Abstract
Data on COVID-19 vaccine acceptability among parents of children with multisystem inflammatory syndrome (MIS-C) are limited. In this cohort of children with MIS-C, enrolled in the Swissped RECOVERY trial (NCT 04826588), comparing intravenous immunoglobulins or methylprednisolone, who, in accordance with Swiss guidelines, were recommended for SARS-CoV-2 vaccination, 65% (73/112) of parents reported being vaccinated against SARS-CoV-2 before the MIS-C, while 70% were vaccinated after the MIS-C episode of their child. None of the children were vaccinated before the occurrence of the MIS-C, and only 9% (5/56) received the COVID-19 vaccine after the MIS-C. The predominant barriers to COVID-19 vaccination were concerns over potential side effects and insufficient support from their doctors. This emphasizes the crucial role of health care providers in promoting COVID-19 vaccination among children.
Keywords: vaccine acceptance, SARS-CoV-2, COVID-19 vaccine, MIS-C, PIMS
Vaccination against SARS-CoV-2 has demonstrated remarkable efficacy in averting multisystem inflammatory syndrome (MIS-C) following SARS-CoV-2 infection in adolescents and children.1,2 In Switzerland, SARS-CoV-2 vaccination was recommended for children 16 years of age and above starting from May 2021 onward, followed by an extension to children 12 years old and above from June 2021. Subsequently, from December 2021, children 5 years of age and above were included in the vaccination recommendation.3 Several countries, including the United States,4 Canada5 and Switzerland, recommended SARS-CoV-2 vaccination for children who had experienced MIS-C. The rationale behind this recommendation rested on the potential to prevent recurrence of MIS-C through vaccination, considering that COVID-19 vaccination did not exhibit evidence of being a risk factor for MIS-C, while SARS-CoV-2 infection was unequivocally recognized as a risk factor for MIS-C. In addition, the vaccine was well tolerated in MIS-C children and was not associated to any relapse of MIS-C episode.6,7 In other countries, the vaccination strategy for children post-MIS-C was debated. In France, for instance, SARS-CoV-2 vaccination was initially contraindicated in this population but their position was revised in February 2022 to recommend vaccination.8 Limited research has been conducted on parental vaccination and attitudes toward vaccination of children who have experienced MIS-C.
METHODS
This is an ancillary study nested in the prospective Swissped RECOVERY trial of the Swiss National Clinical Trials Portal (SNCTP000004720) and clinicaltrial.gov (NCT 04826588), an investigator-initiated randomized multicenter open-label 2-arm trial in which children hospitalized with MIS-C were randomly assigned 1:1 to intravenous immunoglobulins or intravenous methylprednisolone at 10 Swiss pediatric hospitals.9,10 Cohort characteristics and outcomes have been published previously.9 Included children were followed until 6 months after randomization, and their families received a questionnaire (Questionnaire, Supplemental Digital Content 2, http://links.lww.com/INF/F338) regarding COVID-19 vaccination at 6 months after hospital discharge. For parents, the criterion for not being opposed to vaccination was having received at least 1 dose of the SARS-CoV-2 vaccine. This approach was adopted due to the absence of information regarding parental demographics, underlying health conditions, occupation or historical exposure, factors that would have enabled us to determine the requisite number of vaccine doses for considering vaccination as complete.
Data were summarized using the number (percentage) for categorical variables and the median (interquartile range) for continuous variables. Summary statistic comparisons between groups were performed using the Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. All analyses were based on the complete case data only and were performed using the statistical software R (version 4.0.3, R Development Core Team, Vienna, Austria11). A significance level of 5% was used for all statistical analysis.
RESULTS
Between May 21, 2021 and April 15, 2022, a total of 75 patients were included in the intention-to-treat analysis. Detailed information on the cohort, including baseline characteristics, is presented in the original publication.9 The vaccination questionnaire was completed for 56 children presenting with a MIS-C (74%; 56/75), resulting in 56 patients and 112 parents with available data for this analysis. We have included the patient characteristics from the original manuscript in Table, Supplemental Digital Content 3, http://links.lww.com/INF/F339 (“Total patients”) to observe any important difference that could suggest a bias in our analysis. No big differences between the 2 populations were observed.
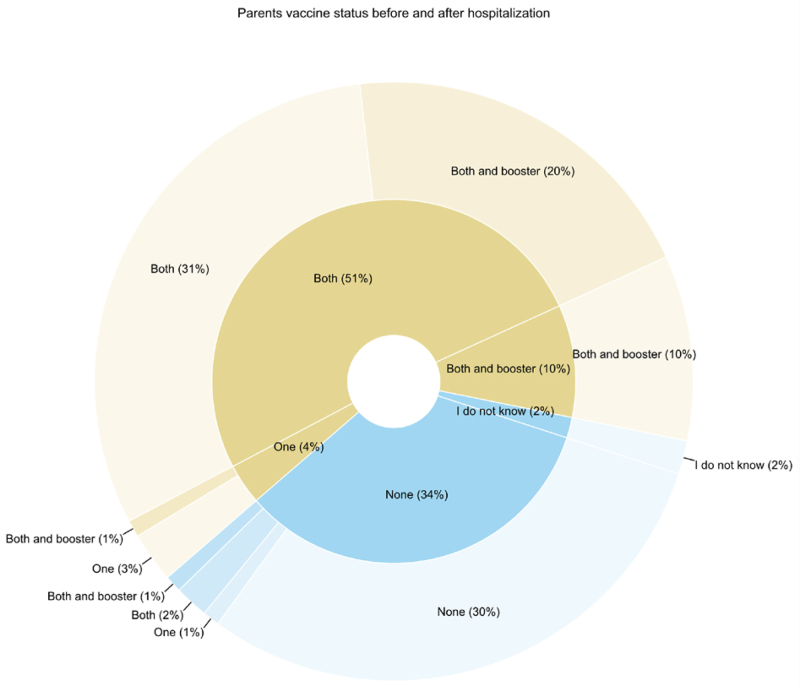
Before the occurrence of MIS-C in their offspring, 65% (73/112) of parents reported being vaccinated against SARS-CoV-2 and 70% (78/112) after the MIS-C episode. Among the 73 parents who had previously been vaccinated, 21% (15/73) received additional doses of the vaccine after the MIS-C episode of their child. In the group of parents who had initially chosen not to get vaccinated, 11% (4/37) got vaccinated (1 parent received a single dose, 2 received 2 doses and 1 received 3 doses) (Fig. 1). Most individuals within a couple shared the same vaccination status and there was no indication that the timing of the MIS-C occurrence influenced the vaccination status of the parents (Figure, Supplemental Digital Content 4, http://links.lww.com/INF/F340).
FIGURE 1.
Comparative pie chart illustrating parental vaccination status before their child’s MIS-C episode (inner circle) and 6 months post-MIS-C (outer circle). Due to rounding, the total percentage may sum to 101%. Light shades denote parental vaccination status before MIS-C, while darker shades represent status 6 months post-MIS-C. Notably, 2 parents indicated uncertainty regarding their SARS-CoV-2 vaccination. It is presumed that 1 parent completed the questionnaire, potentially explaining the inability to ascertain the other parent’s vaccination status in this instance.
SARS-CoV-2 vaccination was recommended for children 5 years old and above in December 2021, while it had been recommended for children 12 years old and older since June 2021. In our study, none of the children were vaccinated before the occurrence of MIS-C, and only 9% (5/56) of children received vaccination after the MIS-C episode. The parents provided various reasons for not vaccinating their children before the MIS-C and after, which are summarized in Figure, Supplemental Digital Content 5, http://links.lww.com/INF/F341. Parents indicated that half of the children (27/54) were not eligible for vaccination before the MIS-C, and for 44% (24/54), vaccination was felt unnecessary by the parents. Safety concerns were reported for 41% (22/54) of children, and for 4% (2/54) and 6% (3/54), the parents’ decision not to vaccinate their child was influenced by relatives/close friends and their doctor, respectively. Following the MIS-C episode, the reasons cited by parents for not vaccinating their child remained unchanged with respect to their former concerns about safety. There was a decrease in the number of parents reporting eligibility as a reason and an increase in the percentage of parents who felt that vaccination was unnecessary for their children. Notably, 23% (11/47) of parents indicated that their doctor advised against vaccinating their children after the MIS-C episode.
We assessed selected clinical factors (Table, Supplemental Digital Content 3, http://links.lww.com/INF/F339) that could potentially impact the decision of parents to vaccinate or not vaccinate their children after experiencing MIS-C. Our findings indicate that the only significant factor influencing this decision is the age at time of randomization (Table, Supplemental Digital Content 3, http://links.lww.com/INF/F339).
DISCUSSION
Sixty-five percent of parents were vaccinated against SARS-CoV-2 before the MIS-C episode of their child. This number is comparable to previous reports from Switzerland,12 US,13 Ireland and United Kingdom.14 We observed that it was rare for parents who had not been vaccinated before the MIS-C episode to change their mind after witnessing their child being hospitalized with MIS-C as a severe complication of COVID-19. This is in contradiction with previous studies reporting that higher personal and family risk for COVID-19 was associated with stronger COVID-19 vaccination willingness.15,16 Additionally, most couples shared the same vaccination status, indicating a consistent alignment within families and a lack of discrepancies in vaccination attitudes.
Concerning the vaccination of children, our study started 1 month after the COVID-19 vaccine was eligible for children 12 years of age and above in Switzerland and 7 months before the COVID-19 vaccine was eligible for children 5 years old and above. In our study, only 9% of the children had received the COVID-19 vaccine after their MIS-C episode. The sole differentiating factor between vaccinated and unvaccinated children was age, wherein vaccinated children had a higher median age in comparison to unvaccinated children, which could be explained by the fact that age recommendations for vaccination were progressively introduced, with younger patients being vaccinated later. However, given that only a small number of children were vaccinated at all in our study, this might not be relevant. In addition, most children in our study and in the original study were older than 5 years and so eligible for vaccination. Other studies also reported that parental willingness to vaccinate their children against SARS-CoV-2 decreased with the younger age of the child.17–19 A significant concern expressed by parents both before and after the MIS-C episode was related to safety. The apprehensions included fears regarding potential side effects and the perception that the vaccine was not well researched, similar to other studies.6,20 External factors and the environment also played a role in parents’ decision-making, including advice from family members or doctors. Indeed, other studies reported that pediatricians were among the most trusted source of information for COVID-19 vaccination.19,21
In conclusion, our study found that the acceptance of COVID-19 vaccines among parents of children with MIS-C was comparable to that of the general Swiss population, and it did not increase after the MIS-C episode in their child. A minority of MIS-C children received the COVID-19 vaccine after their MIS-C episode, primarily among the older age group. The main reasons cited for COVID-19 vaccine refusal were concerns about potential side effects and inadequate encouragement from doctors. These findings highlight the vital role of health care providers in promoting COVID-19 vaccination among children and in effectively addressing parental concerns to enhance vaccine acceptance.
SWISSPED RECOVERY GROUP:
Henrik Koehler, MD, Spyridoula Gysi, MD, Indra Janz, MD, Andreas Bieri, MD, Kantonsspital Aarau, Aarau, Switzerland; Birgit Donner, MD, Jürg Hammer, MD, Ulrich Heininger, MD, Clemens von Kalckreuth, MD, Malte Kohns, MD, Nicole Mettauer, MD, Alexandra Meyer, MD, Diana Reppucci, MD, Chloé Schlaeppi, MD, Daniel Trachsel, MD, Nina Vaezipour, MD, Andreas Woerner, MD, Andreas Zutter, MD, University Children`s Hospital Basel (UKBB), University of Basel, Basel, Switzerland; Federica Vanoni, MD, Lisa Kottanattu, MD, Calogero Mazzara, MD, Alessia Severi Conti, MD, Clinic of Pediatrics, Pediatric Institute of Southern Switzerland, EOC, Bellinzona, Switzerland; Christoph Aebi, MD, Philipp Agyeman, MD, Andrea Duppenthaler, MD, Martin Glöckler, MD, Sabine Pallivathukal, MD, Thomas Riedel, MD, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Petra Zimmermann, MD, PhD, Hong-Phuc Cudré-Cung, MD, PhD, Mladen Pavlovic, MD, University of Fribourg, Fribourg Hospital, Fribourg, Switzerland; Alice Bordessoule, MD, Anne-Laure Martin, MD, Angelo Polito, MD, Noemie Wagner, MD, Marie Rohr, MD, Arnaud L’Huillier, MD, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland; Vivianne Amiet, MD, Thomas Ferry, MD, David Longchamp, MD, Julia Natterer, MD, Rebecca Oppenheim, PhD, Michael Hofer, MD, University Hospital Lausanne, Lausanne, Switzerland; Michael Buettcher, MD, Katharina Wechselberger, MD, Alex Donas, MD, Sara Germann, MD, Michaela Lütolf Erni, MD, Daniela Kaiser, MD, Katharina Schwendener Scholl, MD, Hans Peter Kuen, MD, Katja Hrup, MD, Janine Stritt, MD, Children`s Hospital, Hospital Lucerne, Lucerne, Switzerland; Douggl G. N. Bailey, MD, Tanja Wachinger, BSc, Ingrid Beck, MD, André Birkenmaier, MD, Bjarte Rogdo, MD, Philip Lorenz, MD, Ivo Iglowstein, MD, Konstanze Zöhrer, MD, Martin Flade, MD, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland; Seraina Prader, MD, Jana Pachlopnik Schmid, MD, PhD, Michelle Seiler, MD, Patrick Meyer Sauteur, MD, PhD, Barbara Brotschi, MD, Kathrin Weber, MD, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Zurich, Switzerland; Elizabeth Whittaker, MD, PhD, Section of Paediatric Infectious Diseases, Imperial College London, London, United Kingdom; Saul N. Faust, MD, PhD, NIHR Southampton Clinical Research Facility and Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Institute for Life Sciences, University of Southampton, Southampton, United Kingdom.
Supplementary Material
Footnotes
This study was funded by grants from NOMIS Foundation, Vontobel Foundation and the Gaydoul Foundation. Supported by grants from the NOMIS Foundation, the Vontobel Foundation and the Gaydoul Foundation (to L.J.S.).
The authors have no conflicts of interest to disclose.
See collaborator group, Supplemental Digital Content 1, http://links.lww.com/INF/F337.
All authors conceptualized and approved this manuscript. G.B.-R., J.T. and C.S. performed data analysis and wrote the manuscript; M.C.A., S.B.-D., S.G., M.-H.P., D.W. and N.S. critically revised the manuscript. T.W., A.A., L.J.S. and J.A.B. lead the trial and revised the manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Carlos Sanchez, Email: Carlos.Sanchez@ukbb.ch.
Maya C. Andre, Email: Maya.Andre@ukbb.ch.
Sabrina Bressieux-Degueldre, Email: sabrina.bressieux@chuv.ch.
Serge Grazioli, Email: serge.grazioli@hcuge.ch.
Marie-Helene Perez, Email: marie-helene.perez@chuv.ch.
Daniela Wütz, Email: daniela.wuetz@kispi.uzh.ch.
Nina Schöbi, Email: nina.schoebi@insel.ch.
Tatjana Welzel, Email: tatjana.welzel@ukbb.ch.
Andrew Atkinson, Email: andrew.atkinson@ukbb.ch.
Luregn J. Schlapbach, Email: luregn.schlapbach@kispi.uzh.ch.
Julia A. Bielicki, Email: juliaanna.bielicki@ukbb.ch.
Johannes Trück, Email: johannes.trueck@kispi.uzh.ch.
Henrik Koehler, Kantonsspital Aarau, Aarau, Switzerland.
Spyridoula Gysi, Kantonsspital Aarau, Aarau, Switzerland.
Indra Janz, Kantonsspital Aarau, Aarau, Switzerland.
Andreas Bieri, Kantonsspital Aarau, Aarau, Switzerland.
Birgit Donner, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland
Jürg Hammer, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland
Ulrich Heininger, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Clemens von Kalckreuth, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Malte Kohns, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Nicole Mettauer, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Alexandra Meyer,, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Diana Reppucci, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Chloé Schlaeppi, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Daniel Trachsel, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Nina Vaezipour, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Andreas Woerner, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Andreas Zutter, University Children’s Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Federica Vanoni, Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Lisa Kottanattu, Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Calogero Mazzara, Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Alessia Severi Conti, Hospital Basel (UKBB), University of Basel, Basel, Switzerland.
Christoph Aebi, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Philipp Agyeman, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Andrea Duppenthaler, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Martin Glöckler, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Sabine Pallivathukal, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Thomas Riedel, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Petra Zimmermann, University of Fribourg, Fribourg Hospital, Fribourg, Switzerland.
Hong-Phuc Cudré-Cung, University of Fribourg, Fribourg Hospital, Fribourg, Switzerland.
Mladen Pavlovic, University of Fribourg, Fribourg Hospital, Fribourg, Switzerland.
Alice Bordessoule, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Anne-Laure Martin, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Angelo Polito, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Noemie Wagner, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Marie Rohr, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Arnaud L’Huillier, Children’s Hospital, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Vivianne Amiet, University Hospital Lausanne, Lausanne, Switzerland.
Thomas Ferry, University Hospital Lausanne, Lausanne, Switzerland.
David Longchamp, University Hospital Lausanne, Lausanne, Switzerland.
Julia Natterer, University Hospital Lausanne, Lausanne, Switzerland.
Rebecca Oppenheim, University Hospital Lausanne, Lausanne, Switzerland.
Michael Hofer, University Hospital Lausanne, Lausanne, Switzerland.
Michael Buettcher, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Katharina Wechselberger, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Alex Donas, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Sara Germann, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Michaela Lütolf Erni, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Daniela Kaiser, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Katharina Schwendener Scholl, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Hans Peter Kuen, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Katja Hrup, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Janine Stritt, Children’s Hospital, Hospital Lucerne, Lucerne, Switzerland.
Douggl G. N. Bailey, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Tanja Wachinger, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Ingrid Beck, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
André Birkenmaier, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Bjarte Rogdo, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Philip Lorenz, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Ivo Iglowstein, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Konstanze Zöhrer, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Martin Flade, Children’s Hospital of Eastern Switzerland St. Gallen, St. Gallen, Switzerland.
Seraina Prader, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Switzerland.
Jana Pachlopnik Schmid, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Switzerland.
Michelle Seiler, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Switzerland.
Patrick Meyer Sauteur, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Switzerland.
Barbara Brotschi, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Switzerland.
Kathrin Weber, Children’s Research Center, University Children’s Hospital Zurich, University of Zurich (UZH), Switzerland.
Elizabeth Whittaker, Section of Paediatric Infectious Diseases, Imperial College London, London, United Kingdom.
Saul N. Faust, NIHR Southampton Clinical Research Facility and Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Institute for Life Sciences, University of Southampton, Southampton, United Kingdom.
Collaborators: Henrik Koehler, Spyridoula Gysi, Indra Janz, Andreas Bieri, Birgit Donner, Jürg Hammer, Ulrich Heininger, Clemens von Kalckreuth, Malte Kohns, Nicole Mettauer, Alexandra Meyer,, Diana Reppucci, Chloé Schlaeppi, Daniel Trachsel, Nina Vaezipour, Andreas Woerner, Andreas Zutter, Federica Vanoni, Lisa Kottanattu, Calogero Mazzara, Alessia Severi Conti, Christoph Aebi, Philipp Agyeman, Andrea Duppenthaler, Martin Glöckler, Sabine Pallivathukal, Thomas Riedel, Petra Zimmermann, Hong-Phuc Cudré-Cung, Mladen Pavlovic, Alice Bordessoule, Anne-Laure Martin, Angelo Polito, Noemie Wagner, Marie Rohr, Arnaud L’Huillier, Vivianne Amiet, Thomas Ferry, David Longchamp, Julia Natterer, Rebecca Oppenheim, Michael Hofer, Michael Buettcher, Katharina Wechselberger, Alex Donas, Sara Germann, Michaela Lütolf Erni, Daniela Kaiser, Katharina Schwendener Scholl, Hans Peter Kuen, Katja Hrup, Janine Stritt, Douggl G. N. Bailey, Tanja Wachinger, Ingrid Beck, André Birkenmaier, Bjarte Rogdo, Philip Lorenz, Ivo Iglowstein, Konstanze Zöhrer, Martin Flade, Seraina Prader, Jana Pachlopnik Schmid, Michelle Seiler, Patrick Meyer Sauteur, Barbara Brotschi, Kathrin Weber, Elizabeth Whittaker, and Saul N. Faust
REFERENCES
- 1.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators. BNT162b2 mRNA vaccination against coronavirus disease 2019 is associated with a decreased likelihood of multisystem inflammatory syndrome in children aged 5-18 years-United States, July 2021 - April 2022. Clin Infect Dis. 2023;76:e90–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.F.C.o. Switzerland. COVID-19: vaccination recommended and possible for children from the beginning of January. 2021. https://www.admin.ch/gov/en/start/documentation/media-releases.msg-id-86451.html#:~:text=beginning%20of%20January-,COVID%2D19%3A%20vaccination%20recommended%20and%20possible%20for%20children,from%20the%20beginning%20of%20January&text=Bern%2C%2014.12.,aged%20between%20five%20and%20eleven.
- 4.C. Centers for Disease. Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us-appendix.html.
- 5.C. Government. COVID-19 vaccine: Canadian Immunization Guide. 2021.https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html.
- 6.Epalza C, Prieto-Tato L, Pino R, et al. ; EPICO-AEP Working Group. Safety and acceptance of COVID-19 vaccination after multisystem inflammatory syndrome in children (MIS-C) in Spain. J Pediatric Infect Dis Soc. 2022;11:471–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minoia F, Lucioni F, Heshin-Bekenstein M, et al. Approaches to SARS-CoV-2 and other vaccinations in children with a history of multisystem inflammatory syndrome (MIS-C): an international survey. Front Pediatr. 2022;10:1030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conseil d’Orientation de la Stratégie VaccinaleConseil d’Orientation de la Stratégie Vaccinale du gouvernement français. Vaccination des enfants et adolescents ayant développé un PIMS, Note du 8 février. 2022. https://sante.gouv.fr/IMG/pdf/cosv_-_note_du_8_fevrier_-_vaccination_des_enfants_ayant_developpe_un_pims.pdf.
- 9.Welzel T, Atkinson A, Schobi N, et al. ; Swissped RECOVERY Trial Group. Methylprednisolone versus intravenous immunoglobulins in children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS): an open-label, multicentre, randomised trial. Lancet Child Adolesc Health. 2023;7:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welzel T, Schobi N, Andre MC, et al. ; Swissped Recovery Trial. Multicenter randomized trial of methylprednisolone vs intravenous immunoglobulins to treat the pediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2 (PIMS-TS): protocol of the Swissped RECOVERY Trial. Front Pediatr. 2022;10:905046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R Foundation for Statistical Computing. R Care Team: a language and environment for statistical computing. Austria; 2020. [Google Scholar]
- 12.Wisniak A, Baysson H, Pullen N, et al. ; Specchio-COVID19 study group. COVID-19 vaccination acceptance in the canton of Geneva: a cross-sectional population-based study. Swiss Med Wkly. 2021;151:w30080. [DOI] [PubMed] [Google Scholar]
- 13.Malik AA, McFadden SM, Elharake J, et al. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hursh SR, Strickland JC, Schwartz LP, et al. Quantifying the impact of public perceptions on vaccine acceptance using behavioral economics. Front Public Health. 2020;8:608852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard-Rohner G, Caprettini B, Rohner D, et al. Impact of COVID-19 and intensive care unit capacity on vaccination support: evidence from a two-leg representative survey in the United Kingdom. J Virus Erad. 2021;7:100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peirolo A, Posfay-Barbe KM, Rohner D, et al. Acceptability of COVID-19 vaccine among hospital employees in the Department of Paediatrics, Gynaecology and Obstetrics in the University Hospitals of Geneva, Switzerland. Front Public Health. 2021;9:781562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanis P, Vraka I, Siskou O, et al. Willingness, refusal and influential factors of parents to vaccinate their children against the COVID-19: a systematic review and meta-analysis. Prev Med. 2022;157:106994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rane MS, Robertson MM, Westmoreland DA, et al. Intention to vaccinate children against COVID-19 among vaccinated and unvaccinated US Parents. JAMA Pediatr. 2022;176:201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal P, Sinharoy A. The influence of pediatricians’ recommendation on caregivers’ COVID-19 vaccine acceptance for children: a nationwide cross-sectional survey study from USA. Front Pediatr. 2023;11:1149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shmueli L. Parents’ intention to vaccinate their 5- to 11-year-old children with the COVID-19 vaccine: rates, predictors and the role of incentives. BMC public health. 2023;23:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousaf AR, Kunkel A, Abrams JY, et al. COVID-19 vaccine reactogenicity and vaccine attitudes among children and parents/guardians after multisystem inflammatory syndrome in children or COVID-19 hospitalization: September 2021-May 2022. Pediatr Infect Dis J. 2023;42:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.