ABSTRACT.
Incidence of human monkeypox (mpox) has been increasing in West and Central Africa, including in the Democratic Republic of Congo (DRC), where monkeypox virus (MPXV) is endemic. Most estimates of the pathogen’s transmissibility in the DRC are based on data from the 1980s. Amid the global 2022 mpox outbreak, new estimates are needed to characterize the virus’ epidemic potential and inform outbreak control strategies. We used the R package vimes to identify clusters of laboratory-confirmed mpox cases in Tshuapa Province, DRC. Cases with both temporal and spatial data were assigned to clusters based on the disease’s serial interval and spatial kernel. We used the size of the clusters to infer the effective reproduction number, Rt, and the rate of zoonotic spillover of MPXV into the human population. Out of 1,463 confirmed mpox cases reported in Tshuapa Province between 2013 and 2017, 878 had both date of symptom onset and a location with geographic coordinates. Results include an estimated Rt of 0.82 (95% CI: 0.79–0.85) and a rate of 132 (95% CI: 122–143) spillovers per year assuming a reporting rate of 25%. This estimate of Rt is larger than most previous estimates. One potential explanation for this result is that Rt could have increased in the DRC over time owing to declining population-level immunity conferred by smallpox vaccination, which was discontinued around 1982. Rt could be overestimated if our assumption of one spillover event per cluster does not hold. Our results are consistent with increased transmissibility of MPXV in Tshuapa Province.
INTRODUCTION
Mpox (formerly known as monkeypox) is a zoonotic disease that has been endemic in Africa for at least half a century.1 The disease is caused by monkeypox virus (MPXV), an orthopoxvirus in the family Poxviridae.2 The virus is related to variola virus, which causes smallpox,3 and is divided into two clades, clade I and clade II. Clade I has historically circulated in the Congo Basin, whereas clade II has historically circulated in West Africa.4 Vaccination against smallpox using vaccinia virus provides protection against other orthopoxvirus infections; however, routine smallpox immunization ended with the eradication of the disease in 1980. In unvaccinated persons, clade I has an estimated case fatality rate of 11%3 compared with < 3% for clade II.5 The clinical presentation of mpox is similar to that of smallpox with the exception of the presence of lymphadenopathy in the majority of mpox cases. Classical symptoms begin with a prodrome consisting of fever, headache, muscle aches, fatigue, and lymphadenopathy.6 One to 3 days later, a rash develops, which progresses through several stages over the course of 2 to 4 weeks. Complications from the disease include scarring, permanent corneal scarring leading to loss of vision, bronchopneumonia, encephalitis, and sepsis.6
Transmission of MPXV from infected animals to humans occurs via scratches or bites7 and may occur while hunting and preparing wild game or through contact with infectious fomites (e.g., environmental contamination).8 Although the animal reservoir(s) is unknown, small mammals including rodents are thought to play a role in the maintenance and spread of the virus.3 After one or more spillover events from the reservoir, human-to-human transmission can occur through close contact with infectious material from skin lesions, respiratory secretions during prolonged face-to-face contact, and fomites such as linens and bedding.9 Prior to 2022, human transmission chains as long as seven generations had been observed.10
Since 2010, the U.S. CDC and Kinshasa School of Public Health have been providing support for enhanced surveillance of mpox in Tshuapa Province. Tshuapa Province is located in a rural, forested area of the Congo Basin in the Democratic Republic of Congo (DRC). It has an area of approximately 133,000 km2 and a population of approximately 2 million11 spread across 12 health zones (HZs). The climate is equatorial, with a rainy and a dry season.12 The region is characterized by poor infrastructure and widespread poverty.13 Much of the population relies on wild game for protein.14
A key epidemiological parameter used to understand the transmission potential of infectious diseases is the reproduction number. The reproduction number is defined as the average number of secondary infections generated by a single infected individual.15 If the value is above 1, an epidemic is growing, whereas a value below 1 suggests an epidemic is shrinking.16 The reproduction number depends on contact patterns, demographic rates, and population-level immunity, among other factors.17 Thus, estimates of the reproduction number for the same pathogen can vary by geographic location and over time. The basic reproduction number, R0, refers to situations in which a pathogen is introduced into a large, completely susceptible population.18 If a pathogen is introduced into a population with immunity from vaccination or infection, the reproduction number is considered an effective reproduction number, Rt.19 Monitoring Rt for emerging and re-emerging infectious diseases is important for global health security, as increases could precede epidemics or pandemics.
In 1988, a study by Fine et al.20 suggested that an R0 of 0.82 (with an upper limit of 1) could be expected for MPXV in the DRC when vaccine-derived immunity disappears. In contrast, a more recent study by Grant et al.21 estimated an R0 of 2.1 (uncertainty range, 1.5–2.7) for the DRC based on data from the same period (1980–1984); however, the analysis did not account for the fairly large proportion of the population in remote areas that was susceptible to mpox at the time of smallpox eradication as a result of incomplete vaccine coverage.
There is evidence that the incidence of mpox has increased in Central22–24 and West Africa.25 Most published estimates of Rt for MPXV in the DRC are based on data from the 1980s and range from approximately 0.3 to 0.5.20,26–28 In 2022, a global outbreak of mpox caused more than 85,000 cases in all regions of the world, disproportionately affecting gay individuals, bisexual individuals, and men who have sex with men.29–31 Given the changing epidemiology of mpox, updated estimates of the virus’ transmissibility are needed in historically affected countries. The aims of this study were to identify clusters of mpox cases from surveillance data in Tshuapa Province, DRC, and to use the cluster sizes to estimate Rt and the rate at which MPXV spills over into the human population.
MATERIALS AND METHODS
Overview.
The model framework implemented in the R package vimes combines various data types (e.g., temporal, spatial, genetic) to identify clusters of cases in an outbreak. Input data types can include anything for which a pairwise distance can be calculated (e.g., days between symptom onset). Vimes connects all cases on a graph for each data type provided. The graph’s edges are weighted by the pairwise distance between cases. Distances greater than specified cutoffs are removed (or “pruned”). The graphs are merged by intersection, with the resulting graph representing clusters of related cases based on all data types. The size of the clusters can then be used to quantify the pathogen’s transmissibility.
Mpox data.
From 2010 to 2019, trained surveillance officers completed paper-based case report forms and collected clinical specimens during investigations of suspected mpox cases in Tshuapa Province (not all suspected cases were investigated). Case report forms include questions about basic demographic information (e.g., age, race/ethnicity, residence), skin lesion characteristics, general signs and symptoms, and exposure history. Lesion swabs, lesion crusts, or blood were tested at the Institut National de Recherche Biomédicale in Kinshasa, DRC, and the CDC in Atlanta, GA. Sample processing and laboratory diagnostic methods have been described elsewhere.32 Case definitions are provided in the Supplemental Methods.
We extracted information from line list data on the timing (date of fever or rash onset) and location (village or neighborhood of residence during the last 12 months or village in which rash onset occurred) of infection for laboratory-confirmed mpox cases to identify clusters of disease (Supplemental Methods). Cases with missing symptom onset date or missing location were not analyzed.
We calculated the pairwise distance between all cases. For temporal data, the distance was calculated as the difference in days between symptom onset dates of all case pair combinations. For spatial data, the geographic distance between cases was calculated using the Vincenty inverse formula for ellipsoids (implemented using the gdist function in the R package Imap),33 given the large area covered by Tshuapa Province.
Epidemiological parameters.
To run vimes, we needed one key parameter for each data type: the serial interval distribution for temporal data and the spatial kernel for spatial data. The serial interval distribution is the time between symptom onset in a primary case and symptom onset in a secondary case infected by the primary case.34 We used a γ-distributed serial interval with mean 16.0 days and standard deviation of 3.7 days based on smallpox data35 (Supplemental Methods). The spatial kernel is the distribution of geographical distances between primary and secondary cases.34 We estimated the mean transmission distance of 0.13 km for MPXV using active contact tracing data collected by the WHO between 1970 and 1986 and (Supplemental Figure 1) assumed a Rayleigh distribution.
Cutoffs.
Choosing appropriate cutoffs is an important part of using vimes as it affects the number of edges kept in the pruning step and the resulting size of clusters.34 Cutoffs are informed by prior knowledge on the distribution of distances between cases (i.e., the epidemiological parameters described above). For a particular data type, the cutoff can be defined as a quantile of the input distribution. Two cases are considered unrelated if the distance between them is larger than the cutoff.
Underreporting.
The reporting rate is the percentage of infections that are ultimately reported as suspected or confirmed cases of the disease.36 When reporting is low, intermediate cases are missed, and the distance between observed cases can increase. vimes accounts for underreporting by defining the cutoff as fn,π, where fn is the probability density function or probability mass function of expected distances between a primary and secondary case for data type, n, and π is the probability of a geometric distribution that describes the number of unobserved intermediate cases between two observed cases. The method assumes that surveillance and reporting are stable over the estimation period and that the probability of being reported is the same for all cases. Further details can be found in Cori et al.34
Nolen et al.10 found a reporting rate of 41% in a household study after a 2013 mpox outbreak in Bokungu HZ. We adjusted this reporting rate for partially missing data (cases that were missing symptom onset date or location). We considered the surveillance effort for mpox in Tshuapa Province to be relatively stable from 2013 to 2017, based on the total number of suspected mpox cases investigated and the percentage of investigated cases that were confirmed by year and HZ.
Estimation of Rt and spillover rate.
We estimated Rt and the rate of spillover of MPXV using the R package branchr.37 To estimate Rt, the method implemented in branchr assumes that the number of secondary cases follows a Poisson distribution with mean Rt (homogeneous transmission). The likelihood of the observed final size of the outbreak is adjusted by integrating over all possible unobserved cases. This approach assumes that unobserved cases are distributed randomly across the outbreaks.34 For the spillover rate, the number of observed spillovers is known (it is the number of clusters identified with vimes), but the number of unobserved spillovers must be estimated. Conditional on Rt and the reporting rate, the number of unobserved spillovers is estimated by assuming a negative binomial distribution with parameters n, Pobs, where n is the number of observed spillovers and Pobs is the probability of observing a spillover. The total number of spillovers is the estimated number of observed spillovers plus the estimated number of unobserved spillovers. A rate is obtained by dividing this result by the time over which surveillance occurred.34 It is further assumed that only one case per cluster is infected via spillover.
Sensitivity analyses.
We performed a subgroup analysis for each year and for each HZ separately. We also examined the impact of the cutoffs (quantiles) and the assumption regarding reporting rates on our estimates, including the use of a shorter serial interval (Supplemental Methods). Previous studies have identified multiple primary cases of mpox per cluster in the DRC20; thus, we performed a sensitivity analysis on the assumption that only one case was infected via spillover per cluster (Supplemental Methods). As transmission heterogeneity has been identified in mpox spread in the DRC,27 we re-estimated Rt and the spillover rate using an extension of the branchr package that uses a negative binomial distribution to model the number of secondary cases (Supplemental Methods).
Simulations.
As recommended by Cori et al.,34 we performed simulations to find the optimal cutoffs for the specific context of mpox in Tshuapa Province and check that the method can correctly identify mpox clusters as well as accurately estimate Rt and the spillover rate.
Validation.
We searched the Program for Monitoring Emerging Diseases (ProMED) and Google Scholar for documentation of any outbreaks of mpox in Tshuapa Province that may have occurred during the study period. To further evaluate the plausibility of identified clusters, we compared the calculated pairwise differences by data type and cluster38 for singletons (cases not linked to any other cases) and large clusters with 10 or more cases. We would expect large clusters to have smaller pairwise distances compared with the distribution of pairwise distances for singletons.
We also checked the assumption of one spillover event per cluster using exposure history. If reporting were perfect and our assumption were correct, we would expect that patients assigned to larger clusters would report contact with an ill person prior to symptom onset more frequently than singletons.
Availability.
All analyses were conducted in R version 4.1.1. The simulation code is available on GitHub (https://github.com/kcharniga/mpox_in_drc). The mpox line list and geographic data associated with cases are owned by the DRC Ministry of Health. The decision to release these data rests with the Ministry of Health.
RESULTS
Mpox surveillance and reporting.
From 2010 to 2019, a total of 2,993 suspected cases were investigated for mpox in Tshuapa Province (Table 1). Of the investigated cases, 43 (1%) were excluded from the analysis because of inconclusive or missing laboratory results; 2,019 (67%) tested positive for Orthopoxvirus or MPXV, and 931 (31%) tested negative for Orthopoxvirus or MPXV. On average, 299 (range, 11–526) suspected cases were investigated for mpox in Tshuapa Province each year (Table 1). The number of investigated mpox cases increased nonmonotonically from 2010 to 2016 and declined each year after 2016. The percentage of investigated mpox cases that were confirmed was low for the first 3 years (mean, 36%) before stabilizing for the last 7 years (mean, 76%; range, 70–80%).
Table 1.
Human mpox reporting by year from surveillance in Tshuapa Province, DRC, 2010–2019
| Year | Confirmed mpox cases with symptom onset and geographic location (% of confirmed) | Confirmed mpox cases (% of investigated) | Total mpox cases investigated* |
|---|---|---|---|
| 2010 | 3 (60) | 5 (45) | 11 |
| 2011 | 29 (46) | 63 (28) | 224 |
| 2012 | 77 (69) | 112 (34) | 326 |
| 2013 | 228 (69) | 330 (78) | 422 |
| 2014 | 143 (61) | 233 (75) | 309 |
| 2015 | 171 (53) | 324 (77) | 419 |
| 2016 | 205 (54) | 381 (72) | 526 |
| 2017 | 187 (62) | 300 (77) | 388 |
| 2018 | 87 (53) | 164 (70) | 235 |
| 2019 | 55 (51) | 107 (80) | 133 |
| Total | 1,185 (59) | 2,019† (67) | 2,993 |
DRC = Democratic Republic of Congo; mpox = monkeypox.
Total mpox cases investigated include individuals who tested positive or negative for monkeypox virus as well as those who had indeterminate laboratory results. Forty-three individuals had indeterminate laboratory results from 2010 to 2019.
Thirty confirmed mpox cases were missing symptom onset date, and 804 were missing geographic location.
During the period of stable reporting from 2013 to 2017, there were 1,568 laboratory-confirmed cases of mpox reported in Tshuapa Province (Table 2). Of these, 934 (60%) had complete information on symptom onset and geographic location. There were 477 male cases (51%) out of 932 with available data. The mean age of cases was 16 (range, 0–79) years (12 cases were missing age). During this period, the number of suspected mpox cases that were investigated ranged from 40 in Monkoto HZ to 300 in Djolu HZ (Table 2). The percentage of investigated cases that were confirmed ranged from 65% in Mondombe HZ to 90% in Wema HZ, and the percentage of confirmed mpox cases with complete temporal and spatial information ranged from 41% in Ikela HZ to 81% in Djolu HZ.
Table 2.
Human mpox reporting by health zone from surveillance in Tshuapa Province, DRC, 2013–2017
| Health zone | Confirmed mpox cases with symptom onset and geographic location (% of confirmed) | Confirmed mpox cases (% of investigated) | Total investigated | Estimated population size* (2016) |
|---|---|---|---|---|
| Befale | 32 (44) | 72 (73) | 99 | 177,864 |
| Boende | 59 (53) | 111 (70) | 158 | 272,699 |
| Bokungu | 80 (54) | 147 (74) | 198 | 201,658 |
| Busanga | 152 (73) | 208 (76) | 272 | 100,372 |
| Djolu | 166 (81) | 206 (69) | 300 | 235,873 |
| Ikela | 60 (41) | 148 (77) | 191 | 198,471 |
| Lingomo | 99 (55) | 179 (85) | 210 | 135,617 |
| Mompono | 106 (78) | 136 (85) | 160 | 142,956 |
| Mondombe | 59 (48) | 122 (65) | 189 | 173,630 |
| Monkoto | 16 (59) | 27 (68) | 40 | 139,186 |
| Wema | 39 (42) | 92 (90) | 102 | 114,787 |
| Yalifafu | 66 (55) | 120 (83) | 145 | 174,973 |
| Total | 934 (60) | 1,568 (76) | 2,064 | 2,068,084 |
DRC = Democratic Republic of Congo; mpox = monkeypox.
We used population projections for Tshuapa Province from 2015 (Annuaire Statistique 2015, Institut National de la Statistique, the DRC) based on the 1984 census and applied these to demographic estimates for each health zone (unpublished, Division Provinciale de la Santé, Tshuapa). As for other years, data for 2016 were extrapolated assuming an annual growth of 3.3%.
Cluster identification and parameter estimation.
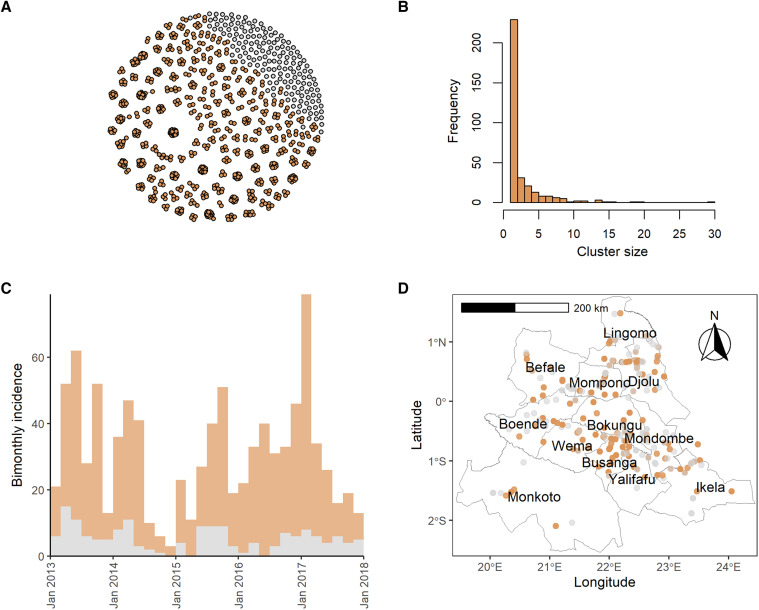
Using vimes, we identified 334 clusters, 161 (48%) of which were singletons (Figure 1 and Supplemental Figure 2). The largest cluster size was 30, and the mean cluster size was 2.8. We estimated an Rt of 0.82 (95% CI: 0.79–0.85) and an annual spillover rate of MPXV into the human population of 132 (95% CI: 122–143) (Supplemental Table 1). We obtained these results assuming a reporting rate of 25% (41% from Nolen et al.10 times 60% of confirmed cases with complete data) and a cutoff corresponding to the 95%1/3 (or 98.3%) quantile of the input distributions. These cutoffs correspond to 233 days and 0.7 km.
Figure 1.
Clusters of human mpox cases in Tshuapa Province, DRC, 2013–2017 identified using the R package vimes. (A) Nodes represent cases, whereas edges represent possible epidemiological links. (B) Frequency of cluster sizes. (C) Bimonthly incidence of human mpox cases by date of symptom onset. Mpox reporting tends to decline in December/January each year, likely because of the holidays and healthcare worker trainings. (D) Map of Tshuapa Province showing the geographic locations of human mpox cases. We adjusted the transparency of the points to improve visualization, but overplotting still occurs for some villages. We assumed a reporting rate of 25% and used pruning cutoff distances associated with the 98.3% quantiles of the input distance distributions. For (A), (C), and (D), cases in orange belong to clusters containing two or more cases, whereas cases in gray are not connected to other cases, using both temporal and spatial data. For (D), we downloaded shapefiles of health zones in the DRC from the Humanitarian Data Exchange. The shapefiles were prepared by the American Red Cross and are available under an Open Database License. DRC = Democratic Republic of Congo; mpox = monkeypox.
Sensitivity analyses.
As expected, using higher cutoffs led to fewer, larger clusters in sensitivity analyses. Using cutoffs ranging from the 90% and 98.3% quantiles of the input distributions (Supplemental Figure 5, Supplemental Table 4), the mean cluster sizes ranged from 2.2 to 3.6, and the maximum cluster size was stable (between 29 and 30). Other summary statistics can be found in Supplemental Table 5. For a given reporting rate, higher cutoffs led to higher estimates of Rt and lower spillover rates (Supplemental Figure 6). The estimated Rt for the most extreme combinations considered (90% quantile/50% reporting rate and 98.3% quantile/10% reporting rate) was 0.67 (95% CI: 0.63–0.71) and 0.92 (95% CI: 0.90–0.94), respectively, and the estimated annual spillover rate was 123 (95% CI: 116–130) and 151 (95% CI: 136–168), respectively. Using cutoffs corresponding to a shorter serial interval estimated during the global mpox outbreak (Supplemental Table 6) did not have a major impact on results; however, setting the distance cutoff to 10 km increased the estimated Rt to 0.89 (95% CI: 0.86–0.93) (Supplemental Results, Supplemental Figure 8, and Supplemental Table 7).
Results of the analysis by year and HZ can be found in the Supplemental Results (Supplemental Figures 10–13, Supplemental Tables 2 and 3). Although Rt was not different across years or HZ (the CIs overlapped), there was heterogeneity in the annual spillover rate by year and HZ (some CIs did not overlap).
As expected, we found slightly lower estimates of Rt and significantly higher spillover rates when we tested the assumption of one case resulting from spillover per cluster (Supplemental Results). When assuming 0.36 for overdispersion in the offspring distribution, we obtained a similar estimate of Rt and a significantly higher estimate for the annual spillover rate compared with our main results (Supplemental Results).
Simulations.
Our simulation study showed that the optimal cutoff for mpox in Tshuapa Province is defined by the 98.3% quantile (Supplemental Figure 15). Further simulation study results can be found in the Supplemental Results and Supplemental Figure 16.
Validation.
We found two reports of potential mpox outbreaks in Tshuapa Province during the study period. Details can be found in the Supplemental Results.
The calculated pairwise differences by data type and cluster for singletons and clusters with 10 or more cases are shown in Supplemental Figure 17. As expected, we found that the median pairwise differences were significantly lower on the temporal and spatial dimensions in all clusters compared with those dimensions in the singletons. In Supplemental Figure 18, we present the epidemiologic curves associated with these clusters. Although cases occur sporadically over the time series for some clusters, others have uninterrupted incidence.
We evaluated exposure information for the 934 mpox patients included in our cluster analysis to check the assumption of one spillover event per cluster. Nearly half of patients with available data reported contact with a person or persons presenting with similar symptoms in the 3 weeks prior to symptom onset, nearly half reported having touched a wild animal during the 3 weeks prior to symptom onset, and 13% reported both types of contact (Supplemental Results). The distribution of patients who reported contact with ill people or animals was similar regardless of cluster type (singleton versus cluster with size > 1; Supplemental Figure 3). We found some statistically significant differences in the proportion of mpox patients who reported contact with ill people or animals by HZ (Supplemental Figure 4).
DISCUSSION
Our results suggest that the transmissibility of MPXV in the DRC has more than doubled since the 1980s. Although Rt still appears to be less than the critical value of 1 that could permit epidemic spread, further increases of just 0.2 would jeopardize this margin of safety. The estimated Rt from our study is consistent with a study published in 2020. Based on anti-orthopoxvirus seroprevalence data collected in the DRC between 2011 and 2012, Grant et al.21 estimated an Rt of 0.85 (uncertainty range, 0.51–1.25) for MPXV. Increases in Rt since the 1980s20,26–28 could be attributed to declining population-level immunity conferred by smallpox vaccination; behavior change, including increased movement of people; ecological and environmental changes, and/or changes that may predispose the virus to spread more easily between humans.
Although the estimates from our study may not be generalizable outside of the Congo Basin, the underlying factors driving the increase in Rt since the 1980s may provide clues about how the epidemiology of mpox could be changing in other historically endemic countries. These factors may have played a role in the 2022 global mpox outbreak during which Rt was estimated to exceed 1.39,40 Further research is needed to understand why the Rt for MPXV has increased in the DRC.
We assumed surveillance of mpox was relatively stable from 2013 to 2017 based on the total number of suspected cases that were investigated. However, an Ebola outbreak in Tshuapa Province may have affected mpox reporting in the second half of 2014, when 69 cases of Ebola virus disease were reported in Boende HZ near the border with Wema HZ.41 Consequently, all specimen collection for non–Ebola virus disease in Tshuapa Province was halted for at least 6 months. Also, from 2012 to 2015, epidemiological studies of mpox were conducted in Djolu HZ, Busanga HZ, and Bokungu HZ, which may have increased the rate of case investigations. Contact tracing can break down the independence function for case observation and leads to larger clusters being more likely to be reported. Paradoxically, this can cause overestimation of Rt when the observation probability is less than 1.27
We did not include mpox cases reported in 2010–2012 and 2018–2019 owing to unstable reporting. In the first few years of the enhanced surveillance system, the number of suspected mpox cases that were investigated increased while resources were put in place (e.g., reagents and test kits) and healthcare workers were trained to collect samples and correctly identify the disease. Declines in the number of investigated cases in 2018 and 2019 could be attributed to Ebola outbreaks. In 2018, an Ebola outbreak occurred in neighboring Equateur Province with 54 total cases.42 One week after the end of the outbreak was declared, another Ebola outbreak was reported in North Kivu Province. It lasted 2 years and resulted in 3,481 total cases.43 Ebola response efforts may have diverted resources (personnel, funding, laboratory capacity, etc.) from mpox surveillance during this period. Healthcare worker strikes may have also affected mpox reporting in 2018–2019.44,45
The cutoff we used for spatial distance (0.7 km) is conservative, and the mean transmission distance informing this cutoff is likely underestimated. We assumed within-village transmission in the WHO data occurred over a distance of 0 km; if case pairs belonged to different households, the pairwise distance would be nonzero. Also, we were not able to find geographic coordinates for the villages of six case pairs with mismatching villages. Although the estimated transmission distance is based on data collected in the 1970s and 1980s, the transportation infrastructure in the region has not changed substantially since then.
The serial interval estimate we used to inform the temporal cutoff agreed with the historical range observed for MPXV in the DRC46 but ultimately was based on data for variola virus.35 The serial interval has been estimated for MPXV using data from the 2022 global outbreak by several groups.47–49 For example, Madewell et al.47 reported an estimated mean serial interval of 8.5 days (95% credible interval [CrI]: 7.3–9.9) in the United States. Slightly longer serial intervals were reported from Europe, with a mean of 9.5 days (95% CrI: 7.4–12.3) reported in the United Kingdom49 and mean of 10.1 days (95% CI: 6.6–14.7) reported in the Netherlands.48 However, these estimates could differ from those in rural DRC because of viral genetic differences, including different clades, and the route and intensity of exposure. According to data from the 2003 mpox outbreak in the United States, which was linked to the exotic pet trade, people who were exposed to MPXV by noninvasive routes, such as petting an infected animal, experienced slower illness progression and longer incubation period than those with complex exposures, such as a bite or scratch from an infected animal.7 Because the serial interval and incubation periods are correlated, we would expect longer incubation periods (and therefore, longer serial intervals) in rural DRC, where human-to-human transmission seems driven by household exposures,10 compared with the global outbreak. Nevertheless, using an estimated serial interval from the global outbreak did not have a large impact on results.
The gold standard for validating clusters of disease is an epidemiological link between cases.38 Although some mpox case report forms in our study list contacts, identifying those contacts is challenging. The identification of mpox clusters in our study could be improved with more available data types, such as social, ecological, and genetic data. vimes is flexible in that it can use any measure of distances between cases. Genetic data were only available for a small proportion of mpox cases in Tshuapa Province.
We did not find published reports about several large clusters of mpox cases identified in our study, including one consisting of 30 patients from the village of Mbotolongo in Djolu HZ in 2017. Although outbreaks of Ebola in the DRC often garner international media coverage50,51 and resources,52 outbreaks of other diseases, such as mpox,53 measles,54 and yellow fever,55,56 receive less attention. At the same time, we were not able to capture at least one large outbreak (consisting of 13 cases from October to November 2016) in our study because the geographic coordinates of the village (Bowe in Wema HZ) were unknown. The high proportion of confirmed mpox cases in Tshuapa Province with missing location data highlights the need to collect better geographic data in the country. These data could be used to improve other public health programs, such as routine childhood immunizations.57 Although not required for this analysis, demographic data for the DRC are limited. Current population estimates are projections based on the last national census, which was conducted nearly 4 decades ago.58
One limitation of our study is that we used a reporting rate estimated from one HZ at one time point.10 We do not know to what extent the reporting rate varied between HZs or across years during the study period. We do know that reporting was low, especially at the national level. Hoff et al.59 estimated that suspected mpox cases in the DRC were 5 to 15 times higher than what was reported (i.e., reporting rates of 7–20%) in 2013.
An additional limitation is that fever may not be accurately recalled by cases,10 as several other febrile diseases are common in the DRC. Alternatively, we could have used date of rash onset in our study but wanted to capture the earliest date of symptom onset, which is usually marked by fever. Another limitation is the timeliness of the data. Data for 2020–2022 were incomplete at the time of writing. Delays can be attributed to lack of equipment and infrastructure, the need for confirmatory testing at the CDC, and the use of paper forms for data collection, among others. Finally, using exposure information to check the assumption of one spillover event per cluster was complicated by large underreporting and the fact that many people are exposed to both sick people and animals in and around their homes. We found that the proportion of patients reporting contact with animals did not depend on cluster type. One potential explanation is that some large clusters may have resulted from multiple zoonotic introductions; Rt would be overestimated if this were the case. An alternative explanation is that some singletons could have been infected via human-to-human transmission and were part of larger clusters of cases that were unreported. Rt would be underestimated if this occurred. Future studies should continue to characterize the relationship between humans and potential animal reservoirs of MPXV in Central Africa.
Supplemental Materials
ACKNOWLEDGMENTS
We thank all the medical and public health professionals involved in investigating and reporting mpox cases in Tshuapa Province. Thanks to two anonymous reviewers for helpful comments on the manuscript as well as Pierre Nouvellet for reviewing the manuscript and extending the branchr package to account for transmission heterogeneity.
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. Ladnyi I, Ziegler P, Kima E, 1972. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ 46: 593–597. [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization , 2022. Mpox (Monkeypox). Available at: https://www.who.int/news-room/fact-sheets/detail/monkeypox. Accessed December 29, 2022.
- 3. McCollum A, Damon I, 2014. Human monkeypox. Clin Infect Dis 58: 260–267. [DOI] [PubMed] [Google Scholar]
- 4. Happi C, Adetifa I, Mbala P, Njouom R, Nakoune E, Happi A, Ndodo N, Ayansola O, Mboowa G, Bedford T, 2022. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol 20: e3001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beer E, Rao B, 2019. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 13: e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jezek Z, Szczeniowski M, Paluku K, Mutombo M, 1987. Human monkeypox: clinical features of 282 patients. J Infect Dis 156: 293–298. [DOI] [PubMed] [Google Scholar]
- 7. Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, Damon IK, 2006. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis 194: 773–780. [DOI] [PubMed] [Google Scholar]
- 8. Simpson K. et al. , 2020. Human monkeypox – after 40 years, an unintended consequence of smallpox eradication. Vaccine 38: 5077–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization Regional Office for Europe and European Centre for Disease Prevention and Control , 2022. Interim Advice for Public Health Authorities on Summer Events During the Monkeypox Outbreak in Europe, 2022. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Interim-advice-for-public-health-authorities-on-summer-events-mpx.pdf. Accessed June 15, 2022.
- 10. Nolen L, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, Doty J, Hughes C, Kabamba J, Malekani J, 2016. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis 22: 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Institut National de la Statistique , 2015. Annuaire Statistique 2015. Available at: https://www.ins.cd/wp-content/uploads/2021/04/Annuairestatistique-2015-Web.pdf. Accessed October 27, 2020.
- 12.U.S. Central Intelligence Agency, 2023. Congo, Democratic Republic of the. Available at: https://www.cia.gov/the-world-factbook/countries/congo-democratic-republic-of-the/#people-and-society. Accessed January 12, 2023.
- 13. Guagliardo S, Reynolds M, Kabamba J, Nguete B, Lushima R, Wemakoy O, McCollum A, 2018. Sounding the alarm: defining thresholds to trigger a public health response to monkeypox. PLoS Negl Trop Dis 12: e0007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monroe B, Doty J, Moses C, Ibata S, Reynolds M, Carroll D, 2015. Collection and utilization of animal carcasses associated with zoonotic disease in Tshuapa District, the Democratic Republic of the Congo, 2012. J Wildl Dis 51: 734–738. [DOI] [PubMed] [Google Scholar]
- 15. Anderson R, May R, 1991. Infectious Diseases of Humans: Dynamics and Control. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- 16. Van Kerkhove M, Bento A, Mills H, Ferguson N, Donnelly C, 2015. A review of epidemiological parameters from Ebola outbreaks to inform early public health decision-making. Nature Scientific Data 2: 150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keeling M, Rohani P, 2008. Modeling Infectious Diseases in Humans and Animals. Princeton, NJ: Princeton University Press. [Google Scholar]
- 18. Heesterbeek J, 2002. A brief history of R0 and a recipe for its calculation. Acta Biotheor 50: 189–204. [DOI] [PubMed] [Google Scholar]
- 19. O’Driscoll M, Harry C, Donnelly C, Cori A, Dorigatti I, 2021. A comparative analysis of statistical methods to estimate the reproduction number in emerging epidemics, with implications for the current coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis 73: e215–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fine P, Jezek Z, Grab B, Dixon H, 1988. The transmission potential of monkeypox virus in human populations. Int J Epidemiol 17: 643–650. [DOI] [PubMed] [Google Scholar]
- 21. Grant R, Nguyen LL, Breban R, 2020. Modelling human-to-human transmission of monkeypox. Bull World Health Organ 98: 638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitehouse E, Bonwitt J, Hughes C, Lushima R, Likafi T, Nguete B, Kabamba J, Monroe B, Doty J, Nakazawa Y, 2021. Clinical and epidemiological findings from enhanced monkeypox surveillance in Tshuapa Province, Democratic Republic of the Congo during 2011–2015. J Infect Dis 223: 1870–1878. [DOI] [PubMed] [Google Scholar]
- 23. Rimoin A. et al. , 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA 107: 16262–16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoff N, Doshi R, Colwell B, Kebela-Illunga B, Mukadi P, Mossoko M, Spencer D, Muyembe-Tamfum J, Okitolonda-Wemakoy E, Lloyd-Smith J, Rimoin A, 2017. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in the Democratic Republic of the Congo, 2001–2013. Int J Trop Dis Health 25: IJTDH.35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yinka-Ogunleye A. et al. , 2019. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis 19: 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ambrose M, Kucharski A, Formenty P, Muyembe-Tamfum J, Rimoin A, Lloyd-Smith J, 2019. Quantifying transmission of emerging zoonoses: using mathematical models to maximize the value of surveillance data. bioRxiv.
- 27. Blumberg S, Lloyd-Smith J, 2013. Inference of R0 and transmission heterogeneity from the size distribution of stuttering chains. PLOS Comput Biol 9: e1002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lloyd-Smith J, Schreiber S, Kopp P, Getz W, 2005. Superspreading and the effect of individual variation on disease emergence. Nature 428: 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Philpott D, Hughes C, Alroy K, Kerins J, Pavlick J, Asbel L, Crawley A, Newman A, Spencer H, Feldpausch A, 2022. Epidemiologic and clinical characteristics of monkeypox cases – United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 71: 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spicknall I, Pollack EC, Clay PA, Oster AM, Charniga K, Masters N, Nakazawa YJ, Rainisch G, Gundlapalli AV, Gift TL, 2022. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men - United States, 2022. MMWR Morb Mortal Wkly Rep 71: 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. U.S. Centers for Disease Control and Prevention , 2022. Mpox Outbreak Global Map. Available at: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Accessed January 6, 2023.
- 32. Hughes C, Liu L, Davidson W, Radford K, Wilkins K, Monroe B, Metcalfe M, Likafi T, Lushima R, Kabamba J, 2021. A tale of two viruses: coinfections of monkeypox and varicella zoster virus in the Democratic Republic of Congo. Am J Trop Med Hyg 104: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wallace J, 2010. R Package ‘Imap’. CRAN. Available at: https://cran.r-project.org/web/packages/Imap/index.html. Accessed December 22, 2022.
- 34. Cori A, Nouvellet P, Garske T, Bourhy H, Nakouné E, Jombart T, 2018. A graph-based evidence synthesis approach to detecting outbreak clusters: an application to dog rabies. PLOS Comput Biol 14: e1006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishiura H, Eichner M, 2007. Infectiousness of smallpox relative to disease age: estimates based on transmission network and incubation period. Epidemiol Infect 135: 1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibbons C, Mangen M, Plass D, Havelaar A, Brooke R, Kramarz P, Peterson K, Stuurman A, Cassini A, 2014. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nouvellet P, Jombart T, 2018. branchr: R Estimation from Cluster Sizes: R Epidemics Consortium. Available at: https://github.com/reconhub/branchr. Accessed December 22, 2022.
- 38. Soetens L, Backer J, Hahné S, van Binnendijk R, Gouma S, Wallinga J, 2019. Visual tools to assess the plausibility of algorithm-identified infectious disease clusters: an application to mumps data from the Netherlands dating from January 2009 to June 2016. Euro Surveill 24: 1800331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charniga K, Madewell Z, Masters N, Asher J, Nakazawa Y, Spicknall I, 2023. Nowcasting and forecasting the 2022 U.S. Mpox outbreak: support for public health decision making and lessons learned. medRxiv. [DOI] [PubMed]
- 40. Laurenson-Schafer H. et al. , 2023. Description of the first global outbreak of mpox: an analysis of global surveillance data. Lancet Glob Health 11: e1012–e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maganga GK. et al. , 2014. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med 371: 2083–2091. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization , 2018. Ebola Virus Disease Democratic Republic of Congo: External Situation Report 17/2018. Avaialable at: https://apps.who.int/iris/bitstream/handle/10665/273348/SITREP_EVD_DRC_20180725-eng.pdf?ua=1. Accessed August 28, 2023.
- 43. World Health Organization , 2020. Ebola North Kivu/Ituri, Democratic Republic of the Congo, August 2018 - June 2020. Available at: https://www.who.int/emergencies/situations/Ebola-2019-drc-. Accessed January 30, 2023.
- 44. Ravez L-C, Rennie S, Yemesi R, Chalachala J-L, Makindu D, Behets F, Fox A, Kashamuka M, Kayembe P, 2019. Les grèves de médecins en République Démocratique du Congo: quels repères éthiques généralisables? Can J Bioeth/Rev Can Bioeth 2: 63–72. [Google Scholar]
- 45. Russo G, Xu L, McIsaac M, Matsika-Claquin M, Dhillon I, McPake B, Campbell J, 2019. Health workers’ strikes in low-income countries: the available evidence. Bull World Health Organ 97: 460–467H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fenner F, Henderson D, Arita I, Jezek Z, Ladnyi I, 1988. Smallpox and its Eradication. Geneva, Switzerland: World Health Organization. Available at: https://apps.who.int/iris/handle/10665/39485. Accessed August 28, 2023. [Google Scholar]
- 47. Madewell Z. et al. , 2023. Serial interval and incubation period estimates of monkeypox virus infection in 12 U.S. jurisdictions, May–August 2022. Emerg Infect Dis 29: 818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miura F, Backer J, van Rijckevorsel G, Bavalia R, Raven S, Petrignani M, Ainslie K, Wallinga J, Dutch Mpox Response Team , 2022. Time scales of human mpox transmission in the Netherlands. medRxiv. [DOI] [PMC free article] [PubMed]
- 49. Ward T, Christie R, Paton R, Cumming F, Overton C, 2022. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ 379: e073153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Belluz J, 2018. Why the Ebola outbreak in DRC is so difficult to contain. Vox.
- 51. Jones R, 2019. Life amid an Ebola outbreak: combating mistrust – and saving lives. Natl Geogr Mag. Available at: https://www.nationalgeographic.com/culture/article/ebola-democratic-republic-congo. Accessed January 30, 2023. [Google Scholar]
- 52. Mindaoudou A, Lusenge J, Bocoum D, Doucet C, Coulibaly M, 2021. Final Report of the Independent Commission on the Review of Sexual Abuse and Exploitation during the Response to the 10th Ebola Virus Disease Epidemic in DRC. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 53. Cohen J, 2022. Monkeypox is a new global threat. African scientists know what the world is up against. Science News. Available at: https://www.science.org/content/article/monkeypox-is-a-new-global-threat-african-scientists-know-what-the-world-is-up-against. Accessed January 30, 2023.
- 54. Kagumire R, Bolombo F, 2020. Analysis: DRC’s deadly, but ignored, measles epidemic. Aljazeera. Available at: https://www.aljazeera.com/features/2020/3/13/analysis-drcs-deadly-but-ignored-measles-epidemic. Accessed January 30, 2023.
- 55. Ingelbeen B, Weregemere N, Noel H, Tshapenda G, Mossoko M, Nsio J, Ronsse A, Ahuka-Mundeke S, Cohuet S, Kebela B, 2018. Urban yellow fever outbreak – Democratic Republic of the Congo, 2016: towards more rapid case detection. PLoS Negl Trop Dis 12: e0007029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schnirring L, 2016. WHO Says Yellow Fever ‘Serious Concern’ But Not Emergency. CIDRAP News. Minneapolis, MN: Center for Infectious Disease Research & Policy at the University of Minnesota. [Google Scholar]
- 57. Sinai C, Hoff N, 2016. Mapping Unmapped Villages with Congo’s National Vaccination Program. Available at: https://mapforenvironment.org/story/Mapping-unmapped-villages-with-Congo’s-National-Vaccination-Program/12. Accessed January 31, 2023.
- 58. Institut National de la Statistique , 2021. Annuaire Statistique RDC 2020. Available at: https://ins.cd/wp-content/uploads/2022/06/ANNUAIRE-STATISTIQUE-2020.pdf. Accessed April 14, 2023.
- 59. Hoff N, Kebela-Illunga B, Eckhoff P, Mukadi P, Mossoko M, Muyembe-Tamfum J-J, Okitolonda-Wemakoy E, Doshi R, Rimoin A, 2015. A descriptive and quantitative analysis of potential underestimation of human monkeypox cases in the passive surveillance system in the Democratic Republic of Congo. Am J Trop Med Hyg 93: 242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.