, Taiwan, and China (LC-SCRUM-Asia) showed frequencies of oncogenic fusions in ALK, ROS1, RET, or NTRK to be similar in Asian and white populations. 81 In particular, some studies have reported a high prevalence of ALK fusions (10%) among South Asian patients. 82 For ERBB2, the prevalence in Asia is between 1.5% and 4%.81,83 MET exon 14 skipping mutation is found in about 2% of patients, also similar to Western countries. 84 Final data from ongoing national registries would allow us to better understand the real prevalence of molecular driver alterations in NSCLC in Asia.
Lung cancer in the Middle East and North Africa
Lung cancer incidence and mortality
The Middle East and North Africa (MENA) region comprises countries that are part of the Arab League, including the Gulf Cooperation Council countries such as the United Arab Emirates, Oman, Kuwait, Bahrain, Saudi Arabia, and Qatar. In addition, it includes Yemen, Iraq, the Levant region consisting of Jordan, Lebanon, and Syria, as well as North African countries like Egypt, Morocco, Libya, and Algeria. While all these countries fall under the Arab League’s umbrella, they exhibit significant variation in their social and economic standings. 85 In the MENA region, the incidence adjusted by age of lung cancer is reported to be lower than international rates showing the lowest rates in Yemen (4.6 per 100,000) and the highest in Lebanon (18.7 per 100,000). 86 In the MENA region, lung cancer caused 15,396 and 57,114 deaths in women and men, respectively, in 2019. However, accurate data collection regarding cancer incidence is hindered by the absence of comprehensive and up-to-date population registries in many of these countries. 85
Lung cancer carcinogens in the MENA region
With a combined population of approximately 360 million, up to 46% of the population in the MENA region are smokers, 85 with a high prevalence in countries such as Jordan (35.0%), Saudi Arabia (30.4%), and Lebanon (26.3%). 87 The MENA region has also a unique smoking issue with the widespread water pipe/hookah smoking. 88 The overall highest rate of current smoking (cigarette and water pipe) is seen among students in Egypt (46.7%), Kuwait (46%), and KSA (42.3%). 87 Lebanon and Tunisia also face significant issues with hookah smoking among young people. A recent study conducted on 3384 students from 17 universities in Lebanon revealed that 23% of them were current hookah smokers, while 19.2% reported being cigarette smokers. 89 In addition, among 13- to 15-year-olds in Lebanon, the Global Youth Tobacco Survey indicated a current hookah smoking rate of 34.8%, 90 compared to 11.3% for cigarettes. 91
Besides smoking, various environmental factors play a role in lung cancer development in the MENA region. Indoor radon exposure is prevalent in some areas, and a study in Iran reported that high radon concentrations were associated with an increased risk of lung cancer among non-smokers. 92 Median radon exposure in Iran (198 Bq/m3) is one of the highest in the region, 70 consistent with regions with high natural background radiation reported as Ramsar, an Iran city on the Caspian Sea. Particularly, Ramsar has been established as one the most radioactive cities of the world 93 with mean radon levels of 650 Bq/m3 and maximum levels of 3700 Bq/m3 because of the deposition of 226 Radium in local rocks and its use in the construction of houses. 94 Radon exposition also stands as a public health issue in countries such as Uzbekistan and Kyrgyzstan with one of the highest mean radon expositions in the world (219 and 200 Bq/m3, respectively). 70 In addition, in some ex-Soviet Republics in central Asia as Kazakhstan, Kyrgyzstan, Tajikistan, and Uzbekistan, extensive uranium mining and milling activities generated large amounts of uranium tailing materials and waste rock deposits, often dumped in inhabited areas or their close vicinity 95 estimating that about 0.7–7.2% of the total population is exposed to radiation risk. 96
MENA is among the regions worldwide with the highest death rates attributable to air pollution. A systematic analysis for the Global Burden Disease of 2019 97 investigated the effect of PM2.5 and ozone air pollution in 21 countries of the region. Up to 12.8% of all the deaths were attributable to air pollution, particularly, 21.3% of lung cancer deaths with the highest rates reported in Afghanistan, Egypt, and Yemen.
Occupational carcinogens are the third reason for lung cancer deaths in North Africa and the Middle East after smoking and air pollution with a 2.4 age-standardized rate of deaths by lung cancer per 100,000 habitants 98 with different patterns between men and women. Retrospective studies in North Africa revealed as main exposition in occupations like masonry construction and painting (47.1%), agriculture (30.8%), and transportation sectors (12.8%) in men with lung cancer; while women were more exposed to cleaning products (50%) and coal smoke (42.8%). 99
Intrinsic factors related to lung cancer in the MENA region
Genetic contribution to lung cancer development has been studied in a few countries of the region. In Tunisia and Egypt, SNPs in five genes were associated with high lung cancer risk (CYP1A2, CYP1A1, IL-17A/F, IL-8, and TNFα/β). 100 Other genetic polymorphism has been evidenced in Iran cohorts 101 linked to lung cancer risk including C-allele of the rs2245214 ATG5 gene polymorphism or C allele rs2645429 in Farnesyl-Diphosphate Farnesyltransferase 1, among others.
Molecular characterization of lung cancer in the MENA region
Molecular information related to lung cancer profile in the MENA region is limited to EGFR and ALK alterations. In a systematic review and meta-analysis including 1215 patients with NSCLC from the Middle East and Africa, 41.1% of the patients were never-smokers, and 85.8% were diagnosed with adenocarcinoma. In 8 out of 10 studies assessed, EGFR mutations were analyzed by polymerase chain reaction, with an overall prevalence of 21.2%. with an enrichment in the female, non-smoker population with adenocarcinoma histology. 102 The exon 19 deletion was the most observed, in 58% of cases.
Regarding ALK fusion, in real-world data of ALK-positive NSCLC in the MENA, the prevalence of ALK fusions was 8.7% among 448 tissue samples analyzed using Immunohistochemistry. 79
Widespread access to molecular profiling platforms for tumor assessment remains limited in many of the MENA regions due to the cost and limited specialized molecular laboratories; however, the establishment of reference central laboratories in each country could play a pivotal role in facilitating access to these tests, particularly as the technology becomes more cost-effective and convenient over time. 85
Lung cancer in Sub-Saharan Africa
Lung cancer incidence and mortality
Infectious diseases account for the greatest burden of disease across sub-Saharan Africa (SSA); however, the health burden due to cancers is increasing. 103 It is estimated that by 2030, there will be a significant rise in cancer. 104 Importantly, survival from cancer is low in SSA in comparison to other world regions. 105 Viral diseases are important drivers for cancers such as cervical cancer, Burkitt’s lymphoma, Kaposi’s sarcoma, or hepatocellular carcinoma. 103
Population-based data on lung cancer incidence and mortality in SSA are scarce. Lung cancer incidence rates remain low, except for Southern regions. 1 Incidence age-standardized incidence rate per 100,000 is significantly higher in Southern Africa (27.5 in men and 9.3 in women) compared to Eastern (4.2 and 3.0), Middle (3.4 and 1.8), and Western Africa (2.8 and 1.8, respectively). 106 This is likely due to the low prevalence of smoking (10% in men and < 2% in women), as well as the lower life expectancy of the population. 106 However, reporting of lung cancer incidence and mortality in SSA is limited by the lack of reliable registries, and it is likely underestimated. 106 In addition, the case fatality rate is higher, mainly due to late presentation and poor access to treatment. 107 In a population-based study, cancer survival was lowest in the two African countries (The Gambia and Uganda). This was attributed to the poorly developed health services, with limited availability of cancer diagnostic and treatment facilities. 105
Lung cancer carcinogens in SSA
Smoking prevalence varies greatly between SSA countries, accounting for about 6% of cancer deaths in Africa, 104 and it is significantly higher in men. Adult smoking prevalence is less than 10% in men and close to 2% in women in many SSA countries, but cigarette consumption is increasing in parts of this region because of the adoption of Western behaviors associated with economic growth and increased marketing by tobacco companies. 104 High smoking rates are observed among countries in the eastern and southern regions of Africa, mainly among men in Ethiopia, Malawi, Rwanda, and Zambia, and women in Rwanda and rural Zambia. 108
Working conditions and workforce characteristics facilitate occupational exposures in SSA. Lack of protective devices, lack of training in hazard awareness, slow implementation of safety standards, obsolete technologies and machinery, and lack of monitoring of occupational health are some of the issues affecting workers of the SSA countries. 109 Occupational factors associated with increased lung cancer risk in Africa include asbestos (South Africa, Swaziland, Zimbabwe), exposure in mines to aluminum smelters (South Africa, Guinea, Mozambique, Cameroon, Nigeria, Ghana), beryllium (Mozambique), nickel compounds (South Africa, Botswana, Zimbabwe), or silica dust from gold mines (South Africa). 109
Indoor combustion of solid fuels for cooking and heating is the main source of air pollution in SSA. In addition, levels of outdoor pollution have risen in urban areas due to a rapid increase in industrial and motor vehicle diesel exhaust. In SSA, average emissions per vehicle are higher as vehicles are on average older, and cheaper, and lower quality fuels are used. 109
Intrinsic factors related to lung cancer in SSA
Despite the growing evidence suggesting that genetic factors contribute to the risk of developing lung cancer, data on genetic biomarkers for lung cancer risk in African ancestry populations are limited. Analysis of African Americans in North America has been performed, looking for nominal SNPs linked to lung cancer, and there is growing literature pointing toward rs2036527 as an informative polymorphism for smoking exposure and lung cancer risk in African Americans. 110
Molecular characterization of lung cancer in SSA
Since most countries in SSA do not have any modalities for molecular testing, 111 the frequency of molecular alterations in African patients has been understudied. Most data come from studies conducted on African Americans in the United States.
In South Africa, biomarkers can be tested using immunohistochemistry and fluorescence in situ hybridization, and next-generation sequencing is available in the private sector to use in selected cases. 112 EGFR mutation prevalence in SSA patients with NSCLC seems to be similar to that in Western countries, compared to the higher prevalence found in Asian patients. 112 In a retrospective study by Chan et al., EGFR mutations were present in 21.3% of South African patients (18% in Caucasians, 23% in Africans, and 39% in other races). 113
Studies exploring the prevalence of EGFR mutations in lung tumors from US patients with Afro-American ancestry have been inconclusive; some studies found a significantly lower prevalence of EGFR mutations in Afro-American patients compared with Caucasians, whereas other studies did not observe any association of EGFR mutation status with ancestry or self-reported race. 114
In an analysis conducted by Araujo et al., 206 self-reported Afro-Americans from the United States with NSCLC provided samples for molecular analyses. The frequency of driver alterations altogether was lower than that reported in Caucasians but no difference was detected in either EGFR or KRAS mutations; in addition, the frequency of ALK fusions was similar to the lower boundary of the rates reported in unselected populations. 115
The NSCLC cases from African patients might have a different pattern of somatic driver mutations than from Caucasians; further studies conducted specifically in SSA patients with NSCLC are needed.
Lung cancer in Europe
Lung cancer incidence and mortality
Lung cancer is the leading cause of cancer deaths in Europe, 1 corresponding to almost 20% of all cancer deaths. 116 The share of all deaths attributed to lung cancer was 7.0% among males, more than double the share (3.2%) recorded for females. 117 Overall, prevalence and mortality rates in Europe are higher than the global average, and 5-year survival rates stand at a mere 11.2% for men and 13.9% for women. 118
By country, the incidence of lung cancer in men is highest in Central and Eastern Europe such as Hungary (138.3 per 100,000) or Serbia (136.4). Whereas the highest rates in women are seen in Ireland (85.1), Denmark (85.1), and Hungary (76.6). 116 Lung cancer incidence is increasing in European women likely due to different time period in which women initiated the smoking habit.
Lung cancer carcinogens in Europe
Smoking prevalence is still very high in some countries, such as Greece (37%), France (36%), and Bulgaria (36%); while there are others as Sweden with only 7% of smokers in the population. Hand-rolled cigarettes have become more popular among smokers in some European countries, including England (27.3%), France (16.5%), and Finland (13.6%), with overall about 10.4% of current smokers using predominantly hand-rolled cigarettes. 4 The implementation of tobacco control policies is contributing to smoking cessation, 119 which could potentially reduce future lung cancer incidence considerably across Europe. 120
According to the European Indoor Radon Map launched by the Joint Research Centre of the European Commission, more than 30% of the territory has median radon above 100 Bq/m3, and 4.2% is above 300 Bq/m3 with several radon-prone areas,121 such as the Bohemian Massif, the north-west of Spain, the Massif Central, the Fennoscandian shield, the Vosges Mountains, the Central Alps, the North of Estonia and certain volcanic structures in central Italy. 122 The estimated annual indoor radon mean is 78.5 Bq/m3, ranging from 10 (Iceland) to 184 Bq/m3 (Serbia). 123 The European pooling study by Darby et al. demonstrated a linear increase of 16% (range: 5–31%) of lung cancer risk per 100 Bq/m3 of indoor radon across all histologies (adenocarcinoma, squamous, SCLC, and others), highlighting that the risk of death from lung cancer is about 25 times greater for cigarette smokers. 124
Occupational carcinogens affect one in five workers in the European Union (EU): based on EU CAREX (Carcinogen Exposure Database), a substantial proportion of workers in the EU were exposed to carcinogens in the early 1990s. 125 Cancer, and particularly lung cancer, is the main cause of death by occupational exposures in Europe, 126 up to 53% of all work-related deaths. Asbestos exposition is the main contributor to occupational lung cancer deaths in the EU being estimated that 46,919 lung cancer and mesothelioma deaths would be caused by asbestos according to the European Agency for Safety and Health at work, 126 especially relevant in countries such as the United Kingdom, Netherlands, and Italy.
Intrinsic factors related to lung cancer in Europe
Wide populational cohorts in Europe have assessed lung cancer risk in first-degree relatives of cancer with lung cancer – relative risk of 2.36 in the Swedish Family-Cancer Database. 127
Genetic variations and SNPs associated with lung cancer risk have been studied with genome-wide association studies in European ancestry populations. In a study by Hung et al., never-smokers of lung cancer risk in never-smokers of European ancestry were associated with genetic variation in the 5p15.33 TERT-CLPTM1Ll region. 128 Interestingly, top variants previously shown to be associated with lung cancer risk only conferred risk in the presence of tobacco exposure, underscoring the importance of gene–environment interactions in the etiology of lung cancer. 128
On the other hand, some authors have analyzed the clinical and molecular features of European patients with lung cancer carriers of a PGV in cancer predisposition genes. For instance, in 22 patients with Li-Fraumeni syndrome and lung cancer, 90% harbored a somatic driver alteration in the lung tumor, mainly EGFR mutations. 47 Going further, Mezquita et al. characterized molecularly by whole exome sequencing the lung tumors of 22 patients PGV carriers finding a prevalence of oncogene-driven tumor of 68%. 129 Future studies assessing the family and clinical/molecular characteristics of patients with lung cancer harboring germline alterations would help to identify a high-risk population. Recent data from a Spanish cohort show a prevalence of PGV in 7.3% of 55 patients with NSCLC, including young individuals, non-smokers, or those with oncogene-addicted tumors, primarily in DNA repair genes 130
Molecular characterization of lung cancer in Europe
Across Europe, there is a significant variability in the adoption of biomarker testing for advanced NSCLC. Limitations to the use of biomarker testing across Europe include a lack of reimbursement of targeted therapies and testing in some countries. Between 2011 and 2016, the proportion of patients with advanced non-squamous NSCLC who underwent single-gene molecular testing ranged from 65% to 85% across Germany, Italy, and Spain. 131 Molecular testing rates typically increase with time.
The largest study in Europe assessing routine nationwide molecular profiling of patients with advanced NSCLC was performed in France collecting 18,679 molecular analysis data from 17,664 patients with NSCLC. 132 The authors found a prevalence of 29% KRAS mutations, 11% EGFR mutations, 5% ALK fusions, 2% BRAF mutations, 2% PIK3CA mutations, and 1% ERBB2 mutations. Another large cohort in Germany (n = 3717 patients with NSCLC) 133 studied separately molecular biomarkers in non-squamous and squamous NSCLC. The most common alterations found in non-squamous tumors were KRAS mutations (39.2%), TP53 (51.4%), and EGFR (15.1%) while in squamous tumors were TP53 (69.1%), MET (11.1%), and EGFR (4.4%). It is worth noting that up to 29.3% of the squamous tumors were not tested for biomarkers compared to 7.8% of the non-squamous tumors.
Some studies have linked lung cancer carcinogens with certain molecular features. Hill et al. found a consistent relationship between PM2.5 air pollutants and EGFR-driven lung cancer incidence in England, Taiwan, and South Korea based on a mechanistic basis for PM-driven lung cancer. 20 Interestingly, two studies in Spain have reported radon levels above 100 Bq/m3 in patients with EGFR mutant and ALK-positive NSCLC.134,135 In line with this data, large ecological studies performed in France observed a higher prevalence of driver oncogenic alterations (EGFR, BRAF, ALK, ROS1, and ERBB2) in patients with lung cancer living in high radon risk areas according to the Radon map in France.136,137 The 1920 – EORTC Bioradon study 138 is currently ongoing in five European countries in Europe to assess prospectively this potential association between radon and molecular alterations in NSCLC.
Lung cancer in Oceania
Lung cancer incidence and mortality
Oceania, an island continent located in the Pacific Ocean, encompasses more than 10,000 islands. Despite having the largest geographic area among continents, its population is one of the smallest, with the majority concentrated in Australia and New Zealand, accounting for over two-thirds of the total population of the continent. 139
According to Globocan, 16,975 new cases of lung cancer were diagnosed in Oceania in 2020, making it the fourth most common tumor in incidence after breast cancer, prostate cancer, and melanoma. It is also the leading cause of cancer-related death when considering both genders, with 12,012 deaths in 2020. 1 However, there are significant intra-continental epidemiological differences, even within each country: while lung cancer ranks fifth in overall incidence among individuals of European descent in Australia/New Zealand, Māori in New Zealand or Indigenous Australians have higher incidence rates (some of which are the highest in the world140,141). They also have significantly higher mortality rates compared to the non-aboriginal population (three times higher in the case of Māoris) and die at younger ages.141–143 In contrast to Australia/New Zealand, in Polynesia and Micronesia, lung cancer is the most incident tumor, 1 fact not completely explained by tobacco consumption.
Lung cancer carcinogens in Oceania
The epidemiological cancer registry and associated risk factors exhibit significant disparities between the Australia/New Zealand regions and other Pacific populations, where there is a significant lack of epidemiological data. 144 Tobacco smoking is the leading cause of lung cancer, accounting for about 90% of lung cancers in males and 65% in females in Australia/New Zealand. 145 In 2007, approximately 20% of Australians/New Zealanders over the age of 14 were current smokers. 146 These rates are even higher in lower socioeconomic strata, and are of particular concern among Aboriginal Australians, with smoking rates reaching up to 50% in the population aged >18 years. 147
In Australia and New Zealand, the annual average of indoor radon concentrations are 45 Bq/m3, 148 and 23 Bq/m3, 149 respectively. No radon-prone areas have been reported according to national agencies.149,150 In Australia, the average radon levels in homes along the Great Dividing Range are typically higher than levels in homes on the coastal plan, mainly due to differences in the nature of the underlying geology (rock and soil). 150 Data for the rest of the islands in the Pacific continent are limited. Although it is expected that their indoor radon concentrations are low given the absence of granitic bedrocks in Pacific islands’ soil, coverage of volcanic rocks by karst limestone plateau, composed largely of fossilized foraminifera and corals with high amounts of uranium, can increase radon concentrations in some islands. 151
As for other risk factors, air pollution is low on the continent. 152 It is worth noting the nuclear test of Moruroa and Fangataufa between 1966 and 1996. 153 It has been estimated that around 110,000 inhabitants may have received doses greater than 1 mSv/year, constituting about 90% of the total Polynesian population in 1974. 154 This exposure may influence the risk of developing in cancer in the affected area. 155
Finally, concerning work-related risk factors, it is estimated that around 37% of the population were exposed to at least one occupational carcinogen in their current job, 156 such as diesel engine exhaust, silica, or wood dust, according to the Australian Worker Exposure Study. It is worth noting that in the 1950s, Australia had the highest per capita consumption of asbestos in the world. Production and importation were banned in 2003. 157 This situation has led to Western Australia having one of the highest rates of malignant mesothelioma in the world 157
Intrinsic factors related to lung cancer in Oceania
Before the arrival of Europeans over 40 1000 years ago, Oceania was inhabited by migrant groups from Africa, 158 who dispersed across the continent leading to the development of a wide variety of ethnicities. The current count exceeds 1000 distinct ethnic groups. Differences in cancer incidence based on ethnicity have been described. For instance, in Australia, Indigenous Australians have a higher likelihood of being diagnosed with lung cancer compared to non-Indigenous Australians, even when accounting for differences in smoking rates. 143 A similar situation occurs with the Māori population in New Zealand. 142 While high smoking rates contribute to the disparity, other factors may also make these populations more susceptible to lung cancer. Epidemiological research is crucial in identifying occupational exposures, diet, socioeconomic status, and genetic differences that may contribute to these disparities.
A notable case was the discovery of germline mutations in the E-cadherin gene associated with gastric and breast cancer, initially identified in three Māori families. 159 Further genetic studies may help identify specific mutations associated with higher lung cancer incidence in certain ethnic groups.
Molecular characterization of lung cancer in Oceania
When examining the molecular profile of lung cancer in Oceania, it is important to consider the variation in access to molecular characterization across different regions. Australia and New Zealand benefit from established public healthcare systems that provide government-funded access to molecular study techniques and targeted therapies. 143 However, except in French Polynesia or Hawaii, the population in the remaining Pacific Islands faces challenges in accessing specialized healthcare and high-quality molecular diagnosis due to factors such as geographical distance and cultural barriers. 160 Consequently, molecular profiling data in Oceania are incomplete, with a focus on wealthier and more populated regions, while other areas have limited available data.
In the few reported series, the frequency of EGFR mutations in the Australian population with lung cancer ranges between 19% and 28%,161–165 including T790M mutations (9.3%) and exon 20 insertions (4.8%). 165 This reflects a higher prevalence compared to other series in the Caucasian population (10–15%). In the largest series of Australian patients with NSCLC, the authors also described the prevalence of other driver alterations such as KRAS (38.3%), BRAF (5.1%), or ERBB2 (1.7%) mutations. 165 Some studies have attempted to determine the differences in biomarkers prevalence among different ethnicities in northern Australia, 166 but due to the small sample size, no significant differences were found in the prevalence of EGFR or KRAS mutations. In the New Zealand population, in a cohort of 384 patients with NSCLC, 167 the overall prevalence of EGFR mutation was 22.5%, with a higher prevalence among the Asian population (51.8%), followed by Pacific Islanders (29%) and New Zealand Europeans (16.5%), while Māori had the lowest proportion (10.9%). Scarce information is available about gene fusions in Oceania. It has been published an 8.4% of ALK fusion incidence in a retrospective study in New Zealand with a higher incidence among Asian, Pacific, or Māori ethnic groups than in New Zealand Europeans (22.0%, 10.8%, and 6.9%, respectively, versus 4.4% in New Zealand Europeans). 168
Conclusion
Cultural, socioeconomic, and geographic differences in each region can lead to unequal exposure to the mentioned carcinogens in lung cancer. The interaction of all potential risk exposures that contribute to the risk of developing lung cancer, combined with endogenous factors in each individual, such as genetic predisposition, ancestry, sex, and other factors, impacts the development of lung cancer in a multifactorial and complex pathway. These factors may influence the profile of lung cancer, including the pathological, genomic, and immunological biomarkers, which can guide treatment selection and subsequently affect clinical outcomes. One example of this is the differences in clinical and molecular profiles among different populations, such as the higher prevalence of lung cancer in non-smoking Asian populations compared to Western populations, especially among women with adenocarcinoma tumors and those with alterations in EGFR or KRAS. 62 While this evidence remains limited, an increasing number of studies are being conducted to gain a deeper understanding of these geographic and genetic differences across different countries and world regions, generating new hypotheses on the interaction between environmental and genetic factors. Collectively, these efforts will contribute to generate knowledge, improving the understanding of lung cancer across different world regions, and promoting cancer policies and prevention strategies worldwide.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241231260 for Geographic differences in lung cancer: focus on carcinogens, genetic predisposition, and molecular epidemiology by Juan Carlos Laguna, Miguel García-Pardo, Joao Alessi, Carlos Barrios, Navneet Singh, Humaid O. Al-Shamsi, Herbert Loong, Miquel Ferriol, Gonzalo Recondo and Laura Mezquita in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank Ainara Arcocha, Jessica González, and Laura Alcolea for the administrative support.
Footnotes
ORCID iDs: Miguel García-Pardo  https://orcid.org/0000-0001-6339-8501
https://orcid.org/0000-0001-6339-8501
Laura Mezquita  https://orcid.org/0000-0003-0936-7338
https://orcid.org/0000-0003-0936-7338
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Juan Carlos Laguna, Medical Oncology Department, Hospital Clinic of Barcelona, Barcelona, Spain; Laboratory of Translational Genomics and Targeted Therapies in Solid Tumors, IDIBAPS, Barcelona, Spain; Department of Medicine, University of Barcelona, Barcelona, Spain.
Miguel García-Pardo, Department of Medical Oncology, Hospital Universitario Ramón y Cajal, Madrid, Spain; Department of Medicine, University of Barcelona, Barcelona, Spain.
Joao Alessi, Lowe Center for Thoracic Oncology, Dana-Farber Cancer Institute.
Carlos Barrios, School of Medicine, Porto Alegre, Rio Grande do Sul, Brazil.
Navneet Singh, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
Humaid O. Al-Shamsi, Burjeel Medical City, Abu Dhabi, United Arab Emirates
Herbert Loong, Department of Clinical Oncology, The Chinese University of Hong Kong, Hong Kong SAR, China.
Miquel Ferriol, Laboratory of Translational Genomics and Targeted Therapies in Solid Tumors, IDIBAPS, Barcelona, Spain; Barcelona Neural Networking Center, Universitat Politècnica de Catalunya, Barcelona, Spain.
Gonzalo Recondo, Medical Oncology Department, CEMIC, Buenos Aires, Argentina.
Laura Mezquita, Medical Oncology Department, Hospital Clinic of Barcelona, Calle Villarroel 170, Barcelona 08036, Spain; Laboratory of Translational Genomics and Targeted Therapies in Solid Tumors, IDIBAPS, Barcelona, Spain; Department of Medicine, University of Barcelona, Barcelona, Spain.
Declarations
Disclaimer: Authors Laura Mezquita and Herbert Loong are Editorial Board Members of Therapeutic Advances in Medical Oncology, and Miguel Garcia-Pardo is on the Editorial Review Board; therefore, the peer review process was managed by alternative members of the board and the submitting editors were not involved in the decision-making process.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Juan Carlos Laguna: Conceptualization; Data curation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing.
Miguel García-Pardo: Conceptualization; Data curation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing.
Joao Alessi: Writing – original draft; Writing – review & editing.
Carlos Barrios: Writing – original draft; Writing – review & editing.
Navneet Singh: Writing – original draft; Writing – review & editing.
Humaid O. Al-Shamsi: Writing – original draft; Writing – review & editing.
Herbert Loong: Writing – original draft; Writing – review & editing.
Miquel Ferriol: Resources; Writing – original draft.
Gonzalo Recondo: Writing – original draft; Writing – review & editing.
Laura Mezquita: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received no specific funding for this work. Juan Carlos Laguna received support from Contractes Clínic de Recerca ‘Emili Letang-Josep Font’ 2023; Hospital Clínic Barcelona, 2023. Laura Mezquita received support from the Contrato Juan Rodes 2020 (ISCIII, Ministry of Health; JR20/00019); Ayuda de la Acción Estratégica en Salud- ISCIII FIS 2021 (PI21/01653); Ayuda SEOM Juan Rodés 2020 and Beca SEOM Grupo emergente 2022.
JCL: Lectures and educational activities: Kyowa Kirin; Travel, Accommodations, Expenses: Rovi, Pierre-Fabre. MGP: The author declares no conflict of interest. JA: advisory board: BMS and AstraZeneca; consultant: MSD and Janssen. CB: The author declares no conflict of interest. NS: The author declares no conflict of interest. HOAS: Research support: AstraZeneca, Merck. HL: Advisory: Boehringer-Ingelheim, Celgene, Eli-Lilly, Illumina, Janssen, Novartis, Merck Sereno, Pfizer, Takeda, George Clinical; Speakers’ Bureau: AbbVie, Amgen, Bayer, Eisai, Eli-Lilly, Guardant Health, Novartis; Travel Support: Bayer, Boehringer-Ingelheim, MSD, Novartis, Pfizer; Research Funding: MSD, Mundipharma, Novartis; Others: Member, Pharmacy and Poisons (Registration of Pharmaceutical Products and Substances: Certification of Clinical Trial/Medicinal Test) Committee, Pharmacy & Poisons Board of Hong Kong. MF: The author declares no conflict of interest. GR: The author declares no conflict of interest. LM: Lectures and educational activities: Bristol-Myers Squibb, AstraZeneca, Roche, Takeda, Janssen, Pfizer, MSD; Consulting, advisory role: Roche, Takeda, Janssen, MSD; Research Grants: Bristol-Myers Squibb, Amgen, Stilla, Inivata, AstraZeneca, Gilead; Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche, Takeda, AstraZeneca, Janssen.
Availability of data and materials: Not applicable.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer. Chest 2013; 143: (5 Suppl) e1S–e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Cancer Society. Lung cancer risk factors. Smoking & Lung Cancer, https://www.cancer.org/cancer/types/lung-cancer/causes-risks-prevention/risk-factors.html (accessed 20 September 2023).
- 4. Belleau P, Deschênes A, Chambwe N, et al. Correction: genetic ancestry inference from cancer-derived molecular data across genomic and transcriptomic platforms. Cancer Res 2023; 83: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukherjee S, Bandlamudi C, Hellmann MD, et al. Germline pathogenic variants impact clinicopathology of advanced lung cancer. Cancer Epidemiol Biomarkers Prev 2022; 31: 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008; 83: 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hendriks LE, Kerr KM, Menis J, et al.; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2023; 34: 358–376. [DOI] [PubMed] [Google Scholar]
- 8. Fois SS, Paliogiannis P, Zinellu A, et al. Molecular epidemiology of the main druggable genetic alterations in Non-Small Cell Lung Cancer. Int J Mol Sci 2021; 22: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melosky B, Kambartel K, Häntschel M, et al. Worldwide prevalence of epidermal growth factor receptor mutations in Non-Small Cell Lung Cancer: a meta-analysis. Mol Diagn Ther 2022; 26: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun L, Hsu M, Cohen RB, et al. Association between KRAS variant status and outcomes with first-line immune checkpoint inhibitor-based therapy in patients with advanced Non-Small-cell Lung Cancer. JAMA Oncol 2021; 7: 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023; 73: 17–48. [DOI] [PubMed] [Google Scholar]
- 12. Brenner DR, Poirier A, Woods RR, et al.; Canadian Cancer Statistics Advisory Committee. Projected estimates of cancer in Canada in 2022. CMAJ 2022; 194: E601–E607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Report on Tobacco Control for the Region of the Americas 2022. [Internet]. https://iris.paho.org/handle/10665.2/56259 (accessed 18 September 2023).
- 14. Wild CP, Weiderpass E, Stewart BW. (ed.). World cancer report: cancer research for cancer prevention. Vol. 199. IARC, 2020, p. 512. [PubMed] [Google Scholar]
- 15. Radon. American Lung Association. https://www.lung.org/clean-air/at-home/indoor-air-pollutants/radon (accessed 18 September 2023).
- 16. What is the average level of radon found in homes in the U.S.? US EPA [Internet]. https://www.epa.gov/radon/what-average-level-radon-found-homes-us (accessed 18 September 2023).
- 17. United States Environmental Protection Agency. EPA map of radon zones, https://www.epa.gov/radon/epa-map-radon-zones (2014, accessed 18 September 2023).
- 18. Gaskin J, Coyle D, Whyte J, et al. Global estimate of lung cancer mortality attributable to residential radon. Environ Health Perspect 2018; 126: 057009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stanley FKT, Irvine JL, Jacques WR, et al. Radon exposure is rising steadily within the modern North American residential environment, and is increasingly uniform across seasons. Sci Rep 2019; 9: 18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill W, Lim EL, Weeden CE, et al.; TRACERx Consortium. Lung adenocarcinoma promotion by air pollutants. Nature 2023; 616: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide [Internet], https://www.who.int/publications-detail-redirect/9789240034228 (accessed 18 September 2023). [PubMed]
- 22. Gogna P, Narain TA, O’Sullivan DE, et al. Estimates of the current and future burden of lung cancer attributable to PM2.5 in Canada. Prev Med 2019; 122: 91–99. [DOI] [PubMed] [Google Scholar]
- 23. Boing AF, deSouza P, Boing AC, et al. Air Pollution, socioeconomic status, and age-specific mortality risk in the United States. JAMA Netw Open 2022; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981; 66: 1191–1308. [PubMed] [Google Scholar]
- 25. Steenland K, Burnett C, Lalich N, et al. Dying for work: the magnitude of US mortality from selected causes of death associated with occupation. Am J Ind Med 2003; 43: 461–482. [DOI] [PubMed] [Google Scholar]
- 26. Occupational Cancer Research Centre. Burden of occupational cancer in Canada: Major workplace carcinogens and prevention of exposure. Toronto, ON, Occupational Cancer Research Centre, 2019. [Google Scholar]
- 27. Centers for Disease Control and Prevention (CDC). Racial/ethnic disparities and geographic differences in lung cancer incidence – 38 states and the District of Columbia, 1998-2006. MMWR Morb Mortal Wkly Rep 2010; 59: 1434–1438. [PubMed] [Google Scholar]
- 28. Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer 2004; 4: 519–527. [DOI] [PubMed] [Google Scholar]
- 29. Parry EM, Gable DL, Stanley SE, et al. Germline mutations in DNA repair genes in lung adenocarcinoma. J Thorac Oncol 2017; 12: 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorscher S, LoPiccolo J, Chen E, et al. Landscape of pathogenic germline variants in patients with lung cancer. 40(36 Suppl):388570–388570. [Google Scholar]
- 31. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014; 311: 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collisson EA, Campbell JD, Brooks AN, et al.; Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27: 4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5: 850–859. [DOI] [PubMed] [Google Scholar]
- 35. Muthusamy B, Raskina K, Lofgren KT, et al. Quantifying the value of multigene testing in resected early stage lung adenocarcinoma. J Thorac Oncol 2023; 18: 476–486. [DOI] [PubMed] [Google Scholar]
- 36. Adib E, Nassar AH, Abou Alaiwi S, et al. Variation in targetable genomic alterations in non-small cell lung cancer by genetic ancestry, sex, smoking history, and histology. Genome Med 2022; 14: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piñeros M, Laversanne M, Barrios E, et al. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg Heal Am 2022; 13: None. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Air quality and health, Centre for Environment & Health (BON). Environment, Climate Change and Health GRC. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Heal Organ 2021. [PubMed] [Google Scholar]
- 39. Interactive global map of 2022 PM2.5 concentrations by city. https://www.iqair.com/world-air-quality-report (accessed 18 September 2023).
- 40. Arrieta O, Campos-Parra AD, Zuloaga C, et al. Clinical and pathological characteristics, outcome and mutational profiles regarding non-small-cell lung cancer related to wood-smoke exposure. J Thorac Oncol 2012; 7: 1228–1234. [DOI] [PubMed] [Google Scholar]
- 41. McClintock TR, Chen Y, Bundschuh J, et al. Arsenic exposure in Latin America: biomarkers, risk assessments and related health effects. Sci Total Environ 2012; 429: 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marshall G, Ferreccio C, Yuan Y, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst 2007; 99: 920–928. [DOI] [PubMed] [Google Scholar]
- 43. Martinez VD, Buys TP, Adonis M, et al. Arsenic-related DNA copy-number alterations in lung squamous cell carcinomas. Br J Cancer 2010; 103: 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferreccio C, González C, Milosavjlevic V, et al. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 2000; 11: 673–679. [DOI] [PubMed] [Google Scholar]
- 45. Giraldo-Osorio A, Ruano-Ravina A, Varela-Lema L, et al. Residential radon in Central and South America: a systematic review. Int J Environ Res Public Health 2020; 17: 4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cranford HM, Koru-Sengul T, Lopes G, et al. Lung cancer incidence by detailed race-ethnicity. Cancers 2023; 15: 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mezquita L, Jové M, Nadal E, et al. High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-Fraumeni syndrome. J Thorac Oncol 2020; 15: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 48. Sandoval RL, Masotti C, de Macedo MP, et al. Identification of the TP53 p.R337H variant in tumor genomic profiling should prompt consideration of germline testing for Li-Fraumeni syndrome. J Glob Oncol 2021; 7: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barbosa M, Cordeiro de Lima V, Formiga M, et al. High prevalence of EGFR mutations in lung adenocarcinomas From Brazilian patients harboring the TP53 p.R337H variant. Vol. 21. Clinical Lung Cancer, Vol. 21, 2020, p. e37–e44. [DOI] [PubMed] [Google Scholar]
- 50. Mascarenhas E, Gelatti AC, Araújo LH, et al. Comprehensive genomic profiling of Brazilian non-small cell lung cancer patients (GBOT 0118/LACOG0418). Thorac Cancer 2021; 12: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arrieta O, Cardona AF, Martín C, et al. Updated frequency of EGFR and KRAS mutations in Non Small-Cell Lung Cancer in Latin America: the Latin-American Consortium for the investigation of Lung Cancer (CLICaP). J Thorac Oncol 2015; 10: 838–843. [DOI] [PubMed] [Google Scholar]
- 52. Martin C, Cuello M, Barajas O, et al. Real-world evaluation of molecular testing and treatment patterns for EGFR mutations in non-small cell lung cancer in Latin America. Mol Clin Oncol 2022; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raez LE, Cardona AF, Arrieta O, et al. Lung cancer disparities in Hispanics: molecular diagnosis and use of immunotherapy. J Glob Oncol 2020; 6: 784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Freitas HC, Torrezan GT, Cunha IWD, et al. Mutational portrait of lung adenocarcinoma in Brazilian patients: past, present, and future of molecular profiling in the Clinic. Front Oncol 2020; 10: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gejman R, González S, Muñoz-Medel M, et al. Prevalence of EGFR mutations and clinico-pathological characteristics of Chilean lung cancer patients. Asian Pacif J Cancer Prev 2019; 20: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berois N, Touya D, Ubillos L, et al. Prevalence of EGFR mutations in lung cancer in Uruguayan population. J Cancer Epidemiol 2017; 2017: 6170290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carrot-Zhang J, Soca-Chafre G, Patterson N, et al. Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discov 2021; 11: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blaquier J, Cerini M, Denninghoff V, et al. P86.18 prevalence, clinical characteristics and survival of patients with KRAS mutant lung cancer in Argentina. J Thorac Oncol 2021; 16: S680. [Google Scholar]
- 59. Arrieta O, Cardona A, Bramuglia G, et al.; on behalf of the CLICaP. Molecular epidemiology of ALK rearrangements in Advanced Lung Adenocarcinoma in Latin America. Oncology 2019; 96: 207–216. [DOI] [PubMed] [Google Scholar]
- 60. Sepúlveda-Hermosilla G, Freire M, Blanco A, et al.; NIRVANA team. Concordance analysis of ALK gene fusion detection methods in patients with Non-Small-Cell Lung Cancer from Chile, Brazil, and Peru. J Mol Diagn 2021; 23: 1127–1137. [DOI] [PubMed] [Google Scholar]
- 61. Sharma R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol 2022; 27: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lam DC, Liam CK, Andarini S, et al. Lung cancer screening in Asia: an expert consensus report. J Thorac Oncol 2023; 18: 1303–1322. [DOI] [PubMed] [Google Scholar]
- 63. Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, et al. The incidence and mortality of lung cancer and their relationship to development in Asia. Transl Lung Cancer Res 2015; 4: 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mackay JM, Dorotheo EU, Assunta M, et al. Tobacco control in Asia-Pacific: wins, challenges and targets. Tob Control 2022; 31: 146–149. [DOI] [PubMed] [Google Scholar]
- 65. Yang JJ, Yu D, Wen W, et al. Tobacco smoking and mortality in Asia: a pooled meta-analysis. JAMA Netw Open 2019; 2: e191474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tobacco. https://www.who.int/southeastasia/health-topics/tobacco (accessed 27 September 2023).
- 67. Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109: 132–137. [DOI] [PubMed] [Google Scholar]
- 68. World Health Organization (WHO) [Internet]. https://www.who.int/es (accessed 9 July 2023).
- 69. Radon database [Internet]. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/gho-phe-radon-database (accessed 27 September 2023).
- 70. Janik M, Bossew P, Hasan MM, et al. Indoor radon Research in the Asia-Pacific region. Atmos 2023; 14: 948. [Google Scholar]
- 71. Liu X, Mubarik S, Wang F, et al. Lung Cancer Death attributable to long-term ambient particulate matter (PM2.5) exposure in East Asian countries during 1990-2019. Front Med 2021; 8: 742076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guan WJ, Zheng XY, Chung KF, et al. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet 2016; 388: 1939–1951. [DOI] [PubMed] [Google Scholar]
- 73. Ge C, Peters S, Olsson A, et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. A pooled exposure–response analysis of 14 case–Control Studies. Am J Respir Crit Care Med 2020; 202: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ge C, Peters S, Olsson A, et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. A pooled analysis of case–Control Studies. Am J Respir Crit Care Med 2020; 202: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. The Lancet respiratory medicine null. The world is failing on silicosis. Lancet Respir Med 2019; 7: 283. [DOI] [PubMed] [Google Scholar]
- 76. Peng W, Li B, Li J, et al. Clinical and genomic features of Chinese lung cancer patients with germline mutations. Nat Commun 2022; 13: 1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hu X, Yang D, Li Y, et al. Erratum to prevalence and clinical significance of pathogenic germline BRCA1/2 mutations in Chinese non-small cell lung cancer patients. Cancer Biol Med 2019; 17: 513–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yamamoto H, Yatabe Y, Toyooka S. Inherited lung cancer syndromes targeting never smokers. Transl Lung Cancer Res 2018; 7: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jazieh AR, Gaafar R, Errihani H, et al. Real-world data on the prevalence of anaplastic lymphoma kinase-positive Non-Small-Cell lung cancer in the Middle East and North Africa. J Glob Oncol 2021; 7: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lim TKH, Skoulidis F, Kerr KM, et al. KRAS G12C in advanced NSCLC: prevalence, co-mutations, and testing. Lung Cancer 2023; 184: 107293. [DOI] [PubMed] [Google Scholar]
- 81. Matsumoto S, Zhou C, Kuo CH, et al. Establishment of the first international large-scale, genomic screening platform to identify patients with rare oncogene drivers in non-small cell lung cancer (NSCLC) in East Asia. J Clin Oncol 2020; 38: 9605–9605. [Google Scholar]
- 82. Roy M, Singh N, Bal A, et al. A brief report on the mutational landscape in non-small cell lung cancer of South Asian patients: comparison at a US and an Indian Institution. Lung India 2022; 39: 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ren S, Wang J, Ying J, et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open 2022; 7: 100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mazieres J, Vioix H, Pfeiffer BM, et al. MET exon 14 skipping in NSCLC: a systematic literature review of epidemiology, clinical characteristics, and outcomes. Clin Lung Cancer 2023; 24: 483–497. [DOI] [PubMed] [Google Scholar]
- 85. Jazieh AR, Algwaiz G, Errihani H, et al. Lung cancer in the Middle East and North Africa Region. J Thorac Oncol 2019; 14: 1884–1891. [DOI] [PubMed] [Google Scholar]
- 86. Cancer today [Internet]. [Cited 2023. sep 27]. Available from: http://gco.iarc.fr/today/home.
- 87. Nasser AMA, Geng Y, Al-Wesabi SA. The prevalence of smoking (cigarette and waterpipe) among university students in some Arab countries: a systematic review. Asian Pac J Cancer Prev 2020; 21: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Al-Shamsi HO, Jaffar H, Mahboub B, et al. Early diagnosis of lung cancer in the United Arab Emirates: challenges and strategic recommendations. Clin Pract 2021; 11: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Salameh P, Salamé J, Waked M, et al. Waterpipe dependence in university students and effect of normative beliefs: a cross-sectional study. BMJ Open 2014; 4: e004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Global Tobacco Surveillance System Data. CDC. https://www.cdc.gov/tobacco/global/gtss/gtssdata/index.html (accessed 27 September 2023).
- 91. Maziak W, Taleb ZB, Bahelah R, et al. The global epidemiology of waterpipe smoking. Tob Control 2015; 24 (Suppl 1) i3–i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sherafat S, Nemati Mansour S, Mosaferi M, et al. First indoor radon mapping and assessment excess lifetime cancer risk in Iran. MethodsX 2019; 6: 2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Abbasi S, Mortazavi SAR, Mortazavi SMJ. Martian residents: mass media and Ramsar high background radiation areas. J Biomed Phys Eng 2019; 9: 483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mortazavi SMJ, Ghiassi-nejad M, Niroomand-rad A, et al. How should governments address high levels of natural radiation and radon – lessons from the chernobyl nuclear accident and Ramsar, Iran. RISK: Health, Safety & Environment (1990-2002), 2002, p.13. https://scholars.unh.edu/risk/vol13/iss1/4 [Google Scholar]
- 95. Stegnar P, Shishkov I, Burkitbayev M, et al. Assessment of the radiological impact of gamma and radon dose rates at former U mining sites in Central Asia. J Environ Radioact 2013; 123: 3–13. [DOI] [PubMed] [Google Scholar]
- 96. Yurov V, Eremin E, Guchenko S. Ecological and radiation safety of Central Asia. E3S Web Conf 2021; 311. [Google Scholar]
- 97. Abbasi-Kangevari M, Malekpour MR, Masinaei M, et al.; GBD 2019 North Africa and the Middle East Air Pollution Collaborators. Effect of air pollution on disease burden, mortality, and life expectancy in North Africa and the Middle East: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Planet Heal 2023; 7: e358–e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Khanmohammadi S, Saeedi Moghaddam S, Azadnajafabad S, et al.; GBD 2019 NAME Tracheal, Bronchus and Lung Cancer Collaborators. Burden of tracheal, bronchus, and lung cancer in North Africa and Middle East countries, 1990 to 2019: Results from the GBD study 2019. Front Oncol 2023; 12: 1098218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Erefai O, Soulaymani A, Mokhtari A, et al. Occupational exposures and lung cancer in Morocco: an epidemiologic study. Mater Today Proc 2023; 72: 3480–3483. [Google Scholar]
- 100. Burden of lung cancer and associated risk factors in Africa by region. Semantic Scholar, https://www.semanticscholar.org/paper/Burden-of-Lung-Cancer-and-Associated-Risk-Factors-Alex-Urman/7689d6f55aa995b6eb2507d5eb2db7002ae9d8c8 (accessed 11 October 2023).
- 101. Salehiniya H, Bahadori M, Ghanizadeh G, et al. Epidemiological study of lung cancer in Iran: a systematic review. Iran J Public Health 2022; 51: 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benbrahim Z, Antonia T, Mellas N. EGFR mutation frequency in Middle East and African non-small cell lung cancer patients: a systematic review and meta-analysis. BMC Cancer 2018; 18: 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Olaleye O, Ekrikpo U. Epidemiology of cancers in Sub-Saharan Africa. In: Adedeji OA. (ed.) Cancer in Sub-Saharan Africa: current practice and future. Cham: Springer International Publishing, 2017, pp.3–19. [Google Scholar]
- 104. Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer 2012; 118: 4372–4384. [DOI] [PubMed] [Google Scholar]
- 105. Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol 2010; 11: 165–173. [DOI] [PubMed] [Google Scholar]
- 106. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Global Health 2019; 85: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Okonta KE, Echieh PC, Abubakar U, et al. Management of lung cancer in Africa: underdiagnosis and poor access to treatment – a close look at Nigeria and West African sub-region. J Pan Afr Thorac Soc 2021; 2: 122–129. [Google Scholar]
- 108. Brathwaite R, Addo J, Smeeth L, et al. A systematic review of tobacco smoking prevalence and description of tobacco control strategies in Sub-Saharan African countries; 2007 to 2014. PLos One 10: e0132401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McCormack VA, Schüz J. Africa’s growing cancer burden: environmental and occupational contributions. Cancer Epidemiol 2012; 36: 1–7. [DOI] [PubMed] [Google Scholar]
- 110. David SP, Wang A, Kapphahn K, et al. Gene by environment investigation of incident lung cancer risk in African-Americans. EBioMedicine 2016; 4: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Niyibizi BA, Muhizi E, Ndoli DA, et al. Lung cancer in Rwanda. J Thorac Oncol 2022; 17: 1074–1077. [DOI] [PubMed] [Google Scholar]
- 112. van Eeden R, Tunmer M, Geldenhuys A, et al. Lung cancer in South Africa. J Thorac Oncol 2020; 15: 22–28. [DOI] [PubMed] [Google Scholar]
- 113. Chan SW, Maske CP, Ruff P. EGFR mutations in Non-Small Cell Lung Cancer in South Africa. Ann Oncol 2015; 26: i1. [Google Scholar]
- 114. Campbell JD, Lathan C, Sholl L, et al. Comparison of prevalence and types of mutations in lung cancers among black and white populations. JAMA Oncol 2017; 3: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Araujo LH, Lammers PE, Matthews-Smith V, et al. Somatic mutation spectrum of Non-Small-Cell lung cancer in African Americans: a pooled analysis. J Thorac Oncol 2015; 10: 1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dyba T, Randi G, Bray F, et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer 2021; 157: 308–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cancer statistics – specific cancers. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cancer_statistics_-_specific_cancers (accessed 29 May 2023).
- 118. Bussell M, Lovell A. MA24.05 lung cancer in Europe: strengthening policy responses one country at a time. J Thorac Oncol 2019; 14: S347–S348. [Google Scholar]
- 119. Schaap MM, Kunst AE, Leinsalu M, et al. Effect of nationwide tobacco control policies on smoking cessation in high and low educated groups in 18 European countries. Tob Control 2008; 17: 248–255. [DOI] [PubMed] [Google Scholar]
- 120. Gredner T, Mons U, Niedermaier T, et al. Impact of tobacco control policies implementation on future lung cancer incidence in Europe: an international, population-based modeling study. Lancet Reg Heal Eur 2021; 4: 100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tollefsen T, Cinelli G, Bossew P, et al. From the European indoor radon map towards an atlas of natural radiation. Radiat Prot Dosimetry 2014; 162: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Riudavets M, Garcia de, Herreros M, Besse B, et al. Radon and lung cancer: Current Trends and Future Perspectives. Cancers 2022; 14: 3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Garcia M, Garcia de Herreros M, Auclin E, et al. OA13.04 prevalence of molecular alterations in NSCLC and Estimated Indoor radon in Europe: RADON EUROPE Study. J Thorac Oncol 2022; 17(Suppl.): S34–S35. [Google Scholar]
- 124. Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kauppinen T, Toikkanen J, Pedersen D, et al. Occupational exposure to carcinogens in the European Union. Occup Environ Med 2000; 57: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Takala J. Eliminating occupational cancer in Europe and globally. Working Papers. https://ideas.repec.org//p/etu/wpaper/14226.html (2015, accessed 11 October 2023).
- 127. Frank C, Sundquist J, Yu H, et al. Concordant and discordant familial cancer: familial risks, proportions and population impact. Int J Cancer 2017; 140: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 128. Hung R, Spitz M, Houlston R, et al. Lung Cancer Risk in never-smokers of European descent is associated with genetic variation in the 5p15.33 TERT-CLPTM1Ll region. J Thorac Oncol 2019; 14: 1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mezquita L, Iurchenko A, Benitez JC, et al. Abstract 448: high prevalence of pathogenic germline variants in patients with oncogene-driven non-small cell lung cancer. Cancer Res 2021; 81(Suppl): 448–448. [Google Scholar]
- 130. Zurera M, Lastra R, Mezquita L, et al. 24P preliminary data on INHERITY LC: Germline mutations of a cohort of selected non-small cell lung cancer (NSCLC) patients. Ann Oncol 2022; 33: S553. [Google Scholar]
- 131. Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021; 154: 161–175. [DOI] [PubMed] [Google Scholar]
- 132. Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016; 387: 1415–1426. [DOI] [PubMed] [Google Scholar]
- 133. Griesinger F, Eberhardt W, Nusch A, et al. Biomarker testing in non-small cell lung cancer in routine care: analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP registry (AIO-TRK-0315). Lung Cancer 2021; 152: 174–184. [DOI] [PubMed] [Google Scholar]
- 134. Mezquita L, Benito A, Ruano-Raviña A, et al. Indoor radon in EGFR- and BRAF-Mutated and ALK-Rearranged Non-Small-Cell lung cancer patients. Clin Lung Cancer 2019; 20: 305–312.e3. [DOI] [PubMed] [Google Scholar]
- 135. Ruano-Ravina A, Torres-Durán M, Kelsey KT, et al. Residential radon, EGFR mutations and ALK alterations in never-smoking lung cancer cases. Eur Respir J 2016; 48: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 136. Mezquita L, Barlesi F, Auclin E, et al. OA09.06 molecular alterations and estimated indoor radon in NSCLC patients from the French National Cancer Institute Registry: radon France Study. J Thorac Oncol 2018; 13: S342. [Google Scholar]
- 137. Mezquita L, Barlesi F, Ielsch G, et al. FP09.05 driver oncogenic alterations and indoor radon in NSCLC patients from the IFCT Biomarker Cohort: Bioradon France Study. J Thorac Oncol 2021; 16: S214. [Google Scholar]
- 138. djarvel. Project Bioradon [Internet]. SPECTA, 2022 https://spectaplatform.org/project-bioradon/ (2022, accessed 12 October 2023).
- 139. World Population Prospects – Population Division – United Nations, https://population.un.org/wpp/ (accessed 29 May 2023).
- 140. Health Quality & Safety Commission [Internet]. Lung Cancer. https://www.hqsc.govt.nz/our-data/atlas-of-healthcare-variation/lung-cancer/ (accessed 29 May 2023).
- 141. Harwood M, Aldington S, Beasley R. Lung cancer in Maori: a neglected priority. N Z Med J 2005; 118: U1410. [PubMed] [Google Scholar]
- 142. Kidd J, Cassim S, Rolleston A, et al. Hā Ora: secondary care barriers and enablers to early diagnosis of lung cancer for Māori communities. BMC Cancer 2021; 21: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. John T, Cooper WA, Wright G, et al. Lung cancer in Australia. J Thorac Oncol 2020; 15: 1809–1814. [DOI] [PubMed] [Google Scholar]
- 144. Dachs GU, Currie MJ, McKenzie F, et al. Cancer disparities in indigenous Polynesian populations: Māori, Native Hawaiians, and Pacific people. Lancet Oncol 2008; 9: 473–484. [DOI] [PubMed] [Google Scholar]
- 145. Ridolfo B, Stevenson C. The quantification of drug-caused mortality and morbidity in Australia, 1998. Canberra: AIHW, Report No: AIHW cat no PHE 29 (Drug Statistics Series no 7), http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442459309 (2001). [Google Scholar]
- 146. Australian Institute of Health and Welfare [Internet]. Cancer in Australia: an overview, 2008, Summary. https://www.aihw.gov.au/reports/cancer/cancer-in-australia-an-overview-2008/summary (2008, accessed 2 June 2023).
- 147. Aboriginal and Torres Strait Islander Peoples: Smoking Trends, Australia, 1994 to 2014-15 | Australian Bureau of Statistics [Internet]. https://www.abs.gov.au/statistics/people/aboriginal-and-torres-strait-islander-peoples/aboriginal-and-torres-strait-islander-peoples-smoking-trends-australia/latest-release (2017, accessed 2 June 2023).
- 148. Long SA, Tinker RA. Australian action to reduce health risks from radon. Ann ICRP 2020; 49: 77–83. [DOI] [PubMed] [Google Scholar]
- 149. Ministry of Health NZ [Internet]. Radon (radioactive gas). https://www.health.govt.nz/your-health/healthy-living/environmental-health/radiation-environment/radon-radioactive-gas (accessed 2 June 2023).
- 150. Radon map of Australia. ARPANSA. https://www.arpansa.gov.au/understanding-radiation/radiation-sources/more-radiation-sources/radon-map (accessed 12 October 2023).
- 151. Denton GRW, Namazi S. Indoor radon levels and lung cancer incidence on Guam. Procedia Environ Sci 2013; 18: 157–166. [Google Scholar]
- 152. Statistics c=AU; o=Commonwealth of A ou=Australian B of. Missing Title Information [Internet]. c=AU; o=Commonwealth of Australia; ou=Australian Bureau of Statistics 2023. https://www.abs.gov.au/ausstats/abs@.nsf/ViewContent?readform&view=productsbytopic&Action=Expand&Num=2.8.1 (accessed 22 June 2023).
- 153. Drozdovitch V, De Vathaire F, Bouville A. Radiological impact of atmospheric nuclear weapons tests at Mururoa and fangataufa atolls to populations in Oceania, South America and Africa: comparison with French Polynesia. Asian Pac J Cancer Prev 2021; 22: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Philippe S, Schoenberger S, Ahmed N. Radiation exposures and compensation of victims of French atmospheric nuclear tests in Polynesia. Sci Glob Sec 2022; 30: 62–94. [Google Scholar]
- 155. Le Vu B, de Vathaire F, de Vathaire CC, et al. Cancer incidence in French Polynesia 1985-95. Trop Med Int Health 2000; 5: 722–731. [DOI] [PubMed] [Google Scholar]
- 156. Workplace cancer. Cancer Council, https://www.cancer.org.au/cancer-information/causes-and-prevention/workplace-cancer (accessed 22 June 2023).
- 157. Musk AW, Olsen N, Alfonso H, et al. Pattern of malignant mesothelioma incidence and occupational exposure to asbestos in Western Australia. Med J Aust 2015; 203: 251–252e.1. [DOI] [PubMed] [Google Scholar]
- 158. Wollstein A, Lao O, Becker C, et al. Demographic history of Oceania inferred from genome-wide data. Curr Biol 2010; 20: 1983–1992. [DOI] [PubMed] [Google Scholar]
- 159. Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998; 392: 402–405. [DOI] [PubMed] [Google Scholar]
- 160. Strengthening Pacific health systems. https://www.who.int/westernpacific/activities/strengthening-pacific-health-systems (accessed 17 June 2023).
- 161. Russell PA, Rogers TM, Solomon B, et al. Correlation between molecular analysis, diagnosis according to the 2015 WHO classification of unresected lung tumours and TTF1 expression in small biopsies and cytology specimens from 344 non-small cell lung carcinoma patients. Pathology 2017; 49: 604–610. [DOI] [PubMed] [Google Scholar]
- 162. Stone E, Allen HA, Saghaie T, et al. High proportion of rare and compound epidermal growth factor receptor mutations in an Australian population of non-squamous non-small-cell lung cancer. Intern Med J 2014; 44: 1188–1192. [DOI] [PubMed] [Google Scholar]
- 163. Tan L, Alexander M, Officer A, et al. Survival difference according to mutation status in a prospective cohort study of Australian patients with metastatic non-small-cell lung carcinoma. Intern Med J 2018; 48: 37–44. [DOI] [PubMed] [Google Scholar]
- 164. Houang M, Sioson L, Clarkson A, et al. EGFR mutation specific immunohistochemistry is a useful adjunct which helps to identify false negative mutation testing in lung cancer. Pathology 2014; 46: 501–508. [DOI] [PubMed] [Google Scholar]
- 165. Kim RH, Lapuk A, Harraway J, et al. Prevalence of the EGFR T790M and other resistance mutations in the Australian population and histopathological correlation in a small subset of cases. Pathology 2020; 52: 410–420. [DOI] [PubMed] [Google Scholar]
- 166. Downton TDF, Wing K, Cosentino SB, et al. The molecular characteristics of non-small cell lung cancer in the Northern Territory’s Top End. Asia Pac J Clin Oncol 2023. [DOI] [PubMed] [Google Scholar]
- 167. Aye PS, McKeage MJ, Tin Tin S, et al. Population-based incidence rates and increased risk of EGFR mutated non-small cell lung cancer in Māori and Pacifica in New Zealand. PLoS One 2021; 16: e0251357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. McKeage MJ, Tin Tin S, Khwaounjoo P, et al. Screening for anaplastic lymphoma kinase (ALK) gene rearrangements in non-small-cell lung cancer in New Zealand. Intern Med J 2020; 50: 716–725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241231260 for Geographic differences in lung cancer: focus on carcinogens, genetic predisposition, and molecular epidemiology by Juan Carlos Laguna, Miguel García-Pardo, Joao Alessi, Carlos Barrios, Navneet Singh, Humaid O. Al-Shamsi, Herbert Loong, Miquel Ferriol, Gonzalo Recondo and Laura Mezquita in Therapeutic Advances in Medical Oncology