Abstract
Background:
Current guidance from the US Centers for Disease Control and Prevention recommends empiric treatment for persons exposed to sexually transmitted infections, including Neisseria gonorrhoeae (NG). As an antimicrobial stewardship measure, some clinics now recommend a test and treat strategy, but reliance on urogenital testing only may miss cases.
Methods:
We conducted a descriptive analysis of pharyngeal NG infection in men who have sex with women (MSW) and women seeking care at a sexual health clinic in Seattle, WA, from February 2017 to July 2021 because of sexual contact to a partner diagnosed with gonorrhea. We also explored behavioral factors associated with pharyngeal NG positivity (by culture or nucleic acid amplification test by χ2 analysis.
Results:
Among 352 NG contacts tested for urogenital or pharyngeal infection, 34% were positive for NG at ≥1 anatomic site (27% for MSW and 40% for women). Among 161 NG contacts tested at the pharynx, 30% (n = 48) were positive: 20% of 54 MSW (n = 11) and 35% (n = 37) of 107 women. If only urogenital testing were performed, 36% of MSW NG infections (n = 5) and 19% of female NG infections (n = 9) would have remained unidentified.
Conclusions:
Pharyngeal NG is relatively common among MSW and women who have been exposed to NG, and likely represents an underdiagnosed reservoir of NG infection. If empiric treatment is abandoned in favor of testing and treating, testing the throats of heterosexuals will be necessary.
Current guidance from the US Centers for Disease Control and Prevention (CDC) recommends empirically treating persons exposed to sexually transmitted infections (STIs), including Neisseria gonorrhoeae (NG).1 Among heterosexual cisgender men and cisgender women tested at the time of empiric treatment, only 20% to 40% of these individuals test positive for NG at a urogenital site.2,3 As an antimicrobial stewardship measure, clinics in the United Kingdom and Australia now recommend a test and treat strategy that delays treatment until receipt of a positive test.4-7 However, urogenital testing alone may not identify all NG. Among men who have sex with men (MSM), pharyngeal NG is common and the CDC recommends routine screening at this anatomic site.1,8,9 At present, no guidelines currently exist for pharyngeal gonorrhea testing in cisgender women (henceforth “women” or “female”) or heterosexual men (cisgender men who have sex with cisgender women, henceforth “MSW”).1,8,9 One prior, small study suggests that pharyngeal gonorrhea is common among heterosexual female and MSW sexual contacts of persons infected with NG (henceforth “NG contacts”) presenting to an Australian sexual health clinic (SHC).10 In this report, we examined the burden of pharyngeal NG in MSW and women with and without concurrent urogenital symptoms (henceforth “asymptomatic contacts”) pre-enting to a US SHC as NG contacts, described how many NG infections are missed by urogenital testing alone, and investigated what sexual behaviors are predictive of pharyngeal NG in MSW and female NG contacts.
MATERIALS AND METHODS
Study Design and Study Population
This is a retrospective, cross-sectional study of clinical data describing the prevalence of pharyngeal NG infections in cisgender female and cisgender heterosexual male NG contacts who attended the Public Health Seattle & King County Sexual Health Clinic (PHSKC SHC) between February 1, 2017, and July 31, 2021. Given growing interest in pharyngeal gonorrhea globally, in February 2017, the PHSKC SHC clinic policy changed to test female and heterosexual male contacts to gonorrhea at the pharynx using NG culture if they reported giving oral sex in the past 2 months. In September 2019, clinic policy expanded to test for NG of the pharynx using both nucleic acid amplification test (NAAT) and culture and to routinely test all NG contacts at the pharynx regardless of report of recent oral sex behavior. All cisgender women who reported sexual exposure to NG were included regardless of self-reported sexual orientation or sexual behaviors. We defined cisgender men as heterosexual (MSW) if they reported current male gender and male sex at birth, and both denied male sexual partners within the last year and reported “never” having sex with a male partner. Any male patient who had rectal testing for GC was excluded as an additional marker of likely sexual activity with men. As a programmatic evaluation of deidentified data, Human Subjects Institutional Review Board approval was not required.
Clinical and Laboratory Protocols
All patients presenting to the PHSKC SHC complete a computer-assisted self-interview to help direct their care. This intake computer-assisted self-interview includes questions regarding demographics, sexual orientation, number and gender of partners, sexual behaviors (e.g., oral, vaginal, and anal sex), and contact to sexual partners with an STI, including NG. All patients reporting sexual exposure to a person diagnosed with gonorrhea are evaluated by a clinician, undergo STI testing, and receive antigonococcal treatment at the same visit. During the study period, testing included urogenital gonorrhea and chlamydia testing using an NAAT and pharyngeal NG testing using culture, NAAT, or both (as described previously). Clinicians collected a urethral swab for Gram staining and culture for men with urethral discharge. Pharyngeal NG testing was either self-collected or clinician collected depending on provider and patient preference.11
Specimens for N. gonorrhoeae culture were plated directly onto modified Thayer Martin agar and placed in a candle jar in an incubator in the clinic laboratory before transfer to the PHSKC laboratory and subsequently the Neisseria Reference Laboratory. Cultures at the PHSKC laboratory were identified and confirmed as N. gonorrhoeae by characteristic gonococcal colonial morphology, Gram stain, oxidase reaction, and the cystine-trypticase agar fermentation technique.12 To expedite N. gonorrhoeae culture confirmation and antimicrobial susceptibility testing per the CDC Strengthening the US Response to Resistant Gonorrhea protocol, the Neisseria Reference Laboratory identified and confirmed subcultures of oxidase-positive, gram-negative diplococci received from the PHSKC laboratory as N. gonorrhoeae by characteristic gonococcal colonial morphology, standard biochemical tests, and API NH (bioMérieux, Durham, NC), as described previously,12 and performed antimicrobial susceptibility testing on confirmed N. gonorrhoeae by Etest (bioMérieux).13 The PHSKC uses the Aptima Combo 2 (Hologic, San Diego, CA) NAAT for diagnosis of urogenital and pharyngeal gonorrhea. Neisseria gonorrhoeae contacts were treated per CDC 2015 STD Treatment Guidelines with 250 mg of IM ceftriaxone and 1 g of oral azithromycin for either urogenital or pharyngeal NG until December 2020,8 when CDC guidance changed for both urogenital and pharyngeal NG to a single dose of 500 mg IM of ceftriaxone.9
Analysis
The primary study outcome was pharyngeal NG positivity in female and MSW NG contacts. We considered pharyngeal NG positive if either culture or NAAT was positive. Subanalyses explored pharyngeal NG positivity by study period and in both the presence and absence of urogenital infection and urogenital symptoms. Urogenital symptoms in MSW were self-reported as penile discharge, penile discomfort, testicular discomfort, or pain with urination. Urogenital symptoms in women were self-reported as vaginal discharge, vaginal odor, vaginal discomfort, abnormal bleeding, abdominal pain, or dyspareunia. We explored demographic and behavioral associations (including age, race, ethnicity, number of sex partners within the prior 2 months, drug use, engagement in transactional sex, HIV serostatus, and presenting symptoms) with NG positivity at the pharynx for MSW and women separately.
For categorical variables, we used χ2 test to assess for differences in proportions, and for continuous variables, we used t test for comparison of means. We considered P < 0.05 to be significant. All analyses were conducted in STATA 16.1.
RESULTS
Study Population and Testing Coverage
Between February 2017 and July 2021, there were 352 SHC visits by MSW and female NG contacts: 170 MSW (48%) and 182 women (52%). Two hundred thirty-seven (67%) of these visits occurred between February 2017 and August 2019, corresponding to the period when only NG contacts who reported performing oral sex were supposed to be tested by culture (henceforth “study period 1”); 115 NG-contact visits (33%) occurred between September 2019 and July 202, the period when clinic policy expanded to test all heterosexual male and female NG contacts by both NAAT and culture (henceforth “study period 2”). Within the overall study period (February 2017 and July 2021), mean age was 32 years (interquartile range [IQR], 24–38 years), and NG contacts self-reported their race/ethnicity as predominantly non-Hispanic Black (n = 156; 38%), non-Hispanic White (n = 142; 34%), and Hispanic (n = 47; 11%). None of the women or MSW contacts were living with HIV. On average, study participants had two sexual partners in the past 2 months (IQR, 1–3 months), and the majority (65%) reported performing oral sex in the last 2 months.
All NG contacts were tested at either a urogenital site or the pharynx. More than 97% were tested at a urogenital site (169 [99%] MSW and 175 [96%] women), but fewer (54 [32%] and 107 [59%] MSW and women, respectively) were tested at the pharynx. Table 1 describes the study population by gender and pharyngeal testing status. Pharyngeal testing varied by gender and by period. During study period 1 when clinic policy was to test the pharynxes of only those NG contacts who reported giving oral sex in the last 2 months, less than one-quarter of MSW were tested at the pharynx, whereas nearly 50% of women were tested. Subsequently, from September 2019 to July 2021, when all NG-contact pharynxes were supposed to be tested irrespective of reported oral sex, pharyngeal testing increased to 51% for MSW and to 78% for women. During study period 1, 32% of 78 MSW (n = 25) and 55% of 74 women (n = 41) who reported recently giving oral sex were tested at the pharynx. This was a significantly higher pharyngeal testing rate than among those who did not report recently giving oral sex, 5% of 37 MSW (n = 2) and 26% of 23 women (n = 6). The association between pharyngeal testing and report of giving oral sex was not seen in study period 2, which appropriately followed the clinic pharyngeal testing policy change. When compared with the overall study period, there were no notable differences in association between pharyngeal testing and age, race/ethnicity, or number of sex partners within MSW or female NG contacts when analysis was stratified by study period (data not shown).
TABLE 1.
Demographics and Behavioral Characteristics of All MSW and Female NG Contacts Tested at the Pharynx (Ph)
| Overall Study Period: Feb 1, 2017–July 31, 2021 | MSW NG Contacts (n = 170) |
Female NG Contacts (n = 182) |
|||||
|---|---|---|---|---|---|---|---|
| No Ph Test (n = 116; 68%) |
Ph Test (n = 54; 32%) |
P | No Ph Test (n = 75; 41%) |
Ph Test (n = 107; 59%) |
P | ||
| Date* | Study period 1: Feb 1, 2017–Aug 31, 2019 (MSW, n = 119; female, n = 118) | 91 (76%) | 28 (24%) | <0.01 | 61 (52%) | 57 (48%) | <0.01 |
| Study period 2: Sept 1, 2019–July 31, 2021 (MSW, n = 51; female, n = 64) | 25 (49%) | 26 (51%) | 14 (22%) | 50 (78%) | |||
| Age, mean (IQR), y | 33 (24–39) | 37 (25–45) | 0.01 | 31 (23–37) | 29 (23–34) | 0.26 | |
| Race/Ethnicity* | Non-Hispanic White (MSW, n = 49; female, n = 74) | 26 (53%) | 23 (47%) | 0.02 | 29 (39%) | 45 (61%) | 0.46 |
| Non-Hispanic Black (MSW, n = 89; female, n = 46) | 70 (79%) | 29 (21%) | 22 (48%) | 24 (52%) | |||
| Hispanic (MSW, n = 10; female, n = 24) | 6 (60%) | 4 (40%) | 7 (29%) | 17 (71%) | |||
| Other/Unknown† (MSW, n = 22; female, n = 38) | 14 (64%) | 8 (36%) | 17 (45%) | 21 (55%) | |||
| Urogenital symptoms* | Present (MSW, n = 80; female, n = 97) | 61 (76%) | 19 (24%) | 0.03 | 40 (41%) | 56 (59%) | 0.10 |
| Absent (MSW, n = 90; female, n = 85) | 55 (61%) | 35 (39%) | 35 (41%) | 50 (59%) | |||
| Ever injected drugs‡ | 6 (55%) | 5 (45%) | 0.25 | 10 (43%) | 13 (57%) | 0.75 | |
| Cocaine/Crack use (in the last 12 mo)* | 3 (30%) | 7 (70%) | <0.01 | 0 (0%) | 13 (100%) | <0.01 | |
| Methamphetamine use (in the last 12 mo)* | 4 (40%) | 6 (60%) | 0.05 | 1 (6%) | 15 (94%) | <0.01 | |
| Received money for sex (in the last 12 mo)* | 0 (0%) | 0 (0%) | — | 0 (0%) | 3 (100%) | 0.14 | |
| Performed oral sex in the last 2 mo*,§ | Feb 1, 2017–Aug 31, 2019 (MSW, n = 78; female, n = 74) | 53 (68%) | 25 (32%) | 0.02 | 33 (45%) | 41 (55%) | 0.01 |
| Sept 1, 2019–July 31, 2021 (MSW, n = 20; female, n = 40) | 7 (35%) | 13 (65%) | 0.11 | 8 (20%) | 32 (80%) | 0.40 | |
| No. sexual partners in the last 2 mo, mean (SD) | 2 (1.5) | 3 (4.7) | 0.03 | 2 (1.2) | 2 (1.9) | 0.09 | |
MSW indicates heterosexual cis men; NG, Neisseria gonorrhoeae; Ph, pharyngeal. Statistically significant associations noted in bold.
Row percents.
Mixed race, Native Hawaiian, Pacific Islander, Asian, American Indian, Alaska Native, or unknown/declined to state race.
MSW missing 4 (2%) and women missing 19 (10%).
MSW missing 4 (2%) and women missing 25 (14%).
Test Positivity
Table 2 describes testing patterns and positivity among study subjects. One hundred eighteen NG contacts (34%) were positive at any anatomic site (urogenital or pharynx) including 45 MSW (27%) and 73 women (40%). In the overall study, 48 of all NG contacts (30%) tested at the pharynx (n = 161) were positive at that site, including 11 MSW (20%) and 37 women (35%). In study period 1, 28 of all NG contacts (33%) tested at the pharynx (n = 85) were positive at that site, including 6 MSW (21%) and 22 women (39%). In study period 2, 20 of all NG contacts (26%) tested at the pharynx (n = 76) were positive at that site, including 5 MSW (19%) and 15 women (30%). No significant difference in pharyngeal positivity was found between study periods 1 and 2, nor was a significant difference in pharyngeal positivity found when this analysis was stratified by gender or urogenital symptom status.
TABLE 2.
Testing and Test Positivity of NG Infection in NG Contacts by Anatomic Sites Tested
| Anatomic Site(s) Tested | All MSW and Female NG Contacts |
MSW NG Contacts |
Female NG Contacts |
|||
|---|---|---|---|---|---|---|
| No. Tested | NG Positive, n (%) | No. Tested | NG Positive, n (%) | No. Tested | NG Positive, n (%) | |
| GU only | 191 | 55 (29) | 116 | 30 (26) | 75 | 25 (33) |
| Ph only | 8 | 2 (25) | 1 | 1 (100) | 7 | 1 (14) |
| Both Ph and GU* | 153 | GU + 47 (31) | 53 | GU + 9 (17) | 100 | GU + 38 (38) |
| Ph+ 46 (30) | Ph+ 10 (19) | Ph+ 36 (36) | ||||
| Total | 352 | 118 (34) | 170 | 45 (27) | 182 | 73 (40) |
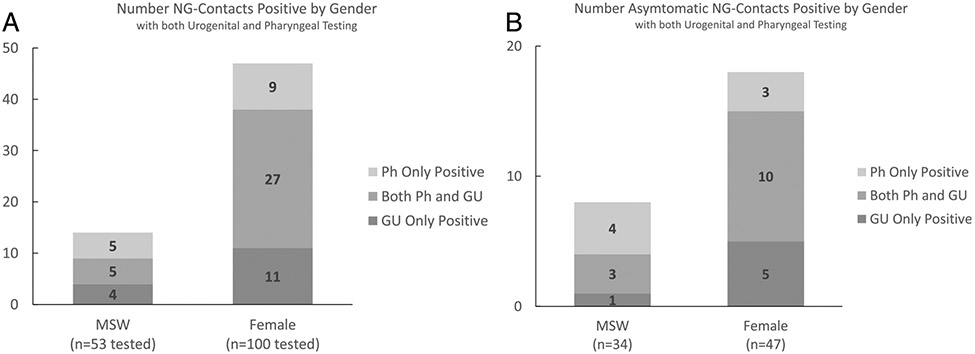
See Figures 1A and B for isolated pharyngeal NG positivity within those tested at both GU and Ph sites.
GU, urogenital; MSW, heterosexual cis men; NG, Neisseria gonorrhoeae; Ph, pharyngeal.
Among NG contacts with both urogenital and pharyngeal testing (n = 153), 46 of NG contacts (30%) tested positive at the pharynx: 10 of 53 MSW (19%) and 36 of 100 women (35%). Pharyngeal positivity was comparable to urogenital positivity. Among NG contacts with both urogenital and pharyngeal testing (n = 153), 47 of NG contacts (31%) were positive at the urogenital site: 9 of 53 MSW (17%) and 38 of 100 women (38%). Testing at both sites increased NG positivity overall by 38%, from 29% to 40%. Within those tested at both the urogenital and pharyngeal site, 5 of the 14 MSW (36%) and 9 of the 47 women (19%) with gonorrhea had pharyngeal infections in the absence of urogenital infections (Fig. 1A). Thus, without pharyngeal testing, 23% (n = 14) of NG infections (n = 61) would have been undiagnosed.
Figure 1.
A, NG contacts with both pharyngeal and urogenital testing, irrespective of urogenital symptom status: 19% (n = 10) of MSW and 36% (n = 36) of female NG contacts were positive at the pharynx. Thirty-six percent (n = 5) of all MSW NG-contact infections (n = 14) were isolated pharyngeal infection, and 19% (n = 9) of all female NG-contact infections (n = 47) were isolated pharyngeal infections. B, NG contacts without urogenital symptoms tested at both the pharyngeal and urogenital sites: Twenty-one percent (n = 7) of MSW and 28% (n = 13) of asymptomatic female NG contacts were positive at the pharynx. Fifty percent (n = 4) of asymptomatic MSW NG-contact infections (n = 8) were isolated pharyngeal infection, and 17% (n = 3) of asymptomatic female NG-contact infections (n = 18) were isolated pharyngeal infection. MSW indicates heterosexual cis men; NG, Neisseria gonorrhoeae; Ph, pharyngeal; U, urogenital.
Asymptomatic NG Contacts
Of the 352 NG contacts included in the study, 175 (50%) were asymptomatic at the time of presentation: 90 MSW and 85 women. Eighty-five asymptomatic NG contacts were tested at the pharynx: 35 MSW and 50 women (Table 1). There were no significant differences in age, race/ethnicity, or average number of sex partners between asymptomatic and symptomatic NG contacts tested at the pharynx.
Pharyngeal test positivity among all asymptomatic contacts tested was 25%: 23% (8/35 tested) and 28% (14/50 tested) among MSW and women, respectively. Table 3 describes demographics and behavioral characteristics of MSW and female contacts positive for NG at the pharynx by urogenital symptom status. Among asymptomatic NG contacts tested at both urogenital and pharyngeal sites, 7 of 34 MSW (21%) and 13 of 44 female NG contacts (30%) were NG positive at the pharynx. Among these asymptomatic contacts, 4 of the 8 MSW (50%) and 3 of the 18 women (17%) had a pharyngeal infection in the absence of urogenital NG (Fig. 1B). Testing at both sites increased NG positivity overall by 40%, from 25% to 35%. Twenty-seven percent (n = 7) of NG infections among the subset of asymptomatic contacts (n = 26) would have gone undiagnosed without pharyngeal testing.
TABLE 3.
Demographics and Behavioral Characteristics of MSW and Female NG Contacts Positive for NG at the Pharynx by Urogenital Symptom Status
| MSW NG Contacts |
Female NG Contacts |
|||
|---|---|---|---|---|
| Total (n = 11) | Asymptomatic (n = 8) | Total (n = 37) | Asymptomatic (n = 14) | |
| Date* | ||||
| Feb 1, 2017–Aug 21, 2019 | 6 (55%) | 4 (50%) | 22 (59%) | 10 (71%) |
| Sept 1, 2019–July 31, 2021 | 5 (45%) | 4 (50%) | 15 (41%) | 4 (29%) |
| Age, mean (IQR), y | 36 (24–45) | 36 (30–43) | 29 (22–33) | 26 (20–31) |
| Race/Ethnicity* | ||||
| Non-Hispanic White | 6 (55%) | 4 (50%) | 15 (%) | 4 (28.5%) |
| Non-Hispanic Black | 4 (36%) | 3 (37%) | 13 (%) | 5 (36%) |
| Hispanic | 1 (9%) | 1 (13%) | 3 (%) | 1 (7%) |
| Other/Unknown† | 0 (0%) | 0 (0%) | 6 (%) | 4 (28.5%) |
| NG positive at Urogenital Site‡ | 5 (50%) | 3 (43%) | 27 (75%) | 10 (77%) |
| Ever injected drugs§ | 2 (18%) | 0 (0%) | 6 (6%) | 1 (7%) |
| Cocaine/Crack use | 2 (18%) | 1 (13%) | 3 (3%) | 1 (7%) |
| Methamphetamine use | 1 (9%) | 0 (0%) | 6 (6%) | 1 (7%) |
| Performed oral sex in the last 2 mo* | 7 (70%) | 4 (57%) | 31 (91%) | 10 (90%) |
| No. sexual partners in the last 2 mo, mean (SD) | 6 (8.5) | 2 (1.8) | 3 (2.6) | 3 (4.1) |
Fifty-four MSWNG contacts were tested at the pharynx over the entire study period, of whom 35 were asymptomatic, and 107 female NG contacts were tested at the pharynx over the entire study period, of whom 50 were asymptomatic.
MSW, n = 1 missing; female, n = 3 missing.
Mixed race, Native Hawaiian, Pacific Islander, Asian, American Indian, Alaska Native, or unknown/declined to state race.
MSW, n = 1 missing; female, n = 1 missing.
MSW, n = 1 missing; female, n = 2 missing.
MSW indicates heterosexual cis men; NG, Neisseria gonorrhoeae.
Sexual Behavior
The majority of both MSW (58%) and women (63%) reported giving oral sex in the last 2 months. Similarly, among asymptomatic NG contacts, those without urogenital symptoms, the majority of both women (54%; n = 27) and MSW (63%; n = 22) reported performing oral sex in the last 2 months (although 26% of asymptomatic female NG contacts were missing these data). Overall, female NG contacts positive at the pharynx were significantly more likely to report giving oral sex compared with those who were NG negative at the pharynx. Ninety-one percent (n = 31) of women NG positive at the pharynx reported giving oral sex in the last 2 months, whereas only 71% of NG-negative female contacts reported recently giving oral sex to a male partner (P = 0.02). In contrast, MSW NG positivity at the pharynx was not associated with giving oral sex within the last 2 months. Sixty-four percent (n = 7) of MSW NG positive at the pharynx and 72% (n = 31) of MSW NG negative at the pharynx reportedly recently giving oral sex (P = 0.90). The lack of association between MSW pharyngeal positivity and cunnilingus held true within a stratified analysis by study period.
DISCUSSION
Among heterosexual male and female contacts to gonorrhea presenting to an urban SHC, 30% were infected with N. gonorrhoeae at the pharynx (33% in study period 1 and 26% in study period 2). It is important to note that, among asymptomatic persons infected with gonorrhea, 27% of cases were only infected at the throat and would have been missed in the absence of testing.
These results are consistent with the prevalence of pharyngeal NG in heterosexual male and female NG contacts reported by a recent study conducted in Australia.10 Chow and colleagues10 found that 18% of MSW and 46% of female asymptomatic NG contacts at the Melbourne SHC had NG in their pharynx. In Melbourne, Australia, only asymptomatic MSW were included in the study population because they use a test and treat strategy.7,10 In the United States, persons reporting sexual exposure to NG receive treatment empirically as a public health control policy known as epidemiologic treatment. However, there have been calls to abandon this practice in the United States as they have in parts of Australia and the United Kingdom, given rapidly rising rates of antimicrobial resistant N. gonorrhoeae and few novel antibiotics in the pipeline.2,4,14-16 Should the United States move to that approach, our analysis reinforces the fact that clinics will need to test the pharynxes of heterosexual men and women to not miss infections.
One method of epidemiologic treatment that will need to be reexamined is expedited partner therapy (EPT). Expedited partner therapy is a form of epidemiologic treatment whereby the sex partner(s) of the infected patient is/are treated with oral medications without an evaluation by a provider.17 The CDC currently only recommends EPT for heterosexual cisgender men and women.1 Until recently, the recommended EPT regimen for gonorrhea was dual therapy with cefixime 400 mg and azithromycin 1 g; this regimen has been shown to decrease reinfection rates in the original patient in a large-scale randomized clinical trial.17 However, in the 2020 Update to the Gonorrhea Treatment Guidelines, the CDC removed co-treatment with azithromycin for EPT because of rising rates of NG azithromycin resistance and consequently increased the dose of cefixime from 400 to 800 mg1,9—a regimen that has not been evaluated for EPT. Based on very limited data from the combined results of 2 small studies (n = 11), the efficacy of 800 mg of cefixime along for pharyngeal gonorrhea is less than 85%.18,19 Insofar as the new recommended EPT regimen results in uneradicated NG infections at the pharynx, this could lead to reinfections and sustain community transmission.20 These data also reinforce the need to ensure that all recommended treatment regimens eradicate NG at the pharynx.
One curious finding in our study was the lack of association between performing oral sex and pharyngeal gonorrhea among MSW. Although this is not a novel finding,21-23 it certainly begs the question: Is there another behavior that is commonly practiced and a relatively efficient mode of gonococcal transmission? Perhaps kissing or oral-anal sex might explain transmission if oral-vaginal sex does not. There is limited evidence that kissing does transmit gonorrhea between oropharynxes,24-26 but further investigation into transmission of NG both to and from the oropharynx is warranted.
Despite being a retrospective analysis of a relatively small number of NG contacts, our study is one of the first in the United States to describe the prevalence of pharyngeal NG in women and heterosexual male NG contacts in the modern NAAT era. One key limitation is that clinic guidelines surrounding pharyngeal testing evolved over the study period. In addition, implementation of pharyngeal testing of heterosexuals was not systematically used by clinic providers—not all persons who reported oral sex were tested at the pharynx during study period 1, nor were all NG contacts tested at the pharynx during study period 2. Evidence for this potential for bias is most notable for MSW (Table 1). It remains unclear if the differences in testing certain MSW were due to clinician practice or patient refusal. Moreover, it remains unclear to what extent possible testing bias affected the positivity rates, particularly in MSW for whom the overall number of positive pharyngeal NG cases was low. Positivity may have also been affected using culture only for NG diagnosis during study period 1. It has been shown that culture of NG from the pharynx is only 28% sensitive27; thus, we may have undercounted cases during that period. However, because no difference in pharyngeal NG positivity was observed between study period 1 and 2, there may exist a relative balance of bias direction between patient population testing strategy and NG laboratory testing method. For instance, during study period 1, prevalence may have been overestimated by patient testing strategy (the testing only of NG contacts who reported recently giving oral sex) while simultaneously being underestimated by NG laboratory testing by culture alone. Regardless, we found a relatively high proportion of pharyngeal gonorrhea among MSW and female NG contacts, even in the absence of urogenital infection.
Lastly, given the relatively high prevalence of pharyngeal NG among heterosexual male and female contact to NG, our findings urge further investigation into the prevalence of pharyngeal gonorrhea among all MSW and women, not just NG contacts; we also suggest additional study into the efficacy of cefixime 800 mg at the pharynx given its use for EPT. Until we understand the burden of pharyngeal infection in MSW and women, and have the drugs to treat it effectively, the elimination of untreated NG reservoirs within the sexually active population will likely be unattainable.
Acknowledgments
Funding provided by the National Institutes of Health: T32 AI007140-43 and by funding from the US Centers for Disease Control and Prevention's Epidemiology and Laboratory Capacity for the Prevention and Control of Infectious Diseases Cooperative Agreement (CK19-1904) under the Strengthening the US Response to Resistant Gonorrhea project.
Footnotes
Conflict of Interest and Sources of Funding: L.A.B. has received research support from Hologic, SpeeDx, and Nabriva, unrelated to the current work. O.O.S. and M.R.G. have received research support from SpeeDx and Hologic, unrelated to the current work. S.E.M., A.B., and C.S.T. have none to declare.
REFERENCES
- 1.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowlinson E, Golden MR, Berzkalns A, et al. Epidemiologic treatment for contacts to Neisseria gonorrhoeae and Chlamydia trachomatis infection in sexually transmitted disease clinic patients in Seattle, WA; 1994 to 2018. Sex Transm Dis 2020; 47:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian S, Foster R, Bourne C, et al. Neisseria gonorrhoeae positivity in clients presenting as asymptomatic contacts of gonorrhoea at a sexual health centre. Sex Health 2020; 17:187–191. [DOI] [PubMed] [Google Scholar]
- 4.Mensforth S, Radcliffe K. Is it time to reconsider epidemiological treatment for gonorrhoea? Int J STD AIDS 2018; 29:1043–1044. [DOI] [PubMed] [Google Scholar]
- 5.Fifer H, Saunders J, Soni S, et al. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020; 31:4–15. [DOI] [PubMed] [Google Scholar]
- 6.Fifer H, Saunders J, Soni S, et al. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae (2019) [Internet]. Available at: https://www.bashhguidelines.org/media/1208/gc-2019.pdf. Accessed November 20, 2020. [Google Scholar]
- 7.Gonorrhoea treatment guidelines—Melbourne Sexual Health Centre (MSHC) [Internet]. Available at: https://www.mshc.org.au/health-professionals/treatment-guidelines/gonorrhoea-treatment-guidelines. Accessed August 24, 2022. [Google Scholar]
- 8.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 9.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow EPF, Chen MY, Williamson DA, et al. Oropharyngeal and genital gonorrhea infections among women and heterosexual men reporting sexual contact with partners with gonorrhea: Implication for oropharyngeal testing of heterosexual gonorrhea contacts. Sex Transm Dis 2019; 46:743–747. [DOI] [PubMed] [Google Scholar]
- 11.Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis 2011; 38:1036–1039. [DOI] [PubMed] [Google Scholar]
- 12.Gose SO, Soge OO, Beebe JL, et al. Failure of azithromycin 2.0 g in the treatment of gonococcal urethritis caused by high-level resistance in California. Sex Transm Dis 2015; 42:279–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raphael BH, Pham CD, Sharpe S, et al. Implementation and evaluation of gradient strip antimicrobial susceptibility testing in US public health laboratories to respond to resistant gonorrhea. Sex Transm Dis 2021; 48(12S):S157–S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low S, Varma R, McIver R, et al. Provider attitudes to the empiric treatment of asymptomatic contacts of gonorrhoea. Sex Health 2020; 17:155–159. [DOI] [PubMed] [Google Scholar]
- 15.Pearce E, Chan DJ, Smith DE. Empiric antimicrobial treatment for asymptomatic sexual contacts of sexually transmitted infection in the era of antimicrobial resistance: Time to rethink? Int J STD AIDS 2019; 30:137–139. [DOI] [PubMed] [Google Scholar]
- 16.Rasul R, McIver R, Patel P, et al. Non-empirical management of asymptomatic chlamydia and gonorrhoea reduces unnecessary antibiotic use fivefold: A before and after study. Sex Transm Infect 2022; sextrans-2021-055382. [DOI] [PubMed] [Google Scholar]
- 17.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005; 352:676–685. [DOI] [PubMed] [Google Scholar]
- 18.Handsfield HH, McCormack WM, Hook EW 3rd, et al. A comparison of single-dose cefixime with ceftriaxone as treatment for uncomplicated gonorrhea. The Gonorrhea Treatment Study Group. N Engl J Med 1991; 325:1337–1341. [DOI] [PubMed] [Google Scholar]
- 19.Singh AE, Gratrix J, Martin I, et al. Gonorrhea treatment failures with oral and injectable expanded spectrum cephalosporin monotherapy vs dual therapy at 4 Canadian sexually transmitted infection clinics, 2010–2013. Sex Transm Dis 2015; 42:331–336. [DOI] [PubMed] [Google Scholar]
- 20.Barbee LA, St Cyr SB. Management of Neisseria gonorrhoeae in the United States: Summary of Evidence From the Development of the 2020 Gonorrhea Treatment Recommendations and the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infection Treatment Guidelines. Clin Infect Dis 2022; 74(Suppl_2):S95–S111. [DOI] [PubMed] [Google Scholar]
- 21.Wiesner PJ, Tronca E, Bonin P, et al. Clinical spectrum of pharyngeal gonococcal infection. N Engl J Med 1973; 288:181–185. [DOI] [PubMed] [Google Scholar]
- 22.Tice AW Jr., Rodriguez VL. Pharyngeal gonorrhea. JAMA 1981; 246:2717–2719. [PubMed] [Google Scholar]
- 23.Bro-Jorgensen A, Jensen T. Gonococcal pharyngeal infections. Report of 110 cases. Br J Vener Dis 1973; 49:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow EPF, Cornelisse VJ, Williamson DA, et al. Kissing may be an important and neglected risk factor for oropharyngeal gonorrhoea: A cross-sectional study in men who have sex with men. Sex Transm Infect 2019; 95:516–521. [DOI] [PubMed] [Google Scholar]
- 25.Cornelisse VJ, Williamson D, Zhang L, et al. Evidence for a new paradigm of gonorrhoea transmission: Cross-sectional analysis of Neisseria gonorrhoeae infections by anatomical site in both partners in 60 male couples. Sex Transm Infect 2019; 95:437–442. [DOI] [PubMed] [Google Scholar]
- 26.Templeton DJ, Jin F, McNally LP, et al. Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia. Sex Transm Infect 2010; 86:90–96. [DOI] [PubMed] [Google Scholar]
- 27.Nash EE, Pham CD, Raphael B, et al. Impact of anatomic site, specimen collection timing, and patient symptom status on Neisseria gonorrhoeae culture recovery. Sex Transm Dis 2021; 48(12S):S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]