Abstract
The extracellular matrix in microenvironments harbors a variety of signals to control cellular functions and the materiality of tissues. Most efforts to synthetically reconstitute the matrix by biomaterial design have focused on decoupling cell-secreted and polymer-based cues. Cells package molecules into nanoscale lipid membrane-bound extracellular vesicles and secrete them. Thus, extracellular vesicles inherently interact with the meshwork of the extracellular matrix. In this Review, we discuss various aspects of extracellular vesicle-matrix interactions. Cells receive feedback from the extracellular matrix and leverage intracellular processes to control the biogenesis of extracellular vesicles. Once secreted, various biomolecular and biophysical factors determine whether extracellular vesicles are locally incorporated into the matrix or transported out of the matrix to be taken up by other cells or deposited into tissues at a distal location. These insights can be utilized to develop engineered biomaterials where EV release and retention can be precisely controlled in host tissue to elicit various biological and therapeutic outcomes.
Keywords: extracellular vesicle, extracellular matrix, biomaterials, nanoscale biophysics, nanotechnology
1. Introduction
The extracellular matrix (ECM) is a network structure consisting of various biomolecular and biophysical components essential to cellular functions, which represents the major acellular component of biological tissues. Tissues are active viscoelastic materials1 that can change their properties depending on pathophysiological conditions. The ECM can determine the rheological properties of tissues both directly as constituents and indirectly by calibrating how cells generate contractile forces and tension via mechanotransduction2,3, which can influence the ability of cells to remodel the ECM4. Understanding how the ECM is remodeled and how the materiality of tissue is dynamically controlled will necessitate biomaterial-based strategies to investigate the interplay between cell-secreted factors and polymer-based cues.
Previous studies with purified ECM proteins have highlighted roles of polymeric networks in determining rheological properties essential to tissue integrity, such as strain-stiffening5. To date, efforts to engineer synthetic ECMs to direct cellular functions have focused on controlling the crosslinking of polymeric networks to tune elasticity6, viscoelasticity7,8 and plasticity9. However, molecular profiling studies of decellularized tissues have shown the presence of soluble proteins tightly bound to fibrous ECM networks10. While cells can secrete soluble proteins directly, cells can also package molecules into nanoscale mediators and secrete them, especially in lipid membrane-bound vesicles, called extracellular vesicles (EVs). The presence of EVs in the ECM was documented several decades ago by electron microscopy studies in the context of vesicle-mediated mineralization11,12. However, ECM-bound vesicles were documented in other tissues only recently13. Recent studies with label-free third harmonic generation microscopy further showed the enrichment of EVs in tissue stromal regions, which consist of dense matrix fibers14,15. However, vesicles can also be found in blood16 and lymph17, suggesting that some secreted EVs from cells can transport out of the ECM18 and end up at a distal location to be taken up by other cells19 or deposited into tissues20.
Here, we provide a comprehensive review on EV-ECM interactions. We review the current knowledge of different cell-secreted nanoscale mediators. We elaborate on the role of membrane trafficking in EV biogenesis and its regulatory mechanisms by the ECM as a key example of how cells leverage biological processes to produce and secrete nanoscale mediators. We examine biomolecular and biophysical determinants of EV-ECM polymer interactions. We highlight recent advances in interfacing EVs with engineered hydrogels as biologically inspired strategies to promote tissue regeneration by controlling transport or retention of EVs. Given the importance of sourcing EVs from cells, we also review the role of biomaterial design in controlling EV production from cells. Finally, we explore future areas of investigations into EVs as essential structural elements of hydrogel-based materials to better recapitulate mechanisms of health and disease, and to develop a novel class of biologically-inspired materials.
2. Diversity of cell-secreted nanoscale mediators
Cell-secreted EVs were previously classified into apoptotic bodies, ectosomes (also called microvesicles or microparticles), and exosomes based on their distinct biogenesis mechanisms21 (Fig. 1). Apoptotic bodies are produced during apoptosis of cells by outward budding of the cell membrane22,23. Ectosomes are also produced by outward budding of the plasma membrane, but may or may not accompany apoptosis24. In contrast, exosomes are secreted when early endosomes become specialized into multivesicular bodies (MVBs) by inward budding of intraluminal vesicles (ILVs). MVBs then fuse with the plasma membrane to release ILVs as exosomes that express tetraspanins25. However, validating specific cell-secreted EVs based on biogenesis pathways requires well-controlled investigations, such as employing live cell imaging techniques fused with genetic approaches26.
Figure 1. Cell-secreted nanoscale mediators.
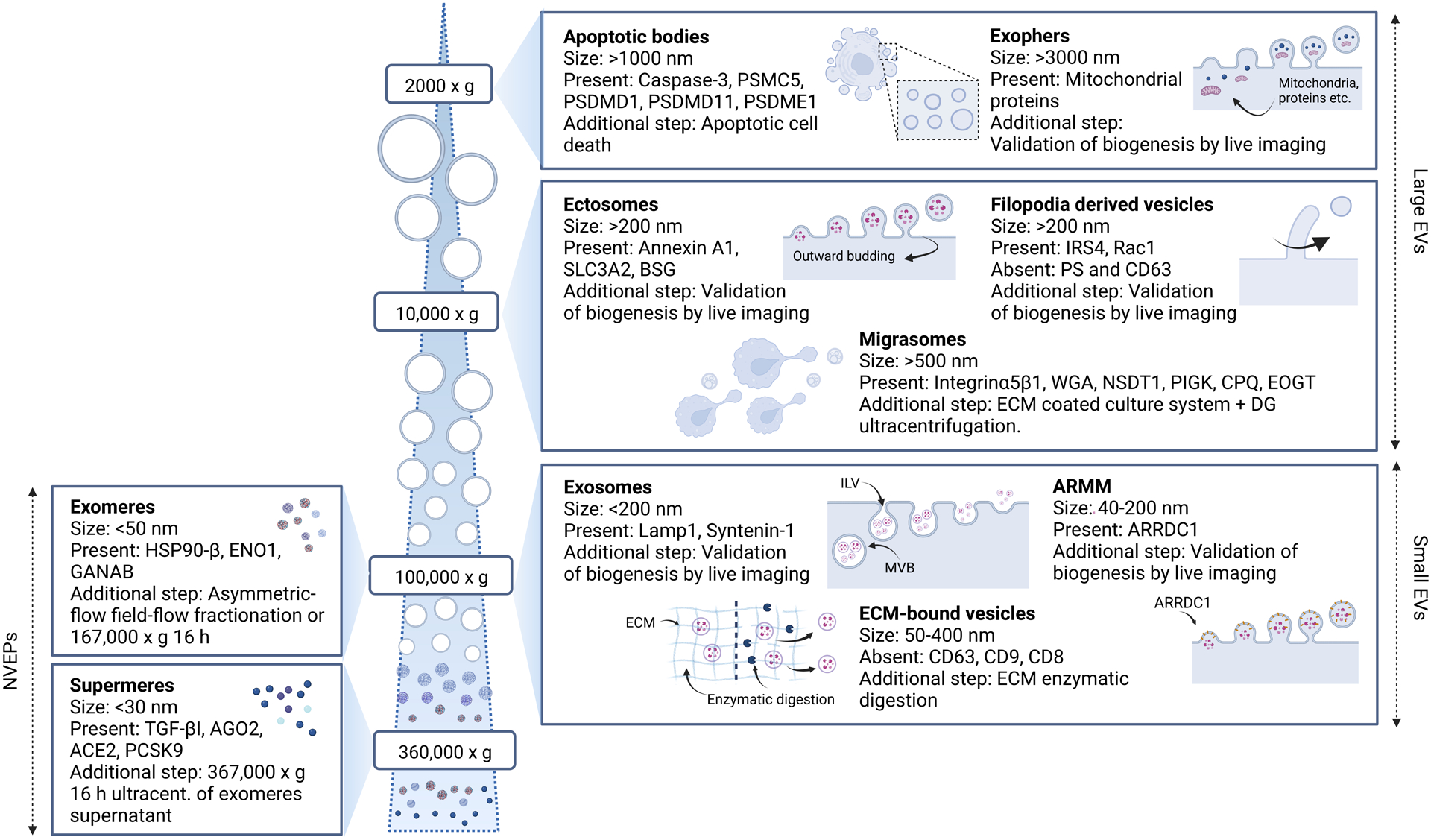
Cells secrete a diverse range of nanoscale mediators with distinct physicochemical properties. In general, these mediators are classified into lipid membrane-bound extracellulr vesicles (EVs) and non-vesicular extracellular nanoparticles (NVEPs), which can generally be separated based on the size by differential ultracentrifugation. Apoptotic bodies and ectosomes (or microvesicles) are large (>200 nm) EVs and produced by membrane budding. More recently described large EVs are associated with specific biological processes, including exophers, migrasomes and filopodia derived vesicles. Exosomes belong to a subpopulation of small (<200 nm) EVs that originate from intraluminal vesicles (ILVs) in multivesicular bodies (MVBs) and are released when MVBs fuse with the plasma membrane. In addition to exosomes, small EVs consist of other subpopulations, including arrestin-domain-containing protein 1 (ARRDC1)-mediated microvesicles (ARMMs) and extracellular matrix (ECM)-bound vesicles. NVEPs, including exomeres and supermeres are generally smaller (<50 nm) than EVs, and can be isolated by additional ultracentrifugation steps.
From a practical point-of-view, EVs are classified into large (>200 nm) and small (<200 nm) EVs27, since most investigators have been using differential centrifugation to separate large EVs (<10,000g) and small EVs (>100,000g), which may include a variety of EV subtypes in addition to apoptotic bodies, ectosomes, and exosomes. For instance, exophers are microscale large EVs that are isolated at ~1,000g and are known to help transport and eliminate defective mitochondria and protein aggregates28. Migrasomes (>500 nm) are large EVs that are produced from long membrane projections during cell migration on a rigid culture substrate29,30. Similarly, filopodia-derived vesicles (>200 nm) are formed by scission of filopodia31. Some of the recently reported small EV subtypes include arrestin-domain-containing protein 1-mediated microvesicles (ARMMs) that are formed by budding32, and ECM-bound vesicles, which are known to be devoid of classical EV markers, tightly bound to the ECM after decellularization of tissues, and released only after enzyme-mediated digestion of the ECM13.
Adding to the complexity, recent studies have also shown the presence of non-vesicular extracellular particles (NVEPs) that do not contain a lipid bilayer in the pellet after ultracentrifugation at 100,000g, which also contains small EVs. These NVEPs can be separated from small EVs by high-resolution iodixanol density gradient fractionation, followed by taking high density fractions33. The supernatant from the first ultracentrifugation can be subject to additional overnight ultracentrifugation at 100,000g to obtain smaller NVEPs (<50 nm)34, called exomeres, which were first described by using the asymmetric-flow field-flow fractionation method35. After isolating exomeres, another round of ultracentrifugation at a higher speed (~360,000g) can be done overnight on the supernatant to obtain even smaller NVEPs (<30 nm), called supermeres36. While some NVEPs were shown to be released via a shared pathway as exosomes33, the biogenesis pathway of NVEPs remains relatively unknown compared to that of EVs.
3. Mechanisms of EV biogenesis in the ECM
EV biogenesis is intricately linked to intracellular transport and secretory pathways, and physicochemical factors in the ECM that regulate these processes (Fig. 2).
Figure 2. Biogenesis mechanisms of EVs in the ECM.
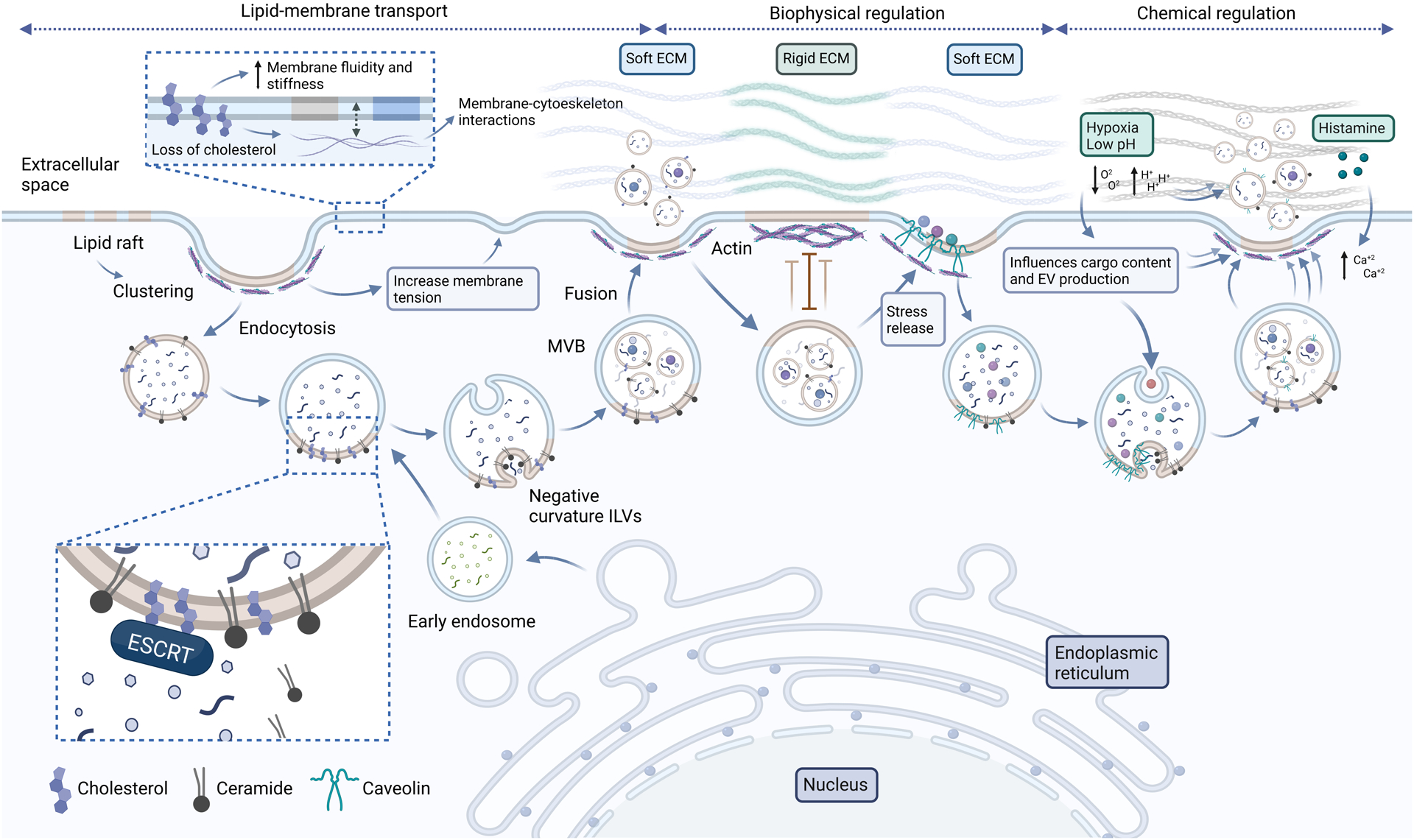
EV biogenesis is tightly linked with the lipid membrane transport process and physicochemical factors of the ECM that regulate this process. Lipid rafts serve as precursors of multivesicular bodies (MVBs) by providing lipids, including cholesterol and ceramide. Cholesterol mediates the recruitment of the endosomal sorting complexes required for transport (ESCRT) and ceramide induces negative curvature to form intraluminal vesicles (ILVs). The loss of membrane during endocytosis of lipid rafts can be counteracted by the gain of membrane during MVB fusion, thereby balancing membrane tension. When the ECM is softer, lipid rafts, including caveolae, are more readily formed because they are not used to counteract mechanical stress. In this case, lipid rafts can package some ECM molecules, which are shuttled into MVBs and released via exosomes. In addition, actin cytoskeletons are less dense in cells on a soft ECM, thereby facilitating MVB fusion and exosome release. The ECM also offers chemical cues that faciliate EV release, including oxygen tension, pH and signaling molecules that activate intracellular calcium levels.
3.1. Lipid membrane transport
The unique structural feature of EVs is that they encapsulate various cargo molecules in the lipid membrane, including proteins, nucleic acids, and various metabolites37. Thus, understanding the role of membrane turnover in the context of the ECM will help understand how EV biogenesis is regulated by the ECM. Lipid rafts are discrete, dynamic nanoscale domains in the external leaflet of the cell membrane, which are present in a metastable state, but become more stable by undergoing clustering in response to external signals, including those present in the ECM38. Some lipid raft domains undergo endocytosis39, and the resulting vesicles fuse with early endosomes40. Lipid rafts are enriched with cholesterol and sphingolipids41. Importantly, cholesterol and ceramide, a simple sphingolipid, are essential for the formation of MVBs by recruiting the endosomal sorting complex required for transport (ESCRT) machinery42 and triggering the negative curvature of the MVB membrane to form ILVs in an ESCRT-independent manner43, respectively. Both cholesterol and ceramide are highly hydrophobic, and intercalate between phospholipid acyl chains of the cell membrane in a competitive manner44,45. Loss of cholesterol increases membrane fluidity46, but also promotes membrane-cytoskeleton interactions47, thereby stiffening the cell membrane48. Thus, endocytosis of lipid rafts may result in temporary increase in the cell membrane tension. However, this increase can be counteracted when MVBs fuse to the cell membrane to release exosomes, the process that can restore the membrane pool and decrease the tension49. Similarly, MVB fusion or exocytosis could potentially serve as a homeostatic mechanism to counteract the loss of plasma membrane during outward budding when microvesicles or apoptotic bodies are formed.
3.2. Biophysical regulation by the ECM
Since cells pull on and sense the resistive force from the ECM2,3, biophysical properties of the ECM can impact membrane trafficking49,50, and hence EV biogenesis. Caveolae represent a subset of lipid rafts that contain the protein caveolin51. Previous studies showed the role of caveolae in mechanosensing, since they enable endothelial cells to be responsive to ECM rigidity52,53 and shear flow54,55, and protect cells from rupture by undergoing flattening and disassembly in response to acute mechanical stress independently of actin and ATP56. Interestingly, caveolin is known to be incorporated into MVBs and exosomes, and required for sorting of some ECM molecules into exosomal cargo, which can then be transported to distal tissues20. Conversely, cells reassemble caveolae in an actin-dependent manner in response to stress release56, and also in a hydrogel matrix that recapitulates the physiological stiffness of soft tissue, where cells maintain low membrane tension57. Consistent with these observations, cells on a soft hydrogel matrix maintain the nanoscale assembly of short actin filaments, which permits MVBs to readily transport and fuse with the plasma membrane to release exosomes—in contrast, cells on a stiffer matrix form an extensive actin network, which serves as a physical barrier for MVB transport and exosome release26.
3.3. Chemical regulation by the ECM
Chemical factors in the ECM can also impact EV biogenesis by modulating membrane trafficking. The ECM is the largest source of free calcium ions58, which bind to lipid rafts to initiate calcium signaling and play essential roles in EV biogenesis, including MVB formation and fusion to the plasma membrane59,60. EV release can be enhanced by soluble extracellular mediators that elevate intracellular calcium, such as histamine61,62. In cancer and tissue injury, some tissues become rigid by increased ECM crosslinking63, which by itself can impede EV production26. However, in these disease conditions, tissues undergo hypoxia, which decreases extracellular pH due to increased anaerobic metabolism64,65. Hypoxia has been shown to increase membrane trafficking by recruiting short actin filaments66, to increase EV number, and to influence EV cargo content that induces pathogenic phenotypes67–69. Low extracellular pH not only enhances the secretion of caveolin-containing EVs but also makes EV membrane less fluid due to increased incorporation of sphingomyelin, another class of sphingolipid70.
4. Biomolecular interactions between EVs and ECM network
The molecular basis of interactions between EVs and ECM polymers can be hypothesized based on biochemical compositions of EVs and the ECM, and chemical bonds that govern interactions between the molecules. EVs contain various protein and lipid molecules, some of which are known to interact with the ECM via covalent or hydrogen bonds (Fig. 3), although most of these interactions remain to be directly confirmed in the context of EV-ECM interactions.
Figure 3. Biomolecular interactions between EVs and the ECM.
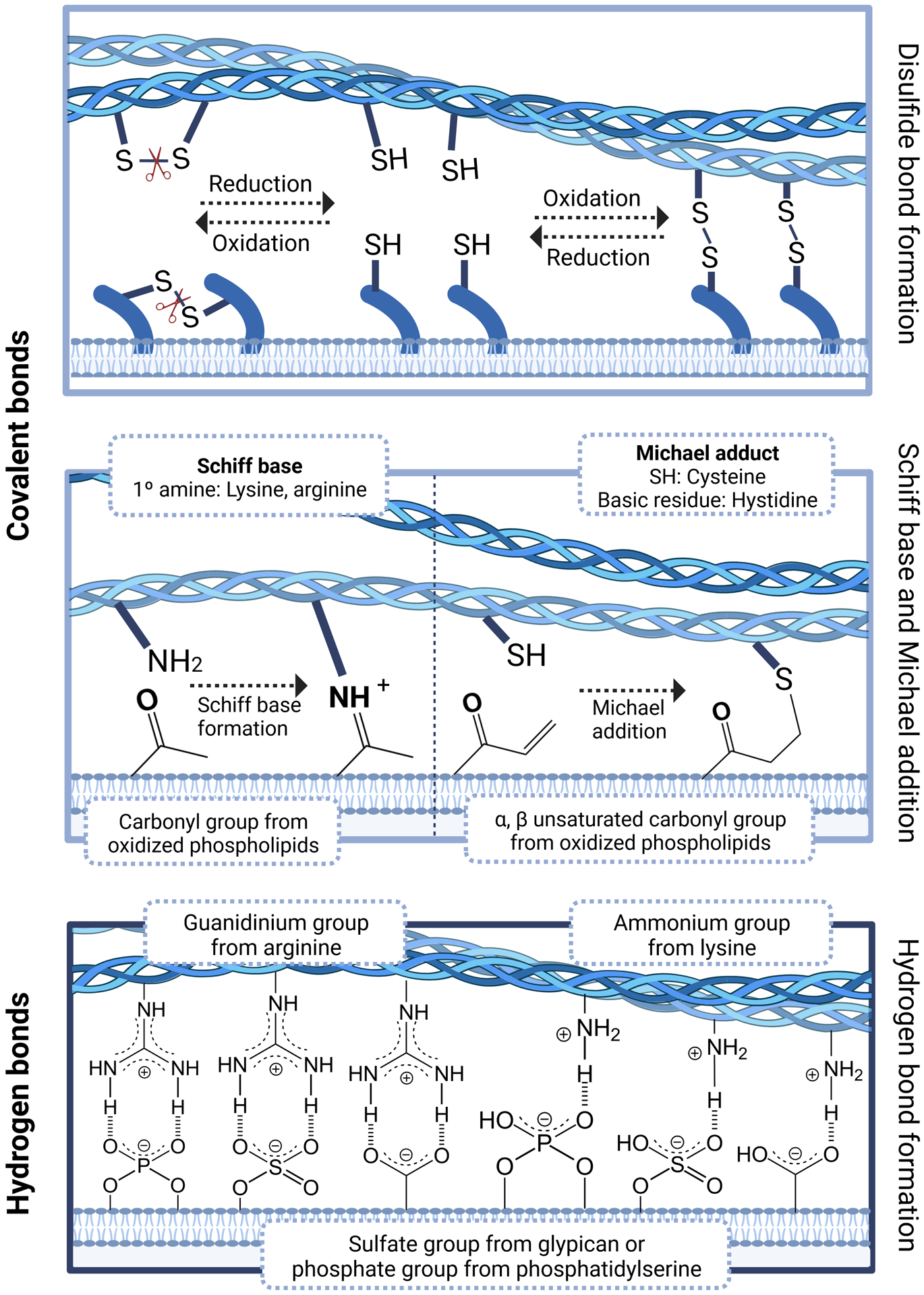
A number of biomolecular interactions can determine whether EVs bind to or are released from the ECM. Disulfide bonds can occur between a cysteine group of an EV membrane protein and that of an ECM protein, and are reversble depending on the redox state of the tissue environment and the availability of an extracellular enzyme that catalyzes this process. In addition, covalent bonds can be formed between a lipid molecule of the EV membrane and an ECM protein as Schiff bases or Michael adducts. EVs can also interact with the ECM via hydrogen bonds between a negatively charged heparin sulfate proteoglycan (e.g. glypican) or a phospholipid (e.g. phosphatidylserine) on the EV membrane and a positively charged amino acid (e.g. arginine or lysine from heparin binding domains) in an ECM protein.
4.1. Covalent bonds
In principle, covalent bonds can facilitate permanent interactions between EVs and the ECM. One way that covalent bonding can occur between EVs and matrix polymers is when proteins on EVs contain cysteines exposed to the extracellular space, which can form disulfide bonds with proteins in the ECM network. This interaction can be facilitated by an extracellular disulfide catalyst secreted by cells as exemplified by covalent incorporation of laminin, which is known to be present in some EVs71, into the ECM72. Since EVs are enclosed by the lipid membrane, they can also form covalent bonds with matrix polymers through lipid-protein interactions. ECM-bound vesicles contain higher levels of oxidized phospholipids than vesicles in fluid73. Oxidized phospholipids that contain carbonyl moieties form Schiff bases by reacting with a primary amine group of lysine or arginine, while those that contain α,β-unsaturated carbonyl groups form Michael adducts by reacting with a thiol group of cysteine or basic residues of histidine74. Indeed, oxidized phospholipids were shown to modify collagen via lipoxidation throughout life, and hence associated with aging75. Thus, some covalent EV-ECM interactions may be subject to regulation by the redox state of their environments, which is altered in various pathological conditions where EVs have been implicated76,77.
4.2. Hydrogen bonds
Hydrogen bonding is ubiquitous in nature and enables the formation of reversible interactions. One potential way for EVs to interact with ECM polymers via hydrogen bonds is through heparin binding domains, which are rich in basic amino acid residues, such as arginine and lysine, and are present in a number of ECM molecules, including fibronectin, vitronectin, collagen, and laminin78. Arginine contains the positively charged guanidinium group, which forms strong hydrogen bonding with negatively charged phosphate, sulfate, and carboxylate groups79. The same principle also applies to lysine, but its interaction with a negatively charged group is weaker than arginine because lysine forms one hydrogen bond, while arginine forms a cyclic structure with a negatively charged group by forming two hydrogen bonds. Thus, some ECM polymers with heparin binding domains may interact with either sulfated molecules on EVs, such as glypican80, or phospholipids on the membrane of vesicles, such as phosphatidylserine, an acidic phospholipid, which is enriched in matrix-bound vesicles secreted from cells in cartilage81. Conversely, this process can be inhibited when ECM polymers themselves are phosphorylated by extracellular enzymes to become more acidic, as occurs in some tissues, such as bones82. In addition, EV membrane contains a number of receptors that can bind to the ECM where hydrogen bonding plays important roles, including integrin αLβ2 (LFA-1)83,84, integrin α4β185,86 and CD4487,88.
5. Biophysical EV-ECM network interactions as a basis of effective EV transport
The ECM consists of a polymer network with meshes that enable the transport of liquid and solutes. The mesh size of the ECM ranges from nanometer to micrometer scales89,90. Unlike small molecules that transport freely through the meshes by diffusion, EVs are often larger and more likely confined in the nanoporous ECM (rmesh/rEV ≤ 1) due to stronger steric hinderance by the polymer. Indeed, the ECM in the interstitium is known to impede the transport of larger (>100 nm) synthetic nanoparticles and drainage into the lymphatic system, thereby serving as a barrier for drug delivery91. Previous studies reported the presence of matrix remodeling enzymes, such as matrix metalloproteinases92 and lysyl oxidases93 in EVs, suggesting the potential of EVs in biochemically modulating the mesh size of the ECM. However, if each EV relies on the ability to degrade the ECM in order to transport, the energy cost of EV transport would be very high. Hence, some EVs may have evolved to rapidly transport in the nanoporous ECM with minimum energy cost by leveraging physical interactions with the network. Transport of EVs in the nanoporous ECM does not necessarily require energy, as long as mechanisms exist to temporarily reduce steric hinderance in the network, thereby restoring thermal motion of EVs. This notion is supported by the hopping diffusion model where trapped particles larger than the mesh size can escape at longer time scales by overcoming free energy barrier between the confinement cages94. Supporting this model, earlier studies show that synthetic nanoparticles exhibit subdiffusive behaviors with infrequent jumps in mucus95,96, which is entangled polymers without covalent crosslinking. In context of ECM-based polymers, a number of studies over the past decades show that the cartilage matrix allows the transport of molecules larger than its pore size (~6 nm)90, including nanoparticles97, the process that is facilitated under mechanical loading due to convective flow98,99. Convective flow is also known to drive the transport of nanoparticles with a certain size range (20–50 nm) in the interstitial matrix by lymphatic drainage91. Recently, it was shown that EVs do not require actomyosin contractility, convective flow or matrix degradation to transport in the viscoelastic ECM18. Understanding the biophysical basis of EV-ECM polymer interactions will inform both fundamental mechanisms behind EV transport in the ECM and engineering strategies to release EVs from or retain EVs in hydrogels (Fig. 4).
Figure 4. Biophysical mechanisms of EV transport in the ECM.
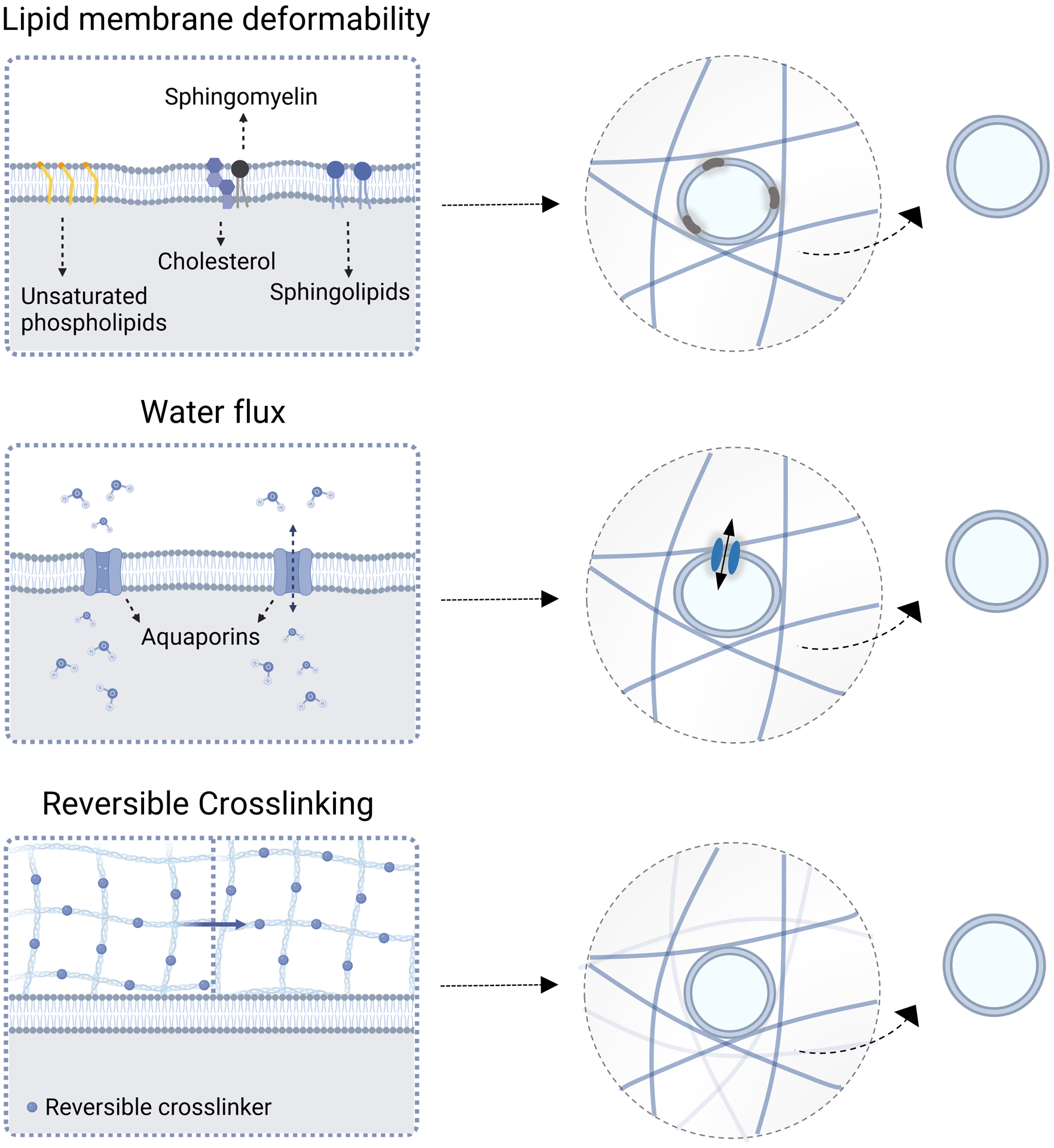
Under certain conditions, EVs can readily transport through a nanoporous network without relying on polymer degradation or convection. EVs contain a distinct set of lipids from cells or ECM-bound vesicles, including unsaturated phosphoslipids and sphingolipids, which can make EVs deformable. The ability of EVs to flux water through aquaporin enables them to deform in the network, thereby helping them resist changes in osmotic pressure. In addition to EV deformability, ECM crosslinking will likely need to be reversible, in order for EVs to bind to the crosslinks and to rearrange the network during the transport process.
5.1. EV biophysical properties
The rigidity of synthetic nanovesicles is known to impact their ability to transport in a confined space by deformation100–103. To date, several studies have reported a broad range of rigidity for EVs. The majority of studies used atomic force microscopy (AFM) to characterize nanoscale vesicle rigidity in terms of Young’s modulus (E in Pa), which is defined by the response of a material to a force applied along a one-dimensional axis. Using the Hertz model of indentation104, E of EVs has generally been reported to be within a megapascal (MPa) range, which varies depending on cell types and subpopulations. EVs from tissue preparations, including saliva105, neuronal synapse106, and blood plasma107 show E < 10 MPa, while EVs secreted from cultured mammalian cells18 and cancer cells108,109 show E > 20 MPa. Within subpopulations, E was shown to be lower for larger EVs than smaller EVs and NVEPs from cancer cells108. Intriguingly, a previous study with synthetic nanovesicles showed that there exists an optimum E ~ 50 MPa where vesicles show the fastest diffusivity through mucus102. This value is similar to E of CD63+ EVs from mesenchymal stromal cells (MSCs) (~100 MPa), which were shown to transport in the crosslinked, viscoelastic ECM18. While the Hertz model has been widely used given its simplicity and independence of particle size, it requires the assumption that EVs are purely elastic and homogeneous in composition. Recently, a modified Canham-Helfrich model was used to account for membrane bending and pressurization from fluid in the vesicle lumen upon AFM probe indentation by measuring vesicle stiffness, size, and tether force110. From this model, bending rigidity (kc in J), the energy to deform a membrane to a different curvature from its initial curvature111, can be directly measured for nanoscale vesicles. Using this model, EVs from red blood cells (RBCs) was shown to be ~15 kbT (kbT = 4.11 × 10−21 J at room temperature)112. Like E, kc is independent of EV geometry. However, a model is yet to be developed to enable the conversion between E and kc for EVs, since the conversion is currently possible only for thin shell vesicles with a hollow lumen, whereas EVs are fluid-filled. Systematic studies are still needed to correlate between E or kc of EVs from different sources and their diffusivity in the ECM.
The relationship between nanoscale particle rigidity and diffusivity raises an important question of what determines the rigidity of EVs. Synthetic phosphatidylcholine-based nanovesicles exhibit E of 2~10 MPa113,114 and kc of ~14 kbT110, the latter of which was also observed in microscale unilamellar vesicles115,116. The similarity of these values to E and kc of EVs warrants further examinations into roles of natural lipid bilayer compositions and lumen fluid properties in determining the rigidity of EVs. Earlier studies with microscale unilamellar vesicles showed that at a constant temperature, the presence of cis-double bonds (unsaturated) in hydrocarbon tails of phospholipids introduces a structural kink, which decreases molecular packing, thereby increasing membrane fluidity and decreasing kc117,118. These observations were confirmed with synthetic nanovesicles by AFM where liposomes with liquid-like, disordered membrane show lower kc119. Culturing MSCs with polyunsaturated acids was shown to increase the content of phospholipids with unsaturated fatty acyl groups in EVs120, suggesting the possibility that kc of EVs could potentially be tuned ex vivo. In contrast, ECM-bound vesicles are enriched in phosphatidylglycerol121, which was previously shown to increase kc of synthetic vesicles122. In addition to phospholipids as a backbone, the bilayer in eukaryotic organisms contains other types of lipids, most notably cholesterol, which is abundant in EVs123. Cholesterol is known to decrease kc of synthetic vesicles in the presence of sphingomyelin124,125. Indeed, sphingolipids are also enriched in EVs34,121,126,127, and their content is higher than ECM-bound vesicles32. Together, lipid membrane compositions could potentially impact the ability of EVs to transport or remain within the nanoporous ECM by tuning their deformability.
In addition to lipids, the membrane of EV subpopulations consists of different transmembrane proteins21,128. It was shown that the rigidity of EVs from RBCs generally decreases with increased protein-to-lipid ratios129, although this relationship will likely depend on how protein insertion impacts membrane order114,130,131. One important class of membrane proteins in natural vesicles is channel proteins that mediate membrane transport, since they regulate fluid content and properties in the vesicle lumen, which can impact vesicle rigidity. To date, a diverse range of ion and water channel proteins have been identified in EVs132. Of these, the aquaporin family is one of the earliest channel proteins discovered in EVs in urine133–135 and RBCs136. The amount of aquaporins in EVs is known to change depending on physiological demands by cells. For instance, more aquaporin-2 is packaged into EVs from the apical plasma membrane of the renal collecting ducts when there is an increased demand to retain water in the body133, while RBCs secrete EVs with less aquaporin-1 under hypertonic conditions136. Interestingly, aquaporin-driven water flux was shown to maintain stability of plant-derived vesicles under hypertonic conditions137, suggesting its role in resisting mechanical deformation. From a biophysical perspective, deformation of EVs would temporarily decrease the internal volume and hence increase the concentration of solutes in the lumen, thereby creating osmotic pressure and increasing vesicle rigidity110. A recent study showed that aquaporin-1 is essential for EVs to transport in the nanoporous ECM, and downregulating aquaporin-1 rigidifies EVs18. Thus, rapid water flux by aquaporins will likely help resist changes in osmotic pressure and rigidification of EVs upon deformation during the transport process.
5.2. ECM biophysical properties
The deformability of EVs alone is less likely sufficient to overcome steric hinderance by the matrix polymer, since extreme deformation of EVs would compromise their structures. Success of EV transport will also require the ability of the ECM polymer to undergo structural reorganization, which is determined in large part by polymer crosslinking. In general, a less permanent form of crosslinking, such as electrostatic and hydrogen bonds, results in a polymeric network that dissipates energy upon external force, leading to viscoelastic properties138. Since most tissues are viscoelastic1, it is possible that EV transport occurs in tissues upon external load. Interestingly, a modeling study showed that in the absence of external force, a weakly crosslinked ECM polymer network can still rearrange if nanostructures in the polymer transiently bind to or interfere with the crosslinks of the polymer, thereby enabling nanoparticle transport in the ECM139. While this concept still remains to be directly tested for EVs in the ECM, a recent study supports this notion, since EVs but not synthetic nanoparticles can transport in ionically crosslinked hydrogels18. This raises an interesting possibility that EVs may be able to transport in viscoelastic hydrogels by influencing their crosslinks.
6. Interfacing EVs with engineered materials
EVs are dispersed and cleared by the liver after systemic injection in vivo in a solution form with half-life less than hours140. Analogous to controlled drug delivery141, material-based strategies, especially engineered hydrogels, can be used to control either release or retention of EVs in a specific tissue of interest. From a macroscopic design point-of-view, implantation142,143, injectable bulk hydrogels144, in situ gelation145–152 and microgels153 have been employed to deliver hydrogels with EVs to the host. The majority of these strategies used EVs from MSCs as a means to restore damaged tissues, since they are known to contain cargo molecules with potential immunomodulatory and regenerative effects154,155.
6.1. Controlled release of EVs to the host
6.1.1. Diffusion.
The ability to gradually release EVs from hydrogels will help control the extent at which EVs become available to occupy tissue over time in order to achieve therapeutic effects. The first important step to achieve this goal is to crosslink hydrogels from polymer solutions while EVs are present so that EVs can gradually diffuse from hydrogels over time (Fig. 5). However, EV transport is generally more sensitive to crosslinking than small molecule transport due to large particle-mesh size ratios. Thus, the choice of crosslinking strategies will determine both kinetics and maximum amount of EV release by diffusion. An earlier study showed a delayed release of EVs from alginate hydrogels with higher molecular weight144. The release might have been facilitated by the use of CaCl2 as an ionic crosslinking agent, which results in a rapid but non-uniform gelation156. Viscoelastic hydrogels from purified alginate can release a significant fraction of EVs at an optimum elasticity when crosslinked with CaSO4, which offers a slower, more uniform gelation, in part because EVs can control deformation via water flux18. In addition to partial or reversible crosslinking of hydrogels, temperature-sensitive crosslinking of hydrogels can be effective in achieving controlled EV release, while offering utility as injectable materials. A recent study loaded EVs in chitosan with glycerol-2-phosphate, which undergoes ionic crosslinking after injection at 37 °C, with an optimum porosity controlled by polymer concentration, EVs were shown to be gradually released and to promote corneal regeneration147. Another study used methylcellulose-based hydrogels with xylitol and polyethylene glycol (PEG) that undergo gelation at 37 °C via hydrogen bonds to control release EVs, while the release rate can be accelerated with lower temperature. This system can potentially be useful in some disease conditions, such as critical limb ischemia where temperature of damaged tissue is known to decrease due to reduced blood flow157.
Figure 5. Biomaterial strategies to control EV release.
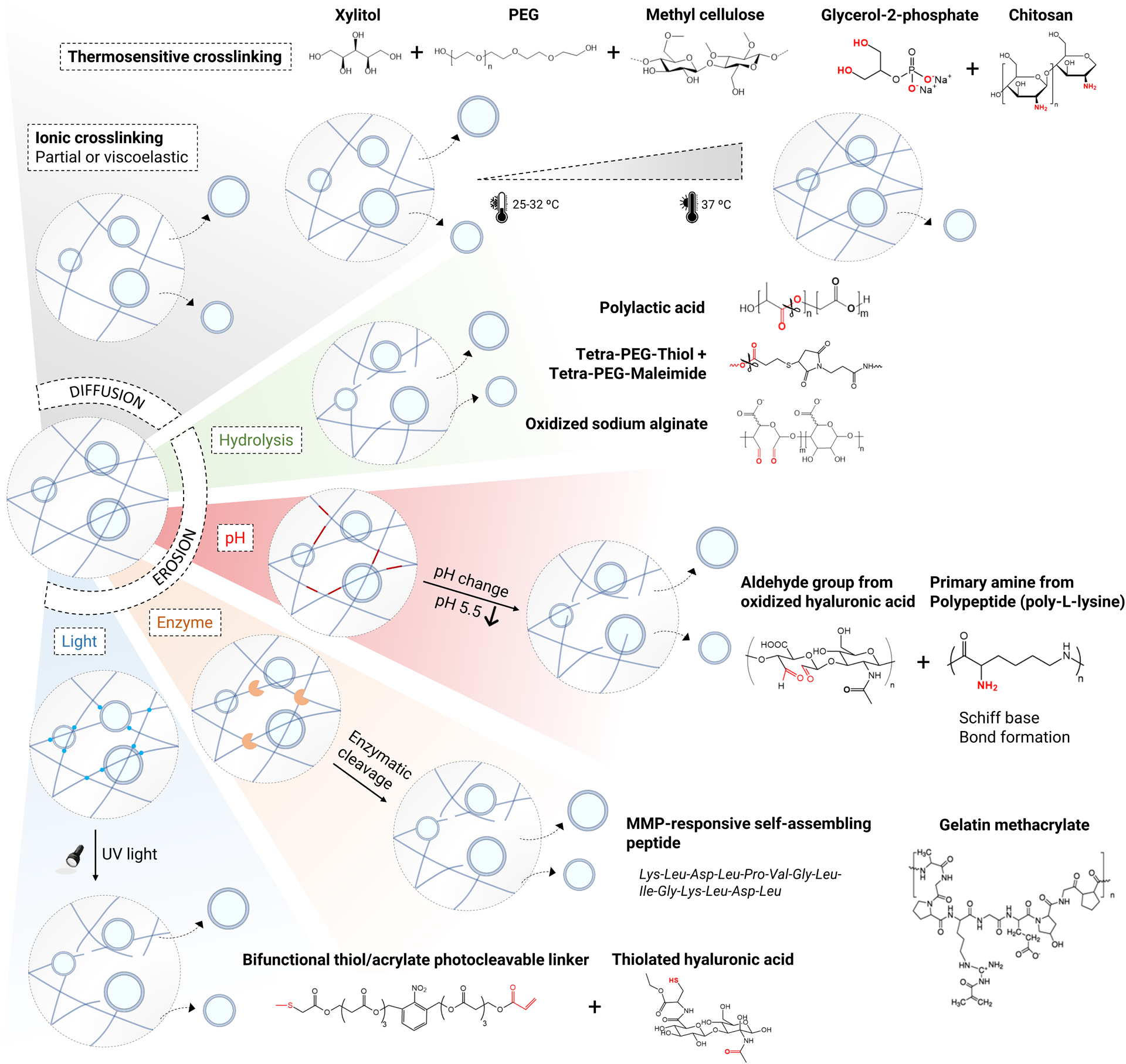
EV release can be controlled by either diffusion or erosion-based mechanisms. EVs can diffuse out in partially-crosslinked or viscoelastic hydrogels. Thermosensitive crosslinking can be used to tune EV diffusion from hydrogels as a function of temperature. For a more complete local release of EVs, erosion of a hydrogel network can be achieved either spontaneously through hydrolytic degradation or conditionally in response to external stimuli. The external stimuli that result in EV release by erosion of a hydrogel network can be classified into those that depend on host tissue conditions, such as pH and presence of enzymes, and those that enable on-demand release, such as light. Specific examples that were previously used to control EV release are shown for each category.
6.1.2. Erosion.
To ensure that EVs are more completely released from hydrogels in a localized manner, several studies have employed strategies to induce the erosion of the polymer backbone, which can be categorized based on degradation mechanisms (Fig. 5). The simplest strategy is to engineer polymer networks so that they can undergo hydrolytic degradation over time to gradually release EVs150,153,158. For example, cleavage of the ester bonds present in poly (lactic acid)-based 3D engineered scaffolds results in sustained release of EVs from human gingival MSCs to treat bone defects158. Similarly, clickable PEG-based hydrogels were used, where cleavage of the ester bonds in PEG-thiol derivatives leads to gradual swelling and sustained release of encapsulated EVs from MSCs over 4 weeks to treat an animal model of chronic liver failure159. In addition, aldehyde-containing oxidized sodium alginate hydrogels with a low degree of oxidation were used to achieve prolonged release of dermal papilla-derived EVs over a period of 7 days, resulting in improved hair growth153.
In many cases, it is desirable to erode the polymer backbone in response to specific conditions in host tissue. In a number of diseases, such as cancer and diabetic wounds, tissue environments become acidic, presenting opportunities to release EVs in a pH sensitive manner. A previous study encapsulated EVs in a hydrogel formed by Schiff base reaction between the aldehyde group of oxidized hyaluronic acid and the primary amine group of a polypeptide, such as ε-poly-L-lysine. Since Schiff bases hydrolyze under weak acidic conditions, this hydrogel system enables EV release in response to low pH, which was shown to be effective in treating an animal model of chronic diabetic wounds149.
Enzyme-based degradation mechanisms can also be employed to erode the polymer backbone and release EVs. In particular, naturally-derived hydrogels or synthetic hydrogels with peptide-based crosslinkers can be used to encapsulate EVs so that they can be released when various cells in host tissue secrete MMPs in pathophysiological conditions. For instance, gelatin-methacrylate hydrogels are known to be degraded by both collagenases and gelatinases160 and were indeed used to encapsulate and locally release EVs for treatment of myocardial infarction151 and cartilage regeneration142. In addition, MMP2-cleavable self-assembling peptides were used to form hydrogels and deliver EVs in the context of renal ischemia-reperfusion injury152.
Light-sensitive degradation of hydrogels addresses a need for noncontact-based strategies to externally trigger EV release independently of host tissue conditions. A recent study used the ortho-nitrobenzyl-based photocleavable linker that contains both thiol and acrylate groups. The linker molecules were first attached to EVs via disulfide bonds and then mixed with cysteine-conjugated hyaluronic acid to induce gelation via thiol-acrylate Michael addition161. The amount of released EVs was shown to be proportional to the number of UV-blue light irradiation, suggesting the utility of this approach in on-demand EV release.
6.2. Strategies to increase EV retention within hydrogels
Previous studies suggest that ECM-bearing EVs deposited on a cell culture surface facilitate cell migration162–164, raising the possibility that EVs can be used as haptotactic cues to recruit cells at the vicinity of hydrogels via juxtacrine interactions. In addition, when EVs are entrapped in hydrogels, soluble factors from EVs can be released in a controlled manner165—some of these factors are chemotactic signals166,167, which can recruit cells from distance. Thus, increasing the retention of EVs in hydrogels offers opportunities to recruit, program, and deploy host cells in a localized manner. Indeed, physical entrapment of EVs in nanoporous hydrogels was shown to increase EV retention in vivo after delivery168–170. However, hydrogels can be engineered to increase the retention of EVs by leveraging non-selective or selective molecular interactions (Fig. 6). The advantage of using non-selective interactions is that they can be generalized to different types of EVs regardless of their subpopulations or sources. Since the EV membrane is negatively charged, positively charged materials can be used to increase the retention of EVs via electrostatic interactions, which were shown to promote regeneration171 and immunomodulation172. EVs can also be grafted to materials more permanently by covalent bonds. One study employed a photoinduced imine crosslinking hydrogel to graft EVs upon gelation and showed sustained EV retention over 2 weeks169. More recently, a copper-free click chemistry strategy was described, where EVs were collected from cells that were metabolically labelled with azide-containing amino acids, and encapsulated in collagen hydrogels that were modified with dibenzocyclooctyne (DBCO) to conjugate EVs, resulting in increased recruitment of macrophages and vascular growth in hydrogels173. On the other hand, selective molecular interactions are desirable if the goal is to elicit specific biological responses by immobilizing a subset of EVs. This has been achieved by grafting peptide sequences that bind to specific integrins present on the EV membrane to promote EV retention and tissue regeneration, including the Arg-Gly-Asp (RGD) peptide174,175 that binds to α5β 1 and αvβ3176 and a laminin-derived peptide177 that binds to α3β1 integrin178.
Figure 6. Biomaterial strategies to promote EV retention.
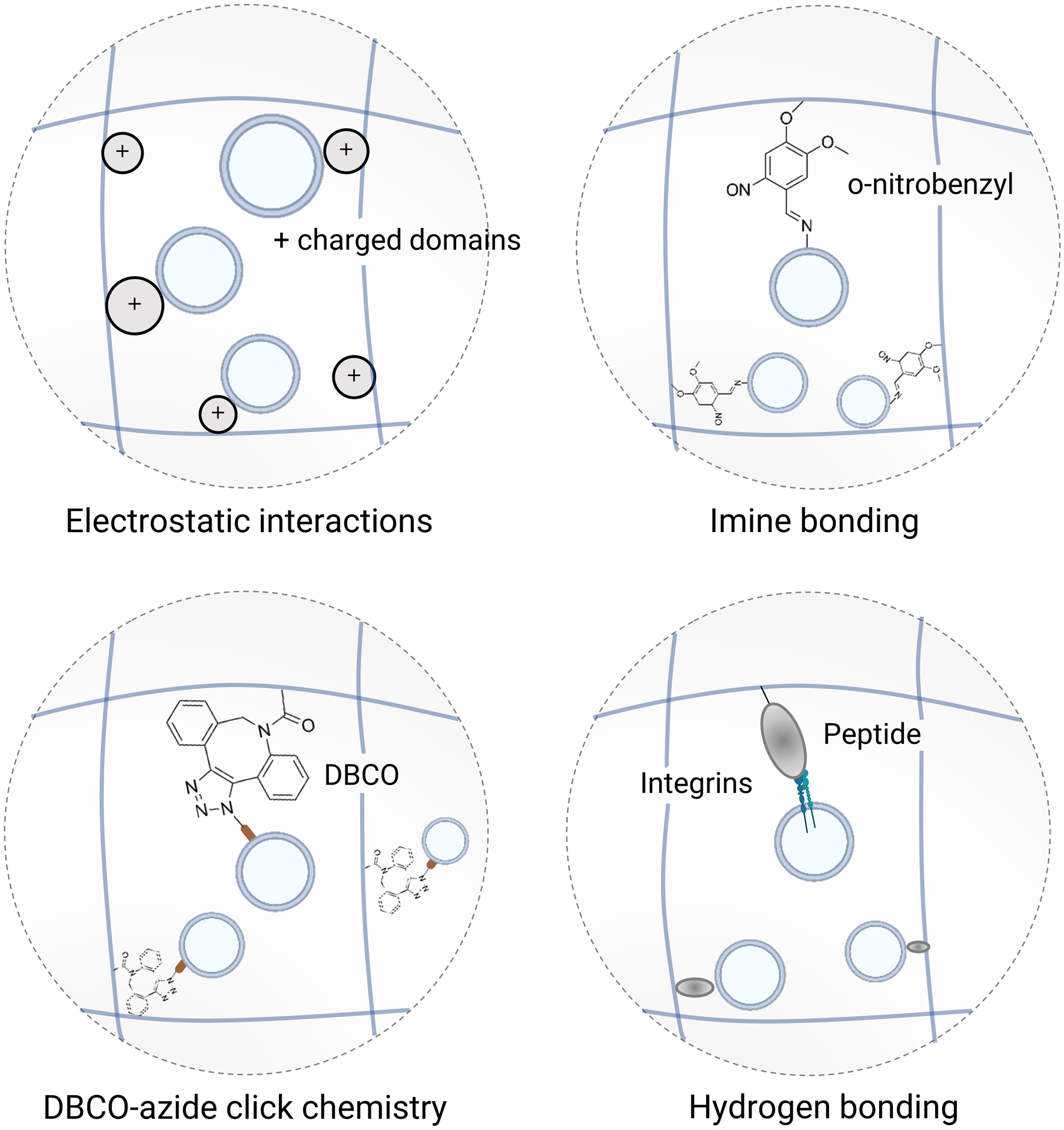
Introducing molecular interactions between EVs and a polymer network helps retain EVs within biomaterials to recruit and locally program cells. These interactions can be general, such as electrostatic interactions, imine bonding, and click chemistry (e.g. dibenzocyclooctyne (DBCO)-azide covalent bonds) of metabolically labelled EVs, in order to accomodate different types of EV subpopulations. Conversely, introducing a molecular sequence to a polymer network, such as an adhesion peptide that binds to integrins, enables the capture of a defined EV subpopulation in order to elicit a specific biological response.
7. Material-based cell culture strategies to control EV secretion from cells
In controlling EV release and retention via engineered materials, most studies to date collected EVs from cells on 2D tissue culture plastic, followed by enrichment of EVs from conditioned media prior to interfacing with materials. However, physicochemical factors of materials used in cell culture can impact the quantity and the properties of EVs from cells (Section 3), which may subsequently influence downstream applications with EVs. Thus, it will be important to understand how materials impact EV production by cells. The insights from this understanding can be helpful not only to improve the production of EVs that will be interfaced with materials, but also to inspire material-based strategies for sustained EV release or retention via cells. Advances in biomaterial design and biomanufacturing strategies have led to tunable engineered systems that recapitulate physical, chemical and structural properties of native tissues—these systems have been leveraged to discover new insights on cellular functions, which cannot be readily studied on standard tissue culture conditions155,179. Recent studies have employed these advances to control and improve EV production.
One important advance is a bioreactor system where cells can be cultured and a medium can be perfused so that EVs can be collected over time. A hollow-fiber bioreactor system (e.g., Fibercell) has emerged as one of the major methods to scale up the production of EVs, since hollow fibers offer a high surface area to attach a large number of cells (over 109) per setup, while enabling the circulation of the medium for nutrient exchange180–183. In addition to concentrating EVs in a small medium volume, the system also enriches for small EV-associated proteins per protein preparation compared to plastic culture. This suggests the potential effect of hollow fiber geometry or mass transfer on increasing small EV secretion or decreasing EV reuptake. It is possible to customize a bioreactor system by replacing hollow fibers with a 3D printed scaffold from a commercial stereolithography instrument, which was shown to increase EV production from endothelial cells184. While these studies used rigid materials to attach cells, employing a hydrogel-based cell culture surface or a scaffold with physiological biophysical properties26 will likely help further increase the yield of EVs from a bioreactor system.
Another emerging approach is to collect EVs from cell spheroids formed in microwells or on non-adhesive materials185. In one study, spheroids from gastric cancer cells were formed in an agarose microwell array and shown to increase the number of EVs per cell, while the average EV size was decreased—spheroid-derived EVs also showed an increased level of microRNAs, which subsequently downregulate proteins involved in the ADP-ribosylation factor 6 pathway that is known to mediate microvesicle shedding186. Thus, this study suggests that cell spheroids produce more small EVs and less large EVs. Consistently, another study showed that MSC spheroids formed by a hanging-drop method or on an anti-adhesive, poly(2-hydroxyethyl methacrylate)-coated surface increase EV number per cell compared to 2D culture183. In a therapeutic context, a recent study formed cell spheroids from lung biopsy tissues on an anti-adhesive surface, followed by cell expansion and collection of EVs, which were shown to be effective in treating preclinical models of fibrotic lung injury187. Overall, these studies suggest the utility of forming spheroids in promoting EV production. Given the diffusion limit of spheroids for nutrient exchange, the size of spheroids will need to be controlled below 100 μm to avoid the necrotic core188. Combining with a bioreactor system or employing vascularization strategies will enable the use of larger spheroids with high viability to increase the yield of EVs. From a mechanistic perspective, micropatterning-based strategies to decouple cell-cell contact and cell-material interactions189 will help dissect their relative contributions to EV production.
In principle, encapsulation in engineered materials provides cells with physiologically relevant cues in 3D microenvironments, which could be optimal for EV production compared to standard culture conditions. One study showed that the amount of EV proteins secreted per cell is increased when the medium is collected from MSCs in 3D collagen gel than cells on 2D plastic culture, and that EVs from MSCs in 3D collagen gel with pore size 1~3 μm190 show improved efficacy in an animal model of traumatic brain injury191. Another study showed that encapsulating HeLa cells in a peptide nanofiber-based hydrogel with pore size ~500 nm increases cell spheroid formation compared to 2D plastic culture, resulting in a more gradual release of EVs with a unimodal size distribution and a similar miRNA expression profile as that of cervical cancer patient plasma192. More studies are warranted to understand how 3D environments improve EV production, since these observations can be attributed to a number of factors arising from differences in the presentation of both physical and biochemical cues by 3D collagen gel vs. 2D plastic culture. Unlike 2D culture where EVs are directly secreted into liquid medium, EVs can interact with a polymeric network in 3D environments, a factor that needs to be taken into consideration in evaluating EV production.
8. Outlook
Understanding EVs in the context of the ECM inspires various strategies to interface EVs with engineered hydrogels as a means to improve the therapeutic efficacy of EVs by locally controlling release or retention. Making advances in this field requires the convergence of multiple fields, including cell and matrix biology, chemistry, membrane biophysics, biomaterial design, and nanotechnology.
The presence of EVs in the ECM is reminiscent of synthetic nanocomposite hydrogels193, materials with distinct properties due to the inclusion of nanoparticles193, which were previously developed to achieve advanced material properties, such as rapid self-healing194 and toughness195. Polymer physics teaches us that nanostructures can crosslink a polymer chain if they bind to the polymer with strong affinity and multivalency, provided that they are small enough to be bridged by the network196. This principle suggests the possibility that some cell-secreted nanoscale mediators may serve as primary or secondary crosslinkers of the ECM polymers, and hence influence ECM structure and ultimately function. Large EVs will likely offer greater multivalency, but small EVs may be better suited to be bridged by the network. Exomeres were shown to be smaller and more rigid than EVs35, suggesting the possibility that NVEPs may remain in nanoporous hydrogels after encapsulation and contribute to mechanical rigidity.
A simple negative feedback loop can be envisioned where cells initially secrete more EVs when the ECM is softer26, but if some EVs are deposited into the ECM20 and stiffen the network by crosslinking, this will limit the ability of cells to further produce EVs in a physiological condition. Testing this possibility will necessitate the development of materials of which properties can be dynamically tuned by incorporation of EVs from material-interfacing cells. This is also important in modeling diseases, such as cancer197 and fibrosis198 where the ECM stiffens in most cases, and EVs play important roles in disease progression199,200. The interplay of cell-secreted EVs, EV-ECM interactions, and their impact on cellular functions will help advance our understanding of pathological processes that accompany substantial structural changes in tissue microenvironments.
It has become clear that cells secrete both EVs and NVEPs with distinct properties33–36. Since this insight has emerged very recently, it is likely that most studies to date interfaced both EVs and NVEPs with biomaterials simultaneously. Thus, future efforts will benefit from the implementation of fractionation strategies to separate or deplete EVs and NVEPs, such as immunoaffinity-based approaches201 prior to interfacing with biomaterials. In addition, biogenesis mechanisms and biomolecular compositions are beginning to be better understood for different types of EVs and NVEPs, offering opportunities to design biomaterials that can release or retain specific subpopulations174,175,177. While the field is still young and rapidly redefined, combining cell-secreted nanoscale mediators with biomaterial design offers a novel platform to advance materials science, biology, and medicine.
Acknowledgments
This work was supported by National Institutes of Health Grants R01-GM141147 and R01-HL141255, and National Science Foundation CAREER Grant 2143857. We acknowledge Dr. Stephen Badylak and Dr. George Hussey for initial discussion of the review.
References
- 1.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ & Shenoy VB Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546, doi: 10.1038/s41586-020-2612-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey JD, Dufresne ER & Schwartz MA Mechanotransduction and extracellular matrix homeostasis. Nature reviews. Molecular cell biology 15, 802–812, doi: 10.1038/nrm3896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romani P, Valcarcel-Jimenez L, Frezza C & Dupont S Crosstalk between mechanotransduction and metabolism. Nature reviews. Molecular cell biology 22, 22–38, doi: 10.1038/s41580-020-00306-w (2021). [DOI] [PubMed] [Google Scholar]
- 4.Lu P, Takai K, Weaver VM & Werb Z Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor perspectives in biology 3, doi: 10.1101/cshperspect.a005058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC & Janmey PA Nonlinear elasticity in biological gels. Nature 435, 191–194, doi: 10.1038/nature03521 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Pelham RJ Jr. & Wang Y Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America 94, 13661–13665, doi: 10.1073/pnas.94.25.13661 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature materials 15, 326–334, doi: 10.1038/nmat4489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron AR, Frith JE & Cooper-White JJ The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993, doi: 10.1016/j.biomaterials.2011.04.003 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Grolman JM, Weinand P & Mooney DJ Extracellular matrix plasticity as a driver of cell spreading. Proceedings of the National Academy of Sciences of the United States of America 117, 25999–26007, doi: 10.1073/pnas.2008801117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao X et al. MatrisomeDB 2.0: 2023 updates to the ECM-protein knowledge database. Nucleic Acids Res, doi: 10.1093/nar/gkac1009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson HC Electron microscopic studies of induced cartilage development and calcification. The Journal of cell biology 35, 81–101, doi: 10.1083/jcb.35.1.81 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonucci E Fine structure of early cartilage calcification. Journal of ultrastructure research 20, 33–50, doi: 10.1016/s0022-5320(67)80034-0 (1967). [DOI] [PubMed] [Google Scholar]
- 13.Huleihel L et al. Matrix-bound nanovesicles within ECM bioscaffolds. Science advances 2, e1600502, doi: 10.1126/sciadv.1600502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu H et al. Concurrence of extracellular vesicle enrichment and metabolic switch visualized label-free in the tumor microenvironment. Science advances 3, e1600675, doi: 10.1126/sciadv.1600675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You S et al. Label-free visualization and characterization of extracellular vesicles in breast cancer. Proceedings of the National Academy of Sciences of the United States of America 116, 24012–24018, doi: 10.1073/pnas.1909243116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proceedings of the National Academy of Sciences of the United States of America 114, 10584–10589, doi: 10.1073/pnas.1709210114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan S, Vannberg FO & Dixon JB Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Scientific reports 6, 24436, doi: 10.1038/srep24436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenzini S, Bargi R, Chung G & Shin JW Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol 15, 217–223, doi: 10.1038/s41565-020-0636-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valadi H et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology 9, 654–659, doi: 10.1038/ncb1596 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Albacete-Albacete L et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. The Journal of cell biology 219, doi: 10.1083/jcb.202006178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzas EI The roles of extracellular vesicles in the immune system. Nature reviews. Immunology, doi: 10.1038/s41577-022-00763-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakarla R, Hur J, Kim YJ, Kim J & Chwae YJ Apoptotic cell-derived exosomes: messages from dying cells. Experimental & molecular medicine 52, 1–6, doi: 10.1038/s12276-019-0362-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang SHM et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nature communications 12, 6495, doi: 10.1038/s41467-021-26834-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cocucci E & Meldolesi J Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25, 364–372, doi: 10.1016/j.tcb.2015.01.004 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Pegtel DM & Gould SJ Exosomes. Annual review of biochemistry 88, 487–514, doi: 10.1146/annurev-biochem-013118-111902 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Lenzini S et al. Cell-Matrix Interactions Regulate Functional Extracellular Vesicle Secretion from Mesenchymal Stromal Cells. ACS nano, doi: 10.1021/acsnano.1c03231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thery C et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles 7, 1535750, doi: 10.1080/20013078.2018.1535750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolas-Avila JA et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 183, 94–109 e123, doi: 10.1016/j.cell.2020.08.031 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Ma L et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res 25, 24–38, doi: 10.1038/cr.2014.135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nature cell biology 21, 991–1002, doi: 10.1038/s41556-019-0367-5 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Nishimura T et al. Filopodium-derived vesicles produced by MIM enhance the migration of recipient cells. Developmental cell 56, 842–859 e848, doi: 10.1016/j.devcel.2021.02.029 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Nabhan JF, Hu R, Oh RS, Cohen SN & Lu Q Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences of the United States of America 109, 4146–4151, doi: 10.1073/pnas.1200448109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeppesen DK et al. Reassessment of Exosome Composition. Cell 177, 428–445 e418, doi: 10.1016/j.cell.2019.02.029 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q et al. Transfer of Functional Cargo in Exomeres. Cell reports 27, 940–954 e946, doi: 10.1016/j.celrep.2019.01.009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nature cell biology 20, 332–343, doi: 10.1038/s41556-018-0040-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nature cell biology 23, 1240–1254, doi: 10.1038/s41556-021-00805-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalluri R & LeBleu VS The biology, function, and biomedical applications of exosomes. Science 367, doi: 10.1126/science.aau6977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lingwood D & Simons K Lipid rafts as a membrane-organizing principle. Science 327, 46–50, doi: 10.1126/science.1174621 (2010). [DOI] [PubMed] [Google Scholar]
- 39.El-Sayed A & Harashima H Endocytosis of gene delivery vectors: from clathrin-dependent to lipid raft-mediated endocytosis. Molecular therapy : the journal of the American Society of Gene Therapy 21, 1118–1130, doi: 10.1038/mt.2013.54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelkmans L, Burli T, Zerial M & Helenius A Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118, 767–780, doi: 10.1016/j.cell.2004.09.003 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Sharma P et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116, 577–589, doi: 10.1016/s0092-8674(04)00167-9 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Boura E, Ivanov V, Carlson LA, Mizuuchi K & Hurley JH Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. The Journal of biological chemistry 287, 28144–28151, doi: 10.1074/jbc.M112.378646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trajkovic K et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247, doi: 10.1126/science.1153124 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Megha & London E Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. The Journal of biological chemistry 279, 9997–10004, doi: 10.1074/jbc.M309992200 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Castro BM, Silva LC, Fedorov A, de Almeida RF & Prieto M Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. The Journal of biological chemistry 284, 22978–22987, doi: 10.1074/jbc.M109.026567 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaus K et al. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proceedings of the National Academy of Sciences of the United States of America 100, 15554–15559, doi: 10.1073/pnas.2534386100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun M et al. The effect of cellular cholesterol on membrane-cytoskeleton adhesion. Journal of cell science 120, 2223–2231, doi: 10.1242/jcs.001370 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Byfield FJ, Aranda-Espinoza H, Romanenko VG, Rothblat GH & Levitan I Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophysical journal 87, 3336–3343, doi: 10.1529/biophysj.104.040634 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diz-Munoz A, Fletcher DA & Weiner OD Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol 23, 47–53, doi: 10.1016/j.tcb.2012.09.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauthier NC, Fardin MA, Roca-Cusachs P & Sheetz MP Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proceedings of the National Academy of Sciences of the United States of America 108, 14467–14472, doi: 10.1073/pnas.1105845108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parton RG & Simons K The multiple faces of caveolae. Nature reviews. Molecular cell biology 8, 185–194, doi: 10.1038/nrm2122 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Yeh YC, Ling JY, Chen WC, Lin HH & Tang MJ Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: reciprocal regulation of caveolin-1 and beta1 integrin. Scientific reports 7, 15008, doi: 10.1038/s41598-017-14932-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno-Vicente R et al. Caveolin-1 Modulates Mechanotransduction Responses to Substrate Stiffness through Actin-Dependent Control of YAP. Cell reports 25, 1622–1635 e1626, doi: 10.1016/j.celrep.2018.10.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu J et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. The Journal of clinical investigation 116, 1284–1291, doi: 10.1172/JCI27100 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sedding DG et al. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circulation research 96, 635–642, doi: 10.1161/01.RES.0000160610.61306.0f (2005). [DOI] [PubMed] [Google Scholar]
- 56.Sinha B et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413, doi: 10.1016/j.cell.2010.12.031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong SW, Lenzini S, Cooper MH, Mooney DJ & Shin JW Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Science advances 6, eaaw0158, doi: 10.1126/sciadv.aaw0158 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carafoli E & Krebs J Why Calcium? How Calcium Became the Best Communicator. The Journal of biological chemistry 291, 20849–20857, doi: 10.1074/jbc.R116.735894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savina A, Furlan M, Vidal M & Colombo MI Exosome release is regulated by a calcium-dependent mechanism in K562 cells. The Journal of biological chemistry 278, 20083–20090, doi: 10.1074/jbc.M301642200 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Savina A, Fader CM, Damiani MT & Colombo MI Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6, 131–143, doi: 10.1111/j.1600-0854.2004.00257.x (2005). [DOI] [PubMed] [Google Scholar]
- 61.Dale P, Head V, Dowling MR & Taylor CW Selective inhibition of histamine-evoked Ca(2+) signals by compartmentalized cAMP in human bronchial airway smooth muscle cells. Cell calcium 71, 53–64, doi: 10.1016/j.ceca.2017.12.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verweij FJ et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. The Journal of cell biology 217, 1129–1142, doi: 10.1083/jcb.201703206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piersma B, Hayward MK & Weaver VM Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer 1873, 188356, doi: 10.1016/j.bbcan.2020.188356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wike-Hooley JL, Van der Zee J, van Rhoon GC, Van den Berg AP & Reinhold HS Human tumour pH changes following hyperthermia and radiation therapy. Eur J Cancer Clin Oncol 20, 619–623, doi: 10.1016/0277-5379(84)90006-3 (1984). [DOI] [PubMed] [Google Scholar]
- 65.Singer AJ & Clark RA Cutaneous wound healing. The New England journal of medicine 341, 738–746, doi: 10.1056/NEJM199909023411006 (1999). [DOI] [PubMed] [Google Scholar]
- 66.Wottawa M et al. Hypoxia-stimulated membrane trafficking requires T-plastin. Acta Physiol (Oxf) 221, 59–73, doi: 10.1111/apha.12859 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Wang T et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proceedings of the National Academy of Sciences of the United States of America 111, E3234–3242, doi: 10.1073/pnas.1410041111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King HW, Michael MZ & Gleadle JM Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12, 421, doi: 10.1186/1471-2407-12-421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Umezu T et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124, 3748–3757, doi: 10.1182/blood-2014-05-576116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parolini I et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of biological chemistry 284, 34211–34222, doi: 10.1074/jbc.M109.041152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang SH et al. Laminin gamma2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin alpha3-dependent uptake by lymphatic endothelial cells. International journal of cancer 144, 2795–2810, doi: 10.1002/ijc.32027 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Ilani T et al. A secreted disulfide catalyst controls extracellular matrix composition and function. Science 341, 74–76, doi: 10.1126/science.1238279 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Hussey GS et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Science Advances 6, eaay4361, doi:doi: 10.1126/sciadv.aay4361 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Domingues RM et al. Lipoxidation adducts with peptides and proteins: deleterious modifications or signaling mechanisms? J Proteomics 92, 110–131, doi: 10.1016/j.jprot.2013.06.004 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Dunn JA, McCance DR, Thorpe SR, Lyons TJ & Baynes JW Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry 30, 1205–1210, doi: 10.1021/bi00219a007 (1991). [DOI] [PubMed] [Google Scholar]
- 76.Borras C et al. Extracellular vesicles and redox modulation in aging. Free radical biology & medicine 149, 44–50, doi: 10.1016/j.freeradbiomed.2019.11.032 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Aparicio-Trejo OE et al. Extracellular Vesicles in Redox Signaling and Metabolic Regulation in Chronic Kidney Disease. Antioxidants (Basel) 11, doi: 10.3390/antiox11020356 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu D & Esko JD Demystifying heparan sulfate-protein interactions. Annual review of biochemistry 83, 129–157, doi: 10.1146/annurev-biochem-060713-035314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walrant A, Bechara C, Alves ID & Sagan S Molecular partners for interaction and cell internalization of cell-penetrating peptides: how identical are they? Nanomedicine (Lond) 7, 133–143, doi: 10.2217/nnm.11.165 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Melo SA et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182, doi: 10.1038/nature14581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wuthier RE Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochimica et biophysica acta 409, 128–143, doi: 10.1016/0005-2760(75)90087-9 (1975). [DOI] [PubMed] [Google Scholar]
- 82.Bailey S et al. The role of extracellular matrix phosphorylation on energy dissipation in bone. eLife 9, doi: 10.7554/eLife.58184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan D et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142, 1–12, doi: 10.1016/j.biomaterials.2017.07.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwards CP, Fisher KL, Presta LG & Bodary SC Mapping the intercellular adhesion molecule-1 and −2 binding site on the inserted domain of leukocyte function-associated antigen-1. The Journal of biological chemistry 273, 28937–28944, doi: 10.1074/jbc.273.44.28937 (1998). [DOI] [PubMed] [Google Scholar]
- 85.Tang TT et al. Employing Macrophage-Derived Microvesicle for Kidney-Targeted Delivery of Dexamethasone: An Efficient Therapeutic Strategy against Renal Inflammation and Fibrosis. Theranostics 9, 4740–4755, doi: 10.7150/thno.33520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.You TJ et al. A 3D structure model of integrin alpha 4 beta 1 complex: I. Construction of a homology model of beta 1 and ligand binding analysis. Biophysical journal 82, 447–457, doi: 10.1016/S0006-3495(02)75409-X (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou L et al. Role of CD44 in increasing the potency of mesenchymal stem cell extracellular vesicles by hyaluronic acid in severe pneumonia. Stem cell research & therapy 12, 293, doi: 10.1186/s13287-021-02329-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerji S et al. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol 14, 234–239, doi: 10.1038/nsmb1201 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Xu Z, Ozcelikkale A, Kim YL & Han B Spatiotemporal Characterization of Extracellular Matrix Microstructures in Engineered Tissue: A Whole-Field Spectroscopic Imaging Approach. J Nanotechnol Eng Med 4, 110051–110059, doi: 10.1115/1.4024130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DiDomenico CD, Lintz M & Bonassar LJ Molecular transport in articular cartilage - what have we learned from the past 50 years? Nat Rev Rheumatol 14, 393–403, doi: 10.1038/s41584-018-0033-5 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Irvine DJ, Swartz MA & Szeto GL Engineering synthetic vaccines using cues from natural immunity. Nature materials 12, 978–990, doi: 10.1038/nmat3775 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shimoda M & Khokha R Metalloproteinases in extracellular vesicles. Biochim Biophys Acta Mol Cell Res 1864, 1989–2000, doi: 10.1016/j.bbamcr.2017.05.027 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Zhu G et al. LOXL2-enriched small extracellular vesicles mediate hypoxia-induced premetastatic niche and indicates poor outcome of head and neck cancer. Theranostics 11, 9198–9216, doi: 10.7150/thno.62455 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai LH, Panyukov S & Rubinstein M Hopping Diffusion of Nanoparticles in Polymer Matrices. Macromolecules 48, 847–862, doi: 10.1021/ma501608x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Georgiades P, Pudney PD, Thornton DJ & Waigh TA Particle tracking microrheology of purified gastrointestinal mucins. Biopolymers 101, 366–377, doi: 10.1002/bip.22372 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Lai SK et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proceedings of the National Academy of Sciences of the United States of America 104, 1482–1487, doi: 10.1073/pnas.0608611104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bottini M et al. Nanodrugs to target articular cartilage: An emerging platform for osteoarthritis therapy. Nanomedicine : nanotechnology, biology, and medicine 12, 255–268, doi: 10.1016/j.nano.2015.09.013 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Evans RC & Quinn TM Solute convection in dynamically compressed cartilage. Journal of biomechanics 39, 1048–1055, doi: 10.1016/j.jbiomech.2005.02.017 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Gardiner B et al. Solute transport in cartilage undergoing cyclic deformation. Comput Methods Biomech Biomed Engin 10, 265–278, doi: 10.1080/10255840701309163 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Yu M et al. Temperature- and rigidity-mediated rapid transport of lipid nanovesicles in hydrogels. Proceedings of the National Academy of Sciences of the United States of America 116, 5362–5369, doi: 10.1073/pnas.1818924116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao J, Su J, Qin L, Zhang X & Mao S Exploring the influence of inhaled liposome membrane fluidity on its interaction with pulmonary physiological barriers. Biomater Sci 8, 6786–6797, doi: 10.1039/d0bm01529f (2020). [DOI] [PubMed] [Google Scholar]
- 102.Yu M et al. Rapid transport of deformation-tuned nanoparticles across biological hydrogels and cellular barriers. Nature communications 9, 2607, doi: 10.1038/s41467-018-05061-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu H et al. Cholesterol-tuned liposomal membrane rigidity directs tumor penetration and anti-tumor effect. Acta Pharm Sin B 9, 858–870, doi: 10.1016/j.apsb.2019.02.010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kontomaris SV, Malamou A & Stylianou A The Hertzian theory in AFM nanoindentation experiments regarding biological samples: Overcoming limitations in data processing. Micron 155, 103228, doi: 10.1016/j.micron.2022.103228 (2022). [DOI] [PubMed] [Google Scholar]
- 105.Sharma S et al. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS nano 4, 1921–1926, doi: 10.1021/nn901824n (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia RA, Laney DE, Parsons SM & Hansma HG Substructure and responses of cholinergic synaptic vesicles in the atomic force microscope. J Neurosci Res 52, 350–355, doi: (1998). [DOI] [PubMed] [Google Scholar]
- 107.Bairamukov V et al. Biomechanical Properties of Blood Plasma Extracellular Vesicles Revealed by Atomic Force Microscopy. Biology (Basel) 10, doi: 10.3390/biology10010004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yurtsever A et al. Structural and mechanical characteristics of exosomes from osteosarcoma cells explored by 3D-atomic force microscopy. Nanoscale 13, 6661–6677, doi: 10.1039/d0nr09178b (2021). [DOI] [PubMed] [Google Scholar]
- 109.Whitehead B et al. Tumour exosomes display differential mechanical and complement activation properties dependent on malignant state: implications in endothelial leakiness. Journal of extracellular vesicles 4, 29685, doi: 10.3402/jev.v4.29685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vorselen D, MacKintosh FC, Roos WH & Wuite GJ Competition between Bending and Internal Pressure Governs the Mechanics of Fluid Nanovesicles. ACS nano 11, 2628–2636, doi: 10.1021/acsnano.6b07302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Evans EA Bending resistance and chemically induced moments in membrane bilayers. Biophysical journal 14, 923–931, doi: 10.1016/S0006-3495(74)85959-X (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vorselen D et al. The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis. Nature communications 9, 4960, doi: 10.1038/s41467-018-07445-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liang X, Mao G & Simon Ng KY Probing small unilamellar EggPC vesicles on mica surface by atomic force microscopy. Colloids Surf B Biointerfaces 34, 41–51, doi: 10.1016/j.colsurfb.2003.10.017 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Li S, Eghiaian F, Sieben C, Herrmann A & Schaap IAT Bending and puncturing the influenza lipid envelope. Biophysical journal 100, 637–645, doi: 10.1016/j.bpj.2010.12.3701 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kucerka N, Tristram-Nagle S & Nagle JF Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J Membr Biol 208, 193–202, doi: 10.1007/s00232-005-7006-8 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Arriaga LR et al. Stiffening effect of cholesterol on disordered lipid phases: a combined neutron spin echo + dynamic light scattering analysis of the bending elasticity of large unilamellar vesicles. Biophysical journal 96, 3629–3637, doi: 10.1016/j.bpj.2009.01.045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olbrich K, Rawicz W, Needham D & Evans E Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophysical journal 79, 321–327, doi: 10.1016/S0006-3495(00)76294-1 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rawicz W, Olbrich KC, McIntosh T, Needham D & Evans E Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophysical journal 79, 328–339, doi: 10.1016/S0006-3495(00)76295-3 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haraya-Takechi Y et al. Atomic Force Microscopic Analysis of the Effect of Lipid Composition on Liposome Membrane Rigidity. Langmuir : the ACS journal of surfaces and colloids 32, 6074–6082 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Holopainen M et al. Polyunsaturated fatty acids modify the extracellular vesicle membranes and increase the production of proresolving lipid mediators of human mesenchymal stromal cells. Biochim Biophys Acta Mol Cell Biol Lipids 1864, 1350–1362, doi: 10.1016/j.bbalip.2019.06.010 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Hussey GS et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Science advances 6, eaay4361, doi: 10.1126/sciadv.aay4361 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mertins O & Dimova R Insights on the interactions of chitosan with phospholipid vesicles. Part II: Membrane stiffening and pore formation. Langmuir : the ACS journal of surfaces and colloids 29, 14552–14559, doi: 10.1021/la4032199 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Skotland T, Hessvik NP, Sandvig K & Llorente A Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res 60, 9–18, doi: 10.1194/jlr.R084343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gracia RS, Bezlyepkina N, Knorr RL, Lipowsky R & Dimova R Effect of cholesterol on the rigidity of saturated and unsaturated membranes: fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 6, 1472–1482 (2010). [Google Scholar]
- 125.Khelashvili G, Johner N, Zhao G, Harries D & Scott HL Molecular origins of bending rigidity in lipids with isolated and conjugated double bonds: the effect of cholesterol. Chem Phys Lipids 178, 18–26, doi: 10.1016/j.chemphyslip.2013.12.012 (2014). [DOI] [PubMed] [Google Scholar]
- 126.Mobius W et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 4, 222–231, doi: 10.1034/j.1600-0854.2003.00072.x (2003). [DOI] [PubMed] [Google Scholar]
- 127.Huotari J & Helenius A Endosome maturation. EMBO J 30, 3481–3500, doi: 10.1038/emboj.2011.286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang Y, Hong Y, Cho E, Kim GB & Kim IS Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. Journal of extracellular vesicles 7, 1440131, doi: 10.1080/20013078.2018.1440131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sorkin R et al. Nanomechanics of Extracellular Vesicles Reveals Vesiculation Pathways. Small 14, e1801650, doi: 10.1002/smll.201801650 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Calo A et al. Force measurements on natural membrane nanovesicles reveal a composition-independent, high Young’s modulus. Nanoscale 6, 2275–2285, doi: 10.1039/c3nr05107b (2014). [DOI] [PubMed] [Google Scholar]
- 131.Fowler PW et al. Membrane stiffness is modified by integral membrane proteins. Soft Matter 12, 7792–7803, doi: 10.1039/c6sm01186a (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pathan M et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res 47, D516–D519, doi: 10.1093/nar/gky1029 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wen H, Frokiaer J, Kwon TH & Nielsen S Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol 10, 1416–1429, doi: 10.1681/ASN.V1071416 (1999). [DOI] [PubMed] [Google Scholar]
- 134.Mc KJ et al. Detection of Na(+) transporter proteins in urine. J Am Soc Nephrol 11, 2128–2132, doi: 10.1681/ASN.V11112128 (2000). [DOI] [PubMed] [Google Scholar]
- 135.Pisitkun T, Shen RF & Knepper MA Identification and proteomic profiling of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America 101, 13368–13373, doi: 10.1073/pnas.0403453101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blanc L et al. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume. Blood 114, 3928–3934, doi: 10.1182/blood-2009-06-230086 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martinez-Ballesta MDC et al. Plasma membrane aquaporins mediates vesicle stability in broccoli. PloS one 13, e0192422, doi: 10.1371/journal.pone.0192422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao X, Huebsch N, Mooney DJ & Suo Z Stress-relaxation behavior in gels with ionic and covalent crosslinks. Journal of applied physics 107, 63509, doi: 10.1063/1.3343265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goodrich CP, Brenner MP & Ribbeck K Enhanced diffusion by binding to the crosslinks of a polymer gel. Nature communications 9, 4348, doi: 10.1038/s41467-018-06851-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lai CP et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS nano 8, 483–494, doi: 10.1021/nn404945r (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J & Mooney DJ Designing hydrogels for controlled drug delivery. Nature reviews. Materials 1, doi: 10.1038/natrevmats.2016.71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu H et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. Journal of extracellular vesicles 9, 1778883, doi: 10.1080/20013078.2020.1778883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen Y et al. Sequential Release of Small Extracellular Vesicles from Bilayered Thiolated Alginate/Polyethylene Glycol Diacrylate Hydrogels for Scarless Wound Healing. ACS nano 15, 6352–6368, doi: 10.1021/acsnano.0c07714 (2021). [DOI] [PubMed] [Google Scholar]
- 144.Lv K et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 9, 7403–7416, doi: 10.7150/thno.32637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Henriques-Antunes H et al. The Kinetics of Small Extracellular Vesicle Delivery Impacts Skin Tissue Regeneration. ACS nano 13, 8694–8707, doi: 10.1021/acsnano.9b00376 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Xing H et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. Journal of nanobiotechnology 19, 264, doi: 10.1186/s12951-021-00991-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tang Q et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 280, 121320, doi: 10.1016/j.biomaterials.2021.121320 (2022). [DOI] [PubMed] [Google Scholar]
- 148.Xing Z et al. Hydrogel Loaded with VEGF/TFEB-Engineered Extracellular Vesicles for Rescuing Critical Limb Ischemia by a Dual-Pathway Activation Strategy. Advanced healthcare materials 11, e2100334, doi: 10.1002/adhm.202100334 (2022). [DOI] [PubMed] [Google Scholar]
- 149.Wang C et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 9, 65–76, doi: 10.7150/thno.29766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mardpour S et al. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS applied materials & interfaces 11, 37421–37433, doi: 10.1021/acsami.9b10126 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Tang J et al. Injection-Free Delivery of MSC-Derived Extracellular Vesicles for Myocardial Infarction Therapeutics. Advanced healthcare materials 11, e2100312, doi: 10.1002/adhm.202100312 (2022). [DOI] [PubMed] [Google Scholar]
- 152.Zhou Y et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J Control Release 316, 93–104, doi: 10.1016/j.jconrel.2019.11.003 (2019). [DOI] [PubMed] [Google Scholar]
- 153.Chen Y et al. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics 10, 1454–1478, doi: 10.7150/thno.39566 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Witwer KW et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. Journal of extracellular vesicles 8, 1609206, doi: 10.1080/20013078.2019.1609206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wong SW, Lenzini S, Giovanni R, Knowles K & Shin JW Matrix biophysical cues direct mesenchymal stromal cell functions in immunity. Acta biomaterialia 133, 126–138, doi: 10.1016/j.actbio.2021.07.075 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuo CK & Ma PX Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials 22, 511–521, doi: 10.1016/s0142-9612(00)00201-5 (2001). [DOI] [PubMed] [Google Scholar]
- 157.Xing Z et al. Hydrogel Loaded with VEGF/TFEB-Engineered Extracellular Vesicles for Rescuing Critical Limb Ischemia by a Dual-Pathway Activation Strategy. Advanced Healthcare Materials 11, 2100334, doi: 10.1002/adhm.202100334 (2022). [DOI] [PubMed] [Google Scholar]
- 158.Diomede F et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther 9, 104, doi: 10.1186/s13287-018-0850-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mardpour S et al. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Applied Materials & Interfaces 11, 37421–37433, doi: 10.1021/acsami.9b10126 (2019). [DOI] [PubMed] [Google Scholar]
- 160.Pepelanova I, Kruppa K, Scheper T & Lavrentieva A Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering (Basel) 5, doi: 10.3390/bioengineering5030055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Henriques-Antunes H et al. The Kinetics of Small Extracellular Vesicle Delivery Impacts Skin Tissue Regeneration. ACS Nano 13, 8694–8707, doi: 10.1021/acsnano.9b00376 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Sung BH, Ketova T, Hoshino D, Zijlstra A & Weaver AM Directional cell movement through tissues is controlled by exosome secretion. Nature communications 6, 7164, doi: 10.1038/ncomms8164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brown M et al. Lymphatic exosomes promote dendritic cell migration along guidance cues. The Journal of cell biology 217, 2205–2221, doi: 10.1083/jcb.201612051 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lan J et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer research 79, 146–158, doi: 10.1158/0008-5472.CAN-18-0014 (2019). [DOI] [PubMed] [Google Scholar]
- 165.Fuhrmann G et al. Engineering Extracellular Vesicles with the Tools of Enzyme Prodrug Therapy. Advanced materials 30, e1706616, doi: 10.1002/adma.201706616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kriebel PW et al. Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. The Journal of cell biology 217, 2891–2910, doi: 10.1083/jcb.201710170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sung BH & Weaver AM Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr 11, 187–195, doi: 10.1080/19336918.2016.1273307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu D et al. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nature communications 12, 1412, doi: 10.1038/s41467-021-21682-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu X et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9, 4430–4438, doi: 10.1039/c7nr00352h (2017). [DOI] [PubMed] [Google Scholar]
- 170.Zhang K et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS applied materials & interfaces 10, 30081–30091, doi: 10.1021/acsami.8b08449 (2018). [DOI] [PubMed] [Google Scholar]
- 171.Li W et al. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS applied materials & interfaces 10, 5240–5254, doi: 10.1021/acsami.7b17620 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Su N et al. Mesenchymal stromal exosome-functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Science advances 7, doi: 10.1126/sciadv.abf7207 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xing Y et al. Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials 281, 121357, doi: 10.1016/j.biomaterials.2021.121357 (2022). [DOI] [PubMed] [Google Scholar]
- 174.Zhang C et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS nano 14, 12133–12147, doi: 10.1021/acsnano.0c05681 (2020). [DOI] [PubMed] [Google Scholar]
- 175.Huang CC et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta biomaterialia 126, 199–210, doi: 10.1016/j.actbio.2021.03.030 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shin JW & Mooney DJ Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proceedings of the National Academy of Sciences of the United States of America 113, 12126–12131, doi: 10.1073/pnas.1611338113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li L et al. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett 20, 4298–4305, doi: 10.1021/acs.nanolett.0c00929 (2020). [DOI] [PubMed] [Google Scholar]
- 178.Kim JM, Park WH & Min BM The PPFLMLLKGSTR motif in globular domain 3 of the human laminin-5 alpha3 chain is crucial for integrin alpha3beta1 binding and cell adhesion. Experimental cell research 304, 317–327, doi: 10.1016/j.yexcr.2004.11.009 (2005). [DOI] [PubMed] [Google Scholar]
- 179.Lenzini S, Devine D & *Shin JW Leveraging Biomaterial Mechanics to Improve Pluripotent Stem Cell Applications for Tissue Engineering. Front Bioeng Biotechnol 7, 260, doi: 10.3389/fbioe.2019.00260 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Watson DC et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105, 195–205, doi: 10.1016/j.biomaterials.2016.07.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Watson DC et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. Journal of extracellular vesicles 7, 1442088, doi: 10.1080/20013078.2018.1442088 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gobin J et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem cell research & therapy 12, 127, doi: 10.1186/s13287-021-02190-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cao J et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem cell research & therapy 11, 206, doi: 10.1186/s13287-020-01719-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Patel DB, Luthers CR, Lerman MJ, Fisher JP & Jay SM Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta biomaterialia 95, 236–244, doi: 10.1016/j.actbio.2018.11.024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui X, Hartanto Y & Zhang H Advances in multicellular spheroids formation. J R Soc Interface 14, doi: 10.1098/rsif.2016.0877 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rocha S et al. 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles. Adv Sci (Weinh) 6, 1800948, doi: 10.1002/advs.201800948 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dinh PC et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nature communications 11, 1064, doi: 10.1038/s41467-020-14344-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alessandri K et al. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proceedings of the National Academy of Sciences of the United States of America 110, 14843–14848, doi: 10.1073/pnas.1309482110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mao AS, Shin JW & Mooney DJ Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials 98, 184–191, doi: 10.1016/j.biomaterials.2016.05.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wolf K et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. The Journal of cell biology 201, 1069–1084, doi: 10.1083/jcb.201210152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang Y et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int 111, 69–81, doi: 10.1016/j.neuint.2016.08.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thippabhotla S, Zhong C & He M 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Scientific reports 9, 13012, doi: 10.1038/s41598-019-49671-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thoniyot P, Tan MJ, Karim AA, Young DJ & Loh XJ Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv Sci (Weinh) 2, 1400010, doi: 10.1002/advs.201400010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Appel EA et al. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nature communications 6, 6295, doi: 10.1038/ncomms7295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu J et al. Synthesis of graphene peroxide and its application in fabricating super extensible and highly resilient nanocomposite hydrogels. ACS nano 6, 8194–8202, doi: 10.1021/nn302874v (2012). [DOI] [PubMed] [Google Scholar]
- 196.Rubinstein M & Colby RH Polymer Physics. Oxford Univ. Press; (2003). [Google Scholar]
- 197.Acharya A, Das I, Chandhok D & Saha T Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev 3, 23–34, doi: 10.4161/oxim.3.1.10095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kurundkar A & Thannickal VJ Redox mechanisms in age-related lung fibrosis. Redox Biol 9, 67–76, doi: 10.1016/j.redox.2016.06.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu R et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 15, 617–638, doi: 10.1038/s41571-018-0036-9 (2018). [DOI] [PubMed] [Google Scholar]
- 200.Brigstock DR Extracellular Vesicles in Organ Fibrosis: Mechanisms, Therapies, and Diagnostics. Cells 10, doi: 10.3390/cells10071596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ter-Ovanesyan D et al. Framework for rapid comparison of extracellular vesicle isolation methods. eLife 10, doi: 10.7554/eLife.70725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


