Abstract
A method has been developed for observing membrane transport in isolated protoplasts. Transport of sugars and amino acids has been studied in protoplasts isolated from the mesophyll of Pisum sativum L. That uptake was not due to passive diffusion through damaged membranes was demonstrated by supplying simultaneously two sugar stereoisomers, the one 3H-labeled and the other 14C-labeled. The protoplast membranes were sufficiently functional to discriminate strongly between these stereoisomers.
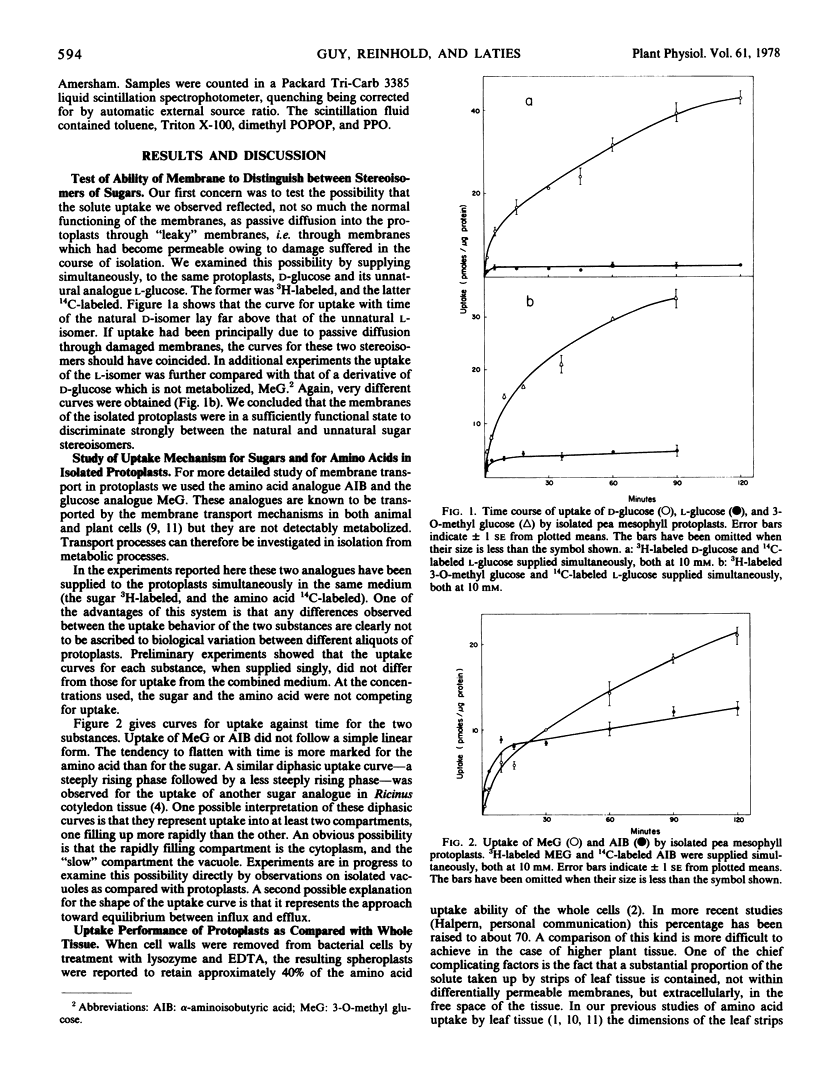
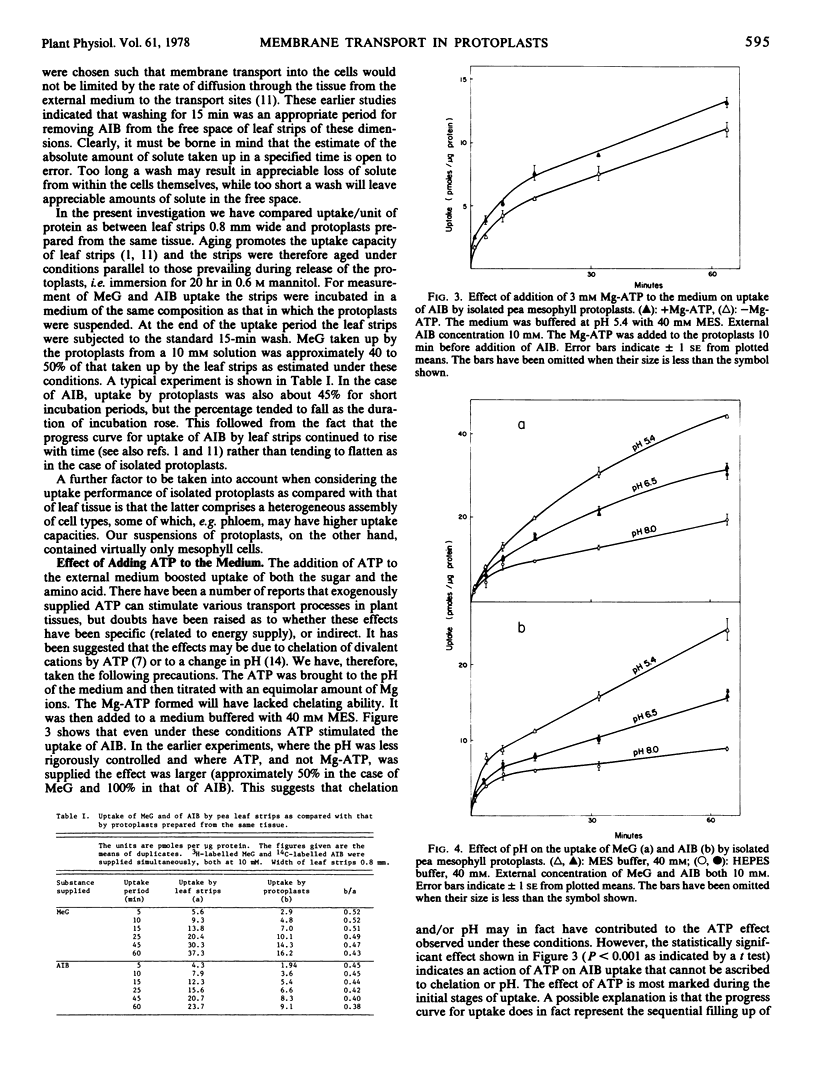
To characterize transport the nonmetabolized glucose analogue 3-O-methyl glucose (MeG) and amino acid analogue α-aminoisobutyric acid (AIB) were employed. When uptake was compared per unit of protein as between leaf strips and protoplasts prepared from the same tissue, it was estimated that the protoplasts had retained approximately 40 to 50% of the uptake ability of the whole cells. Uptake of neither MeG nor AIB by protoplasts was linear with time, but the tendency to flatten was more marked for AIB. Addition of Mg-ATP to buffered medium significantly promoted AIB uptake, an effect not ascribable to either chelation or pH. Transport of both MeG and AIB was markedly pH-dependent, uptake falling with rise in pH.
The stimulatory effect of Mg-ATP and the pH dependence confirm that uptake was not due to a diffusional inward “leak” but involved membrane function.
This work demonstrates the feasibility of using isolated protoplasts for membrane transport studies. The potential advantages of using protoplasts for such studies are pointed out.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L., Reinhold L. Loss of membrane transport ability in leaf cells and release of protein as a result of osmotic shock. Plant Physiol. 1973 Apr;51(4):620–625. doi: 10.1104/pp.51.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash H., Halpern Y. S. Purification and properties of glutamate binding protein from the periplasmic space of Escherichia coli K-12. Biochim Biophys Acta. 1975 Mar 28;386(1):168–180. doi: 10.1016/0005-2795(75)90257-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Reinhold L., Eshhar Z. Transport of 3-o-Methylglucose Into and Out of Storage Cells of Daucus carota. Plant Physiol. 1968 Jul;43(7):1023–1030. doi: 10.1104/pp.43.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]


