Abstract
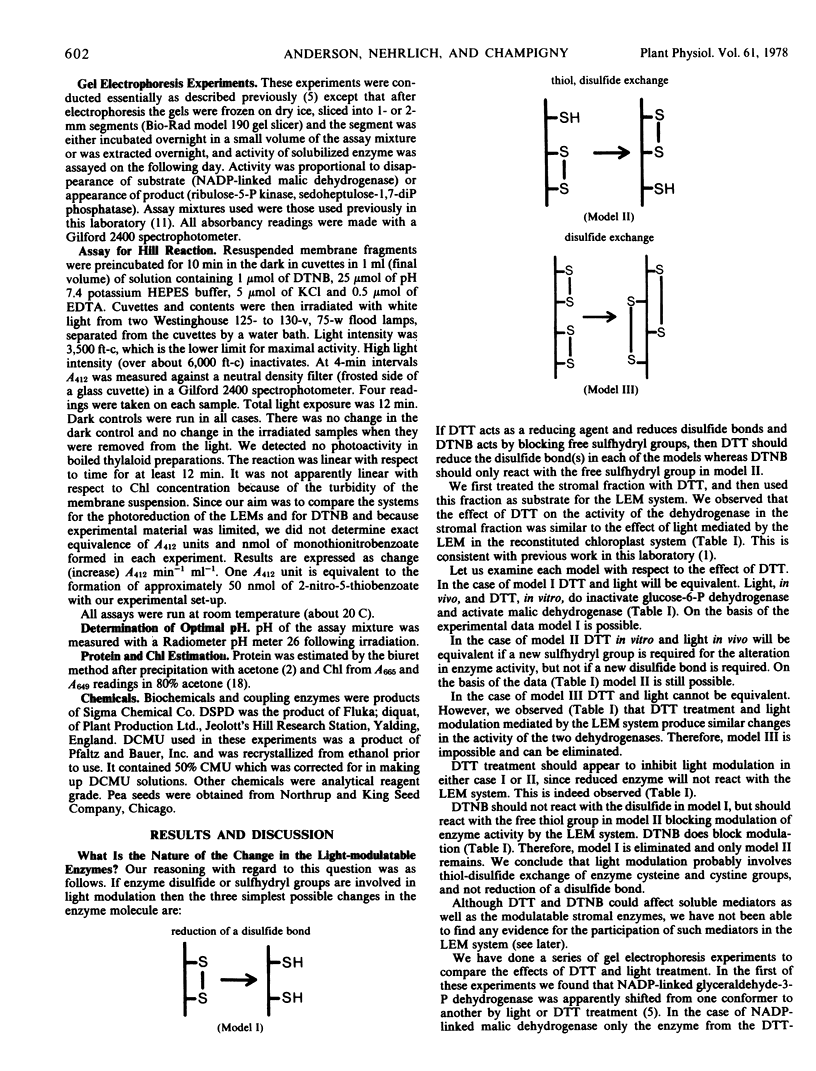
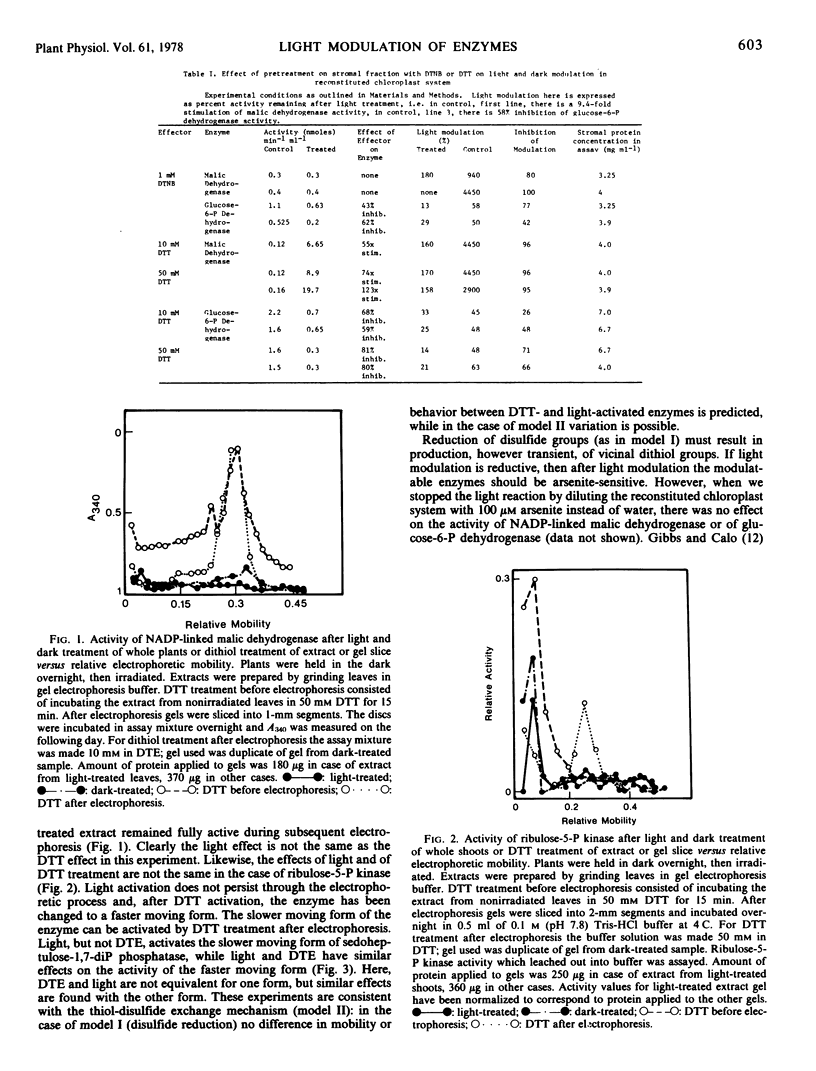
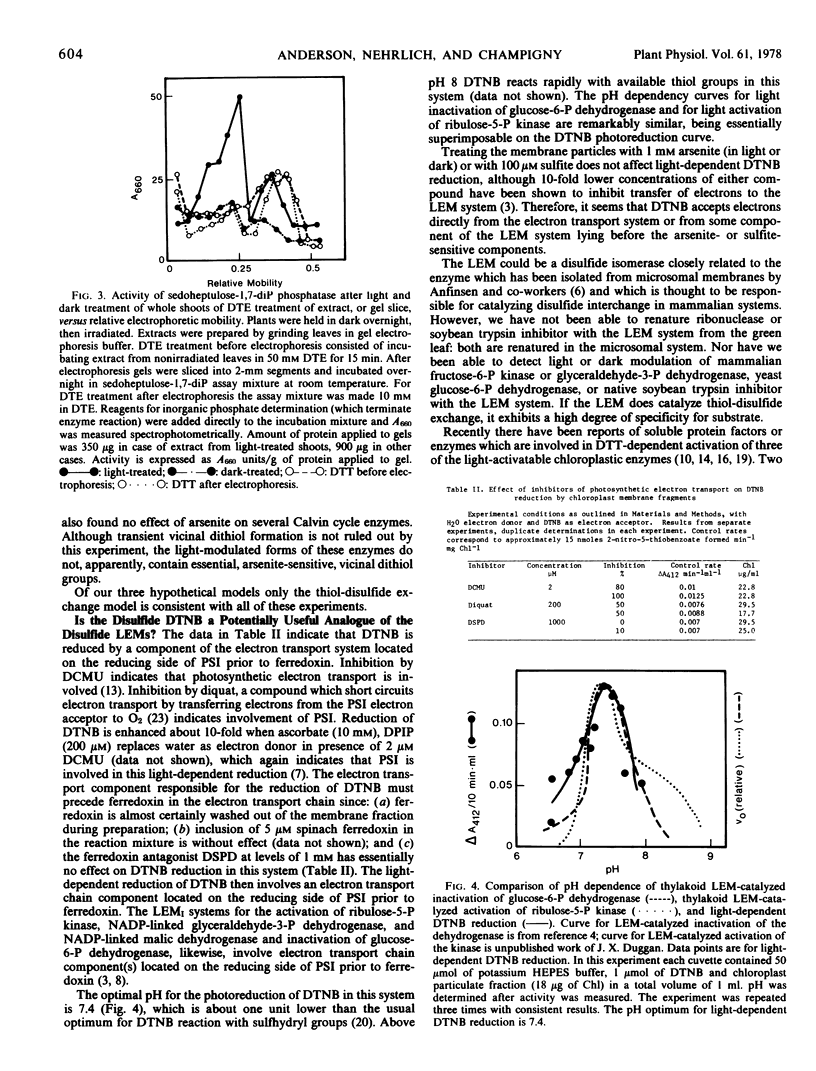
Light and dark modulation experiments with pea (Pisum sativum L.) chloroplast stromal fractions pretreated with dithiothreitol (to reduce protein disulfide bonds) or with 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) (to block sulfhydryl groups) suggest that light modulation involves thiol-disulfide exchange on the modulatable stromal enzyme protein. Light-dependent reduction of DTNB involves a photosynthetic electron transport chain component located on the reducing side of photosystem I prior to ferredoxin; DTNB may be acting as a light effect mediator substitute. The thylakoid-bound light effect mediator system, then, in its light-activated reduced form probably catalyzes thiol-disulfide exchange reactions on stromal enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Duggan J. X. Light modulation of glucose-6-phosphate dehydrogenase: partial characterization of the light inactivation system and its effects on the properties of the chloroplastic and cytoplasmic forms of the enzyme. Plant Physiol. 1976 Aug;58(2):135–139. doi: 10.1104/pp.58.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Lim T. C. Chloroplast glyceraldehyde 3-phosphate dehydrogenase: light-dependent change in the enzyme. FEBS Lett. 1972 Nov 1;27(2):189–191. doi: 10.1016/0014-5793(72)80616-1. [DOI] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Properties of phosphoribulokinase of whole chloroplasts. Plant Physiol. 1974 Feb;53(2):136–139. doi: 10.1104/pp.53.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Buchanan B. B., Wolosiuk R. A. Photosynthetic regulatory protein found in animal and bacterial cells. Nature. 1976 Dec 16;264(5587):669–670. doi: 10.1038/264669a0. [DOI] [PubMed] [Google Scholar]
- GIBBS M., CALO N. Studies on carbon dioxide fixation by chloroplasts, the effect of arsenite and other factors. Biochim Biophys Acta. 1960 Nov 4;44:341–347. doi: 10.1016/0006-3002(60)91570-5. [DOI] [PubMed] [Google Scholar]
- Jacquot J. P., Vidal J., Gadal P. Identification of a protein factor involved in dithiothreitol activation of NADP malate dehydrogenase from French bean leaves. FEBS Lett. 1976 Dec 1;71(2):223–227. doi: 10.1016/0014-5793(76)80937-4. [DOI] [PubMed] [Google Scholar]
- NEWTON J. W. A disulfide photoreduction system in chromatophores of Rhodospirillum rubrum. J Biol Chem. 1962 Oct;237:3282–3286. [PubMed] [Google Scholar]
- Shaltiel S. Hydrophobic chromatography. Methods Enzymol. 1974;34:126–140. doi: 10.1016/s0076-6879(74)34012-8. [DOI] [PubMed] [Google Scholar]
- Trebst A. Measurement of Hill reactions and photoreduction. Methods Enzymol. 1972;24:146–165. doi: 10.1016/0076-6879(72)24065-4. [DOI] [PubMed] [Google Scholar]
- Zweig G., Shavit N., Avron M. Diquat (I,I'-ethylene-2,2'-dipyridylium dibromide) in photo-reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):332–346. [PubMed] [Google Scholar]



