Abstract
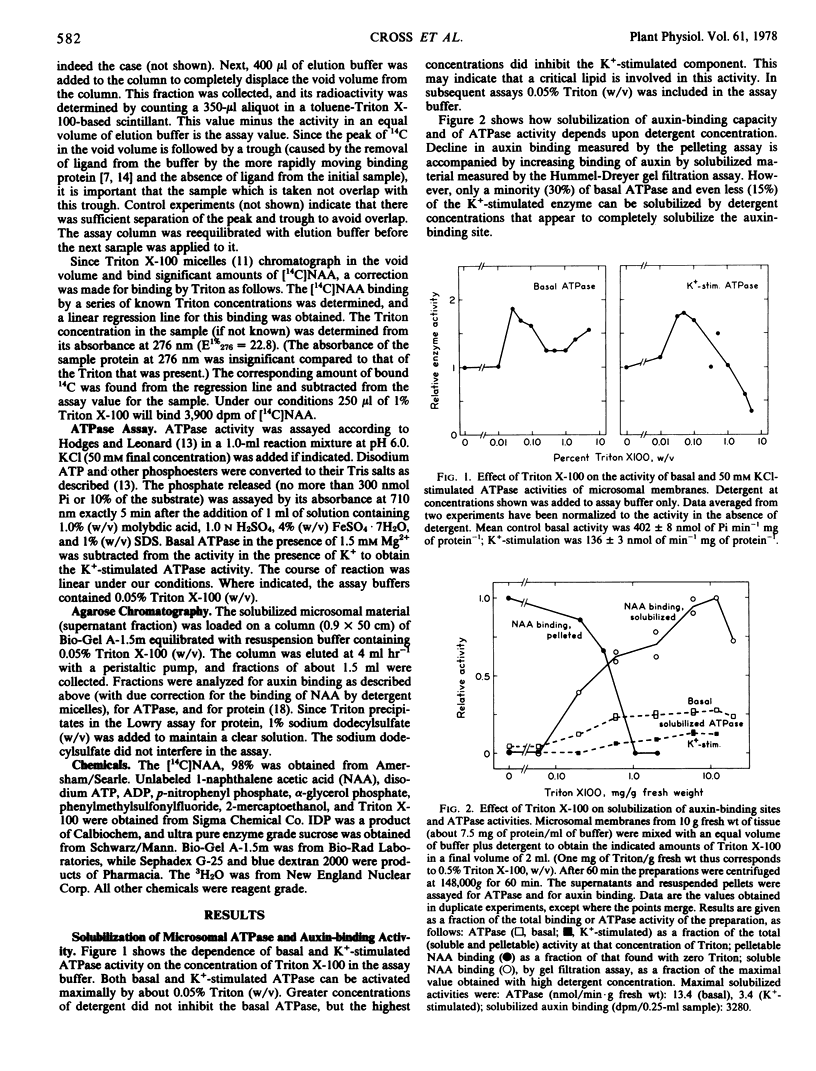
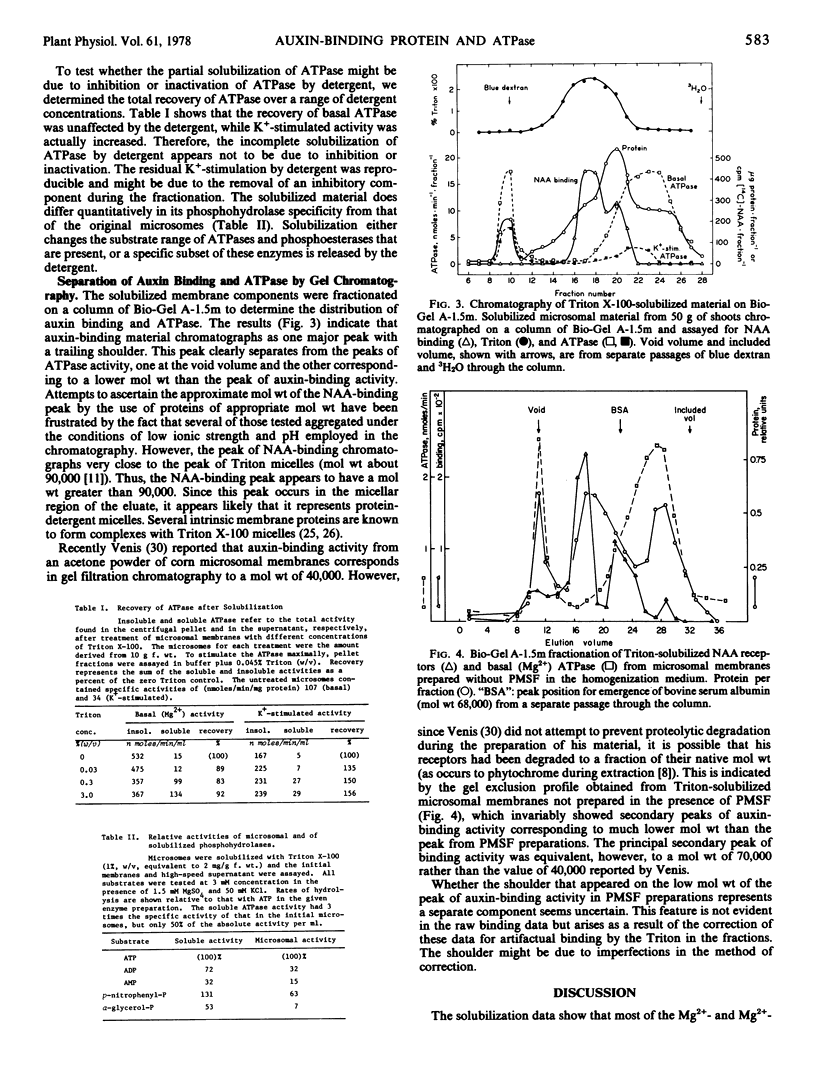
Membrane-localized auxin-binding sites from coleoptiles and primary leaves of Zea mays L. which may be auxin receptors can be fully solubilized by 1 to 1.5 mg of Triton X-100 per mg of membrane protein (about 1 mg per gram of original tissue fresh weight), while 70% of the basal (Mg2+)-ATPase and 85% of the K+-stimulated (Mg2+)-ATPase (pH 6) remain pelletable. Gel exclusion chromatography on Bio-Gel A-1.5m indicates that the solubilized receptors occur as detergent-protein micelles of about 90,000 daltons equivalent molecular weight. Solubilized ATPase activities occur (a) as very large particles excluded from the gel, and (b) as particles of a size substantially smaller than the particles that exhibit auxin binding. The auxin-binding receptor therefore appears not to be an ATPase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Rapid stimulation of K -H exchange by a plant growth hormone. Biochem Biophys Res Commun. 1976 Mar 22;69(2):333–338. doi: 10.1016/0006-291x(76)90526-x. [DOI] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Specificity of Auxin-binding Sites on Maize Coleoptile Membranes as Possible Receptor Sites for Auxin Action. Plant Physiol. 1977 Oct;60(4):585–591. doi: 10.1104/pp.60.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyenolds J. A., Stoeckenius W. Molecular weight of bacteriorhodopsin solubilized in Triton X-100. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2803–2804. doi: 10.1073/pnas.74.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Hollenberg M. D., Bennett V., Cuatrecasas P. Topographic separation of adenylate cyclase and hormone receptors in the plasma membrane of toad erythrocyte ghosts. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2860–2864. doi: 10.1073/pnas.74.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough G. A. The neurospora plasma membrane ATPase is an electrogenic pump. Proc Natl Acad Sci U S A. 1976 May;73(5):1485–1488. doi: 10.1073/pnas.73.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]


