Abstract
Shp-2 is a cytoplasmic tyrosine phosphatase that contains two Src homology 2 (SH2) domains at the N terminus. Biochemical data suggests that Shp-2 acts downstream of a variety of receptor and cytoplasmic tyrosine kinases. A targeted deletion mutation in the N-terminal SH2 (SH2-N) domain results in embryonic lethality of homozygous mutant mice at midgestation. In vitro embryonic stem (ES) cell differentiation assays suggest that Shp-2 might play an important role in hematopoiesis. By aggregating homozygous mutant (Shp-2−/−) ES cells and wild-type (WT) embryos, we created Shp-2−/−-WT chimeric animals. We report here an essential role of Shp-2 in the control of blood cell development. Despite the widespread contribution of mutant cells to various tissues, no Shp-2−/− progenitors for erythroid or myeloid cells were detected in the fetal liver and bone marrow of chimeric animals by using the in vitro CFU assay. Furthermore, hematopoiesis was defective in Shp-2−/− yolk sacs. In addition, the Shp-2 mutation caused multiple developmental defects in chimeric mice, characterized by short hind legs, aberrant limb features, split lumbar vertebrae, abnormal rib patterning, and pathological changes in the lungs, intestines, and skin. These results demonstrate a functional involvement of Shp-2 in the differentiation of multiple tissue-specific cells and in body organization. More importantly, the requirement for Shp-2 is more stringent in hematopoiesis than in other systems.
Mammalian hematopoiesis occurs in successive waves at various anatomical sites during embryogenesis, including the yolk sac and fetal liver, spleen, and bone marrow. It remains to be understood, however, how this developmental scheme functions at the molecular level. An important issue is the identification of genes involved in the developmental decisions leading to the commitment and differentiation of hematopoietic stem and progenitor cells.
Much attention has been paid in recent years to genes that are predominantly expressed in the hematopoietic compartment (22). Inactivation of genes such as those for GATA-1, GATA-2, Pu.1, c-Myb, SCL/tal-1, Ikaros, and AML-1 by gene targeting in mouse embryonic stem (ES) cells has permitted the assessment of their roles in hematopoiesis in mutant mouse models (8, 16, 21, 23, 30, 37). However, the homozygous mutant mice often die early, at midgestation, due to a failure of embryonic hematopoiesis, which precludes detailed analysis of gene functions in blood cell development. Two experimental approaches, i.e., in vitro ES cell differentiation assay and chimeric animal analysis, have been used as alternatives to gain insights into the roles of these genes in hematopoiesis (11, 24, 27). GATA-1 deficiency leads to developmental arrest and cell apoptosis at the proerythroblast stage, and ablation of the GATA-2 gene suppresses the generation of all definitive hematopoietic cell lineages in chimeric animals (37–39). SCL/tal-1 is a helix-loop-helix transcription factor that was identified as a T-cell leukemia oncogene (1). SCL/tal-1−/− ES cells failed to give rise to erythrocytes, myeloid cells, megakaryocytes, and mast cells as well as T and B lymphocytes either in vitro or in vivo in chimeric animals (24, 27). These results suggest that SCL/tal-1 functions very early in the hematopoietic hierarchy, possibly in the specification of ventral mesoderm to blood cell progenitors (24, 27). Flk1 is a receptor tyrosine kinase that binds vascular endothelial growth factor (VEGF) (4, 13), and Flk1−/− embryos die at midsomite stage (32). Chimeric analysis failed to detect the contribution of Flk1−/− ES cells to any hematopoietic progenitor cells in the yolk sac and fetal liver, strongly suggesting an intrinsic requirement for Flk1 during primitive and definitive hematopoiesis (31).
Programming of hematopoietic progenitor cell commitment and differentiation is orchestrated to a large extent by environmental signals, particularly cytokines. Activated cytokine receptors transmit signals from the cell surface into cells to elicit proper cellular responses. However, little is known regarding the intracellular signal transduction mechanisms involved in the control of hematopoiesis. Shp-2 is a widely expressed cytoplasmic tyrosine phosphatase that contains two Src homology 2 (SH2) domains (6, 18). Accumulating biochemical data suggests that Shp-2 participates in signal transmission from a variety of cytokine receptors and might function upstream of extracellular signal-regulated kinase (Erk) (14, 19, 28, 33–36). To define the biological function of Shp-2, we introduced a targeted mutation into the murine Shp-2 locus, which resulted in an internal deletion of 65 amino acids in the N-terminal SH2 (SH2-N) domain of Shp-2 (29). Homozygous Shp-2 mutant mice die at midgestation with multiple defects in mesodermal patterning, while heterozygous mutants appear normal. A similar phenotype was observed through the microinjection of a catalytically inactive mutant mRNA of Shp-2 into Xenopus embryos (34). Taken together, these results suggest that the mutant protein without the intact SH2-N domain does not function in a dominant-negative manner but rather acts as a loss-of-function molecule. To gain further insights into the role of Shp-2 in the differentiation of hematopoietic and other cell lineages, we isolated homozygous mutant (Shp-2−/−) ES cell lines. By performing in vitro ES cell differentiation assays, we demonstrated that the deletion mutation in the Shp-2 locus decreased the ability of primitive and definitive hematopoietic cells to develop in vitro (26).
However, it is not clear if the effect of the Shp-2 mutation on blood cell development is cell autonomous. Since Shp-2 is ubiquitously expressed, it is also of great importance to define the Shp-2 function in the later development of many other systems. In this study, we generated and characterized Shp-2−/−–wild-type (WT) chimeric mice. Although Shp-2−/− cells contributed to a wide variety of tissues, accompanied by pathological changes, hematopoietic progenitor cells of Shp-2−/− origin were barely detectable in the bone marrow and fetal liver by using the CFU assay in vitro. Furthermore, the hematopoietic activity was also defective in Shp-2−/− yolk sacs. These results indicate a more strict requirement for Shp-2 phosphatase in mammalian hematopoiesis than in the differentiation of many other cell types.
MATERIALS AND METHODS
Cell lines and animals.
A targeted mutant allele of Shp-2 was created by homologous recombination in mouse ES cell line R1 that deleted exon 3, coding for amino acid residues 46 to 110 in the SH2-N domain of Shp-2 (29). Shp-2−/− ES cell lines were generated by selection of Shp-2+/− cells in high concentrations of G418 (15, 26). All ES cell lines were maintained in complete ES cell medium containing 15% fetal calf serum (FCS) (Hyclone, Logan, Utah) and 1,000 U of leukemia inhibitory factor (LIF)/ml. CD-1 mice were purchased from Harlan Sprague Dawley (Indianapolis, Ind.).
Generation of chimeric animals.
Shp-2+/+ and Shp-2−/− ES cells of 129/Sv origin were aggregated with WT CD1 embryos to generate chimeric animals as described previously (17). Mouse embryos at the eight-cell stage, 2.5 days postcoitum (d.p.c.), were flushed from the oviduct. After removal of the zonae pellucidae with Tyrode’s solution, individual embryos were aggregated with a gently trypsinized ES cell clump (around eight cells) under a dissection microscope. These aggregates were cultured in M16 medium overnight at 37°C, and the aggregated embryos were then transferred into the uterus of a 2.5-d.p.c. pseudopregnant CD1 foster mother. Chimeric pups were identified by the agouti color of their eyes and subsequently of the coat hairs. Genotyping was performed by Southern blotting or PCR analysis of genomic DNA extracted from embryos or various tissues of chimeric animals (26, 29).
Hematopoietic progenitor assays.
To assess the contribution of Shp-2−/− and Shp-2+/− ES cells to hematopoietic progenitor cells in adult chimeras, bone marrow cells were flushed out from two femurs of chimeric animals and disaggregated into single-cell suspensions. Nucleated cells (5.0 × 104/ml) were seeded into a semisolid CFU assay culture system as described elsewhere (2), which contains α-minimal essential medium (MEM), 30% FCS, 5% pokeweed mitogen-stimulated mouse spleen cell conditioned medium, erythropoietin (2 U/ml; Amgen Corp., Thousand Oaks, Calif.), murine stem cell factor (SCF) (50 ng/ml; Immunex, Seattle, Wash.), glutamine (10−4 M), β-mecaptoethanol (3.3 × 10−5 M), hemin (100 μM; Eastman Kodak, Rochester, N.Y.), and 0.9% methylcellulose. CFU assay cultures were incubated at 37°C in a 5% CO2 moisture-saturated incubator. After 6 to 7 days of incubation, colonies from erythroid (BFU-E), granulocyte-macrophage (CFU-GM), and multipotential (CFU-GEMM) progenitor cells were scored.
To detect hematopoietic progenitor cells in the fetal liver, individual livers of chimeric embryos at 14.5 d.p.c. were dissected free of other tissues in α-MEM plus 15% FCS, disaggregated mechanically into single-cell suspensions, and washed in α-MEM containing 15% FCS. Nucleated cells were subjected to CFU assay as described above.
For the assay of hematopoietic progenitors from yolk sacs, 8.5- to 9.0-d.p.c. yolk sacs were carefully dissected free of maternal tissues under sterile conditions in α-MEM plus 15% FCS and were disaggregated by passing them through a 22-gauge needle with a syringe. After being washed once in a α-MEM, whole yolk sac cells were plated for CFU assay as above. Colonies from whole yolk sac cells were scored after 7 days of incubation.
Skeletal preparation.
Newborn pups were eviscerated, fixed in 100% ethanol for 4 days, kept in acetone for 3 days, and then rinsed with water. The specimens were subsequently stained in alizarin red S (0.005%), alcian blue 8GX (0.015%), acetic acid (5%), and ethanol (85%) containing staining solution for 10 days. After brief rinsing with water, the specimens were kept in 20% glycerol–1% potassium hydroxide at 37°C for 24 h and then at room temperature until tissues were completely cleared. Anatomical changes were examined and photographed under a dissection microscope. Ossified bones were stained red, while the cartilages were blue (12).
Histological analysis.
Whole embryos or surgically removed tissue samples from chimeric mice at different stages were fixed in 10% buffered formalin, dehydrated through graded alcohol solutions, embedded in paraffin, sectioned at 5 μm, and processed for hematoxylin and eosin staining following standard protocols.
RESULTS
Generation of chimeric animals with Shp-2 mutant ES cells.
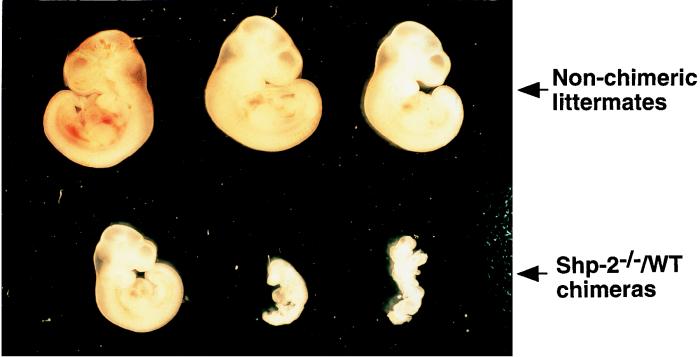
Heterozygous (Shp-2+/−) and homozygous (Shp-2−/−) mutant ES cell lines for the targeted mutation were established as reported previously (26, 29). Chimeric animals were generated by aggregating Shp-2+/− or Shp-2−/− ES cells with WT CD1 embryos (17). The production efficiencies of chimeric mice from Shp-2−/− and Shp-2+/− ES cells at different developmental stages were compared. Genotyping of embryos at 10.5 d.p.c. indicated that 33 of 57 (58%) were Shp-2−/−–WT chimeras, while 11 of 32 (34%) were Shp-2+/−–WT chimeras. Therefore, the chimera-forming efficiency of Shp-2−/− ES cells was much higher than that of Shp-2+/− ES cells. However, the numbers of newborn pups were similar for Shp-2+/−–WT (6 of 20 [30%]) and Shp-2−/−–WT (19 of 68 [28%]) chimeras (identified by the agouti coloring of their eyes). The differences between the frequency in embryos and that in the viable births suggest a partial embryonic lethality of Shp-2−/−–WT chimeras. Indeed, we found that some heavily chimeric animals died in utero between 10.5 and 12.5 d.p.c. (Fig. 1), with a phenotype similar to that of the Shp-2−/− embryos, with severe defects in mesodermal patterning and development of axial structures (29). On the other hand, the higher frequency of Shp-2−/−–WT than Shp-2+/−–WT chimeric embryos detected at 10.5 d.p.c. might reflect a reduced differentiation capacity of Shp-2−/− ES cells in culture. We have reported that Shp-2−/− ES cells had enhanced sensitivity to the differentiation-inhibitory effect of LIF (25). In routine ES cell cultures, a higher proportion of totipotent cells was detected in Shp-2−/− than in WT or Shp-2+/− ES cell populations (25).
FIG. 1.
Heavily chimeric Shp-2−/−–WT embryos were embryo lethal at midgestation. Pregnant foster females at 10.5 d.p.c. were sacrificed, and embryos were dissected from maternal tissues and photographed under a dissecting microscope, followed by genomic DNA extraction and PCR analysis. Chimerism was determined by PCR detection of the Shp-2 mutant allele in embryonic cells.
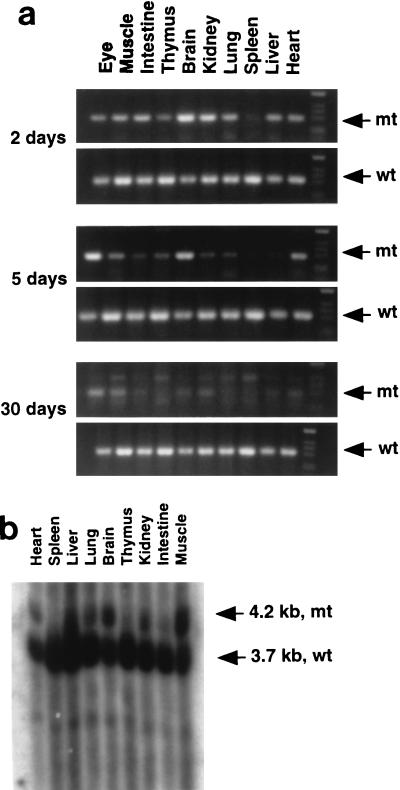
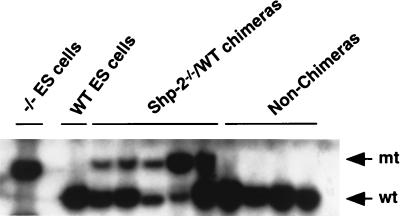
The contribution of Shp-2−/− cells to various tissues in chimeric animals was assessed by PCR and Southern blot analyses that distinguish WT and mutant Shp-2 alleles. Shown in Fig. 2a are representative results for PCR analysis of relative contributions of Shp-2−/− and WT cells to neonatal animals that survived 2, 5, and 30 days. The life span of chimeras seemed to be inversely correlated with the contribution of Shp-2−/− cells. One animal that died 2 days after birth had a relatively high contribution of Shp-2−/− cells to many tissues examined, including brain, heart, kidney, lung, liver, and muscle tissues. The other two mice, which survived for 5 and 30 days, had increasingly lower contributions of mutant cells. Shown in Fig. 2b is the result of Southern blot analysis of various tissues isolated from another chimeric animal that survived for 5 days. Notably, little or no contribution of Shp-2−/− cells in these chimeras was detected in hematopoietic or lymphocytic organs, such as the spleen and thymus, suggesting a block to blood cell development by the Shp-2 mutation in vivo.
FIG. 2.
Contribution of Shp-2−/− cells to various tissues in chimeric mice. (a) Three chimeric mice that survived for 2, 5, and 30 days were dissected. Genomic DNA was extracted from the eyes, muscle, intestine, thymus, brain, kidney, lung, spleen, liver, and heart. Shp-2 mutant (mt) and WT (wt) alleles were detected by PCR with specific pairs of primers (29). The density of DNA bands reflects the relative contributions of Shp-2−/− ES cells versus those of the parental embryo. (b) Tissues from a chimeric pup were dissected, and isolated genomic DNA was subjected to Southern blot analysis with a [α-32P]dCTP-labelled specific probe (29). The relative contributions from mutant cells and the parental embryo were determined by comparing the densities of a 4.2-kb mutant DNA band and a 3.7-kb WT DNA band.
Lack of Shp-2−/− progenitor cells in bone marrow.
As previously reported, we found that the Shp-2 mutation dramatically reduced the development of erythroid and myeloid progenitors from ES cells in vitro (26). By generating chimeric animals, we hoped to examine the effect of the Shp-2 mutation on hematopoiesis in vivo by assessing hematopoietic progenitor cells in the bone marrow and fetal liver. As Shp-2+/− and Shp-2−/− cells contain one and two copies of the neomycin resistance gene, respectively, mutant progenitor cells in chimeras were detected by a combination of G418 selection in CFU assays and PCR analysis of isolated cell colonies for the Shp-2 mutant allele. In preliminary experiments, we set up the CFU assay with cells isolated from Shp-2+/−–WT chimeras by adding different concentrations of G418 in the culture medium. Colonies resistant to 1 mg of G418/ml were individually picked for PCR analysis, and all clones were found to be of Shp-2+/− origin (data not shown). No colonies grew from WT CD1 bone marrow cells under these selection conditions. In subsequent experiments, CFU assays were set up in triplicate cultures with or without G418 (1.25 mg/ml) to assess the relative contribution of Shp-2+/− or Shp-2−/− ES cells versus that of the WT host embryo. This method has been successfully used in evaluating the contribution of SCL/tal-1−/− ES cells to blood cell lineages in chimeric animals (27).
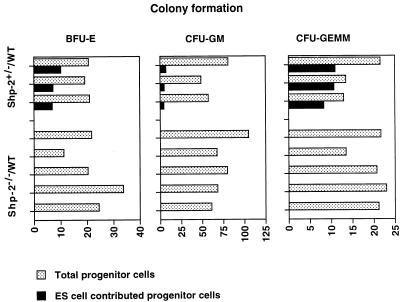
To examine the effect of the Shp-2 mutation on adult blood cell development, we isolated bone marrow cells and set up the CFU assay for BFU-E, CFU-GM, and CFU-GEMM cells. We examined three Shp-2+/−–WT and five Shp-2−/−–WT adult animals; the results are shown in Fig. 3. While 7.9 to 79.3% of the colonies of BFU-E, CFU-GM, and CFU-GEMM cells were derived from Shp-2+/− ES cells, no contribution of Shp-2−/− cells to erythroid or myeloid cell lineages was found. This indicates that Shp-2−/− ES cells failed to contribute to definitive blood cell development even in a normal bone marrow environment.
FIG. 3.
Detection of hematopoietic progenitors in the bone marrow of Shp-2−/−–WT chimeras. Bone marrow cells of two femurs from each of five Shp-2−/−–WT and three Shp-2+/−–WT chimeras were flushed out and disaggregated into single-cell suspensions. Nucleated cells (5.0 × 104/ml) were seeded into semisolid methylcellulose CFU assay culture systems with and without G418 at a concentration of 1.25 mg/ml, as described in the text. After 6 to 7 days of incubation, colonies of BFU-E, CFU-GM, and CFU-GEMM cells grown in cultures without G418 were scored as total progenitor cells, and colonies grown in G418-containing medium were enumerated as mutant ES cell-derived progenitor cells.
The Shp-2 mutation blocks definitive hematopoiesis in the fetal liver.
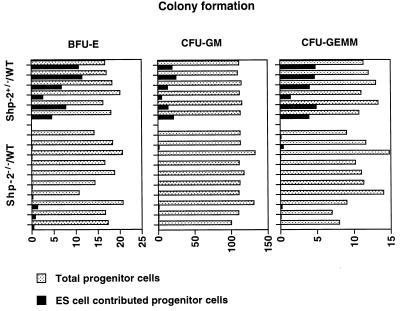
The absence of Shp-2−/− cells in the bone marrow of chimeras described above could be due to the poor contribution of Shp-2−/− cells to surviving chimeras in general (Fig. 2). To further define the effect of the Shp-2 mutation on blood cell development, we examined hematopoietic progenitor cells in the livers of chimeric embryos at 14.5 d.p.c. Fetal livers were carefully dissected free of maternal tissues and other embryonic tissues and disaggregated mechanically into single-cell suspensions. The cells were plated in a CFU assay system which contained combinations of hematopoietic growth factors with or without G418. Other portions of the embryos were used for DNA extraction and Southern blot analysis. As shown in Fig. 4, of 10 Shp-2−/−–WT chimeric embryos examined, none had more than 1% contribution from the Shp-2−/− cells to hematopoietic progenitors in the fetal liver, although the contribution by the mutant ES cells in whole animals was 31 to 63%, according to the Southern blot analysis (Fig. 5). Meanwhile, the contribution of Shp-2+/− ES cells to hematopoietic progenitors in six Shp-2+/−–WT chimeric embryos was from 4.5 to 67.6%, a frequency consistent with the contribution in whole animals. Therefore, despite the significant contribution of Shp-2−/− ES cells to many other tissues in chimeras, Shp-2−/− hematopoietic progenitor cells in the fetal liver and bone marrow were hardly detectable.
FIG. 4.
Definitive hematopoietic cell development in chimeric fetal livers. Shp-2−/−–WT and Shp-2+/−–WT chimeric embryos were dissected at 14.5 d.p.c. Individual fetal livers were isolated in α-MEM plus 15% FCS and disaggregated mechanically into single-cell suspensions. Nucleated cells (2.5 × 104/ml) were plated for CFU assays as described in the legend to Fig. 3. After 6 to 7 days of culture, total and ES cell-contributed BFU-E, CFU-GM, and CFU-GEMM progenitor cells were scored for each fetal liver.
FIG. 5.
Contribution of Shp-2−/− cells in whole embryos. Chimeric and nonchimeric embryos were dissected at 14.5 d.p.c. and subjected to genomic DNA extraction. Southern blot analysis was performed for determination of the relative contribution of Shp-2−/− cells versus that of the parental embryo, as described in the legend to Fig. 2b. Densitometry of the WT (wt) and mutant (mt) bands showed that the percent contribution from ES cells for the five chimeras, from left to right, was 31.1, 35.1, 44.8, 63.0, and 41.4.
Hematopoiesis was deficient in Shp-2−/− yolk sacs.
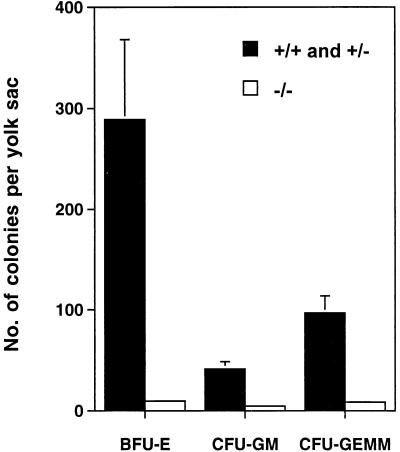
In order to determine at which stage hematopoiesis was blocked by the Shp-2 mutation, embryos were dissected to examine hematopoiesis in yolk sacs at 8.5 to 9.0 d.p.c. Yolk sacs were individually dissected and dissociated into single-cell suspensions for CFU assay. The data shown in Fig. 6 indicates that hematopoietic activity in Shp-2−/− embryos was severely suppressed, although Shp-2+/− embryos had the WT phenotype. We had found previously that Shp-2−/− yolk sacs appeared abnormally thin and wrinkled (29). Thus, Shp-2 plays a critical role in both primitive and definitive blood cell development and might act at a very early stage of hematopoietic stem and/or progenitor cell commitment and differentiation.
FIG. 6.
Defective hematopoiesis in Shp-2−/− yolk sacs. Yolk sacs at 8.5 to 9.0 d.p.c. from Shp-2+/− × Shp-2+/− crosses were carefully dissected free of maternal tissues under sterile conditions in α-MEM plus 15% FCS and were disaggregated by passing them through a 22-gauge needle. After being washed once in α-MEM, whole yolk sac cells were plated for the CFU assay. Error bars indicate standard deviations.
Multiple developmental defects in Shp-2−/−–WT chimeras.
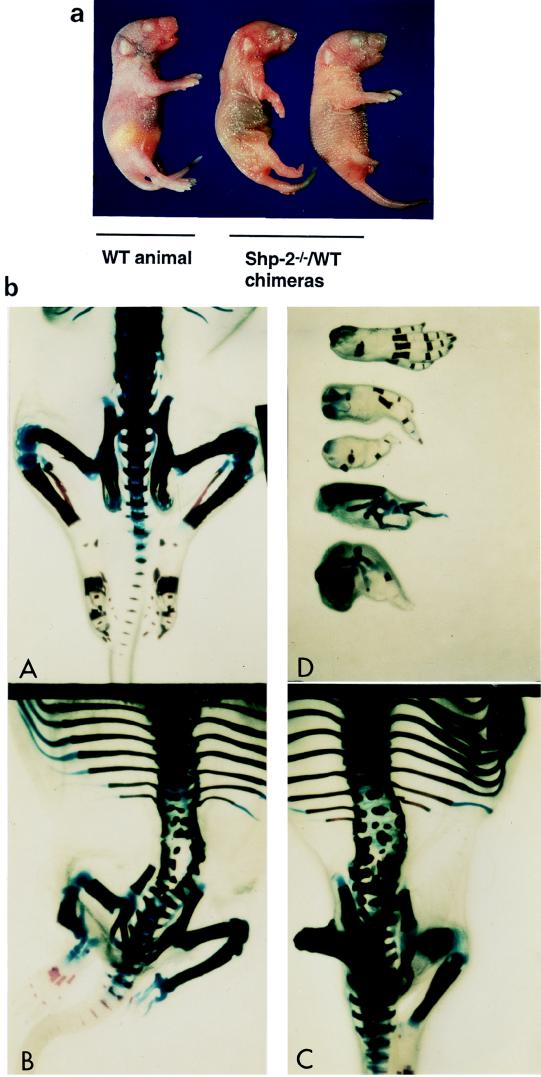
As revealed by PCR and Southern blot analyses (Fig. 2), Shp-2−/− cells contributed to many other nonhematopoietic tissues in chimeras, with high levels in muscle and brain tissues. An obvious defect in terminally developed Shp-2−/−–WT chimeras was the abnormal formation of the hind limbs, lumbar vertebrae, and rib patterning (Fig. 7). The development of hind limbs either stopped with the femur stage or continued to the end with incomplete digit formation. In total, 78% of Shp-2−/−–WT chimeras had short hind legs or abnormal limb features on one or both sides. In many chimeric animals, the structures of lumbar vertebrae were split, with several ossification centers and a large cell mass in one vertebral body. Abnormal patterning of the ribs often resulted in the formation of more or fewer than the 13 pairs found in WT animals. Two costal cartilages were combined before the junction of the costal cartilage and the sternebrae. These results suggest that Shp-2 is involved in mediating the development of terminal and skeletal structures.
FIG. 7.
Abnormalities of terminal and skeletal structures in Shp-2−/−–WT chimeras. (a) Examples from one nonchimeric animal and two chimeric animals are shown. Chimeras were recognized by agouti eye color at birth. The abnormality in hind-leg development was obvious. (b) Newborn Shp-2−/−–WT chimeric pups and nonchimeric controls were eviscerated and fixed in 100% ethanol and acetone. Skeletal specimens were prepared as described in the text. Structural changes were examined and photographed under a dissecting microscope. (c) WT animal; (A and D) typical representatives of Shp-2−/−–WT chimeras; (B) WT mouse hind foot with five fingers and four different abnormal feet from Shp-2−/−–WT chimeric mice.
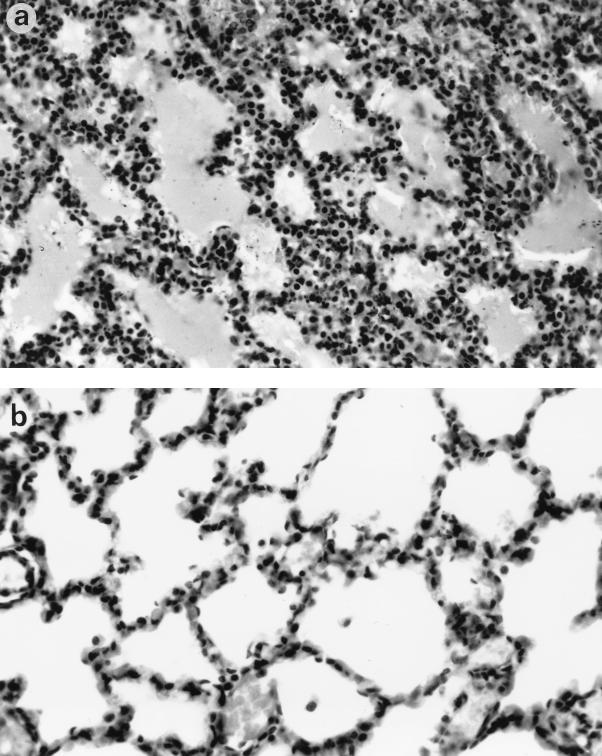
Anatomical and histological examinations also revealed developmental defects in the lung and intestine in chimeras. Consistent with the breathing difficulties observed in the chimeric neonates, atelectasis within the lung tissue of many chimeras was detected (Fig. 8a), similar to what is often seen in immature human babies with respiratory distress syndrome (20). The alveolar septae were thicker and hypercellular compared to the thin alveolar epithelium of a normal lung (Fig. 8b). A severe gastrointestinal problem was also noticed, as many newborn chimeric mice had feeding problems, with no milk observed in the stomach upon dissection. The intestines were also distended, with a very thin muscle layer, and were often dilated with free gas. Twenty-eight of 42 (66.7%) Shp-2−/−–WT chimeric mice were found dead at birth or died in a couple of days with obvious breathing or feeding difficulties. In contrast, none of the chimeric mice derived from Shp-2+/− or WT ES cells were found dead or had any developmental defects. Taken together, these results indicate that Shp-2 is essential for mouse hematopoiesis and is also involved in mediating development of many other tissues.
FIG. 8.
Histopathological changes in the lungs of Shp-2−/−–WT chimeric mice. (a) Mutant lung section, with hypercellular thickened alveolar walls; (b) lung of normal newborn, with thin alveolar walls and well-developed alveolar spaces. Magnification, ×100. Hematoxylin and eosin staining was used.
DISCUSSION
In this report, we present evidence that Shp-2 is involved in mediating the normal development of multiple tissues, with an important role in hematopoiesis. Generation of definitive hematopoietic progenitor cells from Shp-2−/− ES cells was blocked in the fetal liver and bone marrow in chimeric animals. However, Shp-2+/− cells steadily contributed to both erythroid and myeloid cell lineages, in a proportion consistent with their overall contribution in chimeras. This observation, while confirming our previous result that the Shp-2 mutation blocks hematopoietic cell differentiation from ES cells in vitro (26), also indicates that the hematopoietic defect is cell autonomous.
Although the nature of the requirement for Shp-2 in hematopoiesis is unclear at this stage, it is very likely that Shp-2 might act at multiple “checkpoints” of hierarchical blood cell differentiation during embryogenesis. Shp-2 is highly expressed in undifferentiated and differentiating ES cells at various stages as well as in developing mouse embryos (5, 26). Our previous reverse transcriptase PCR analysis detected the expression of several marker genes for hematopoietic progenitor cells, such s CD34 and AIC-2B, in Shp-2−/− embryoid bodies, albeit at reduced levels (26). This would suggest that the Shp-2 mutation did not completely block the commitment of hematopoietic stem and progenitor cells. However, the Shp-2−/− progenitors, if any, are apparently defective in their further development into mature blood cells, as revealed in this study. Presumably, Shp-2 is involved in cytoplasmic signaling pathways that regulate the proliferation and differentiation of hematopoietic stem and progenitor cells. Treatment of ES cells with SCF induced Erk kinase activation, but this induction was completely blocked in Shp-2−/− ES cells (26). We have also found that Shp-2 physically interacts with and gets tyrosine phosphorylated by the c-kit receptor kinase upon SCF binding in blood cells (35). These biochemical data strongly support the notion that Shp-2 is a critical signal transducer downstream of c-kit in mediating hematopoiesis. Further investigation would involve the physical enrichment of any Shp-2−/− progenitor cells and determination of signaling defects that are responsible for the impaired differentiation capacity. Complementation of Shp-2−/− ES cells with constitutively active mutants in the Ras-Erk kinase pathway may also help us to define the molecular basis for Shp-2 function in cytoplasmic signaling for hematopoiesis.
Chimeric animal analysis has provided an unprecedented opportunity to identify the functions of genes involved in the regulation of the developmental behavior of hematopoietic stem cells. This approach has yielded fundamental insights into the molecular mechanism of control of hematopoiesis by several transcription factors, including GATA-2 and SCL/tal-1 (24, 27, 37). Deletion of the genes blocked development of all blood cell lineages in chimeric animals, while no obvious pathological changes were found in other tissues, due to the specific gene expression pattern. We now demonstrate that this system can also be of great value in dissecting functions of a widely expressed gene, such as Shp-2. In this case, chimeric analysis not only reveals a requirement for an essential gene in the control of hematopoiesis but also uncovers its functional involvement in the differentiation and functions of various tissues, which are otherwise masked by the early embryonic lethality of homozygous mutant animals. Thus, this approach will allow detection of a biased suppression of different physiological processes by a specific gene mutation. In this study, we have shown that Shp-2 plays an indispensable role in hematopoiesis, while its functional involvement in other systems could be compensated to a certain degree, resulting in mild-to-severe pathological changes in various organs.
Previous studies have indicated that Shp-2 plays a critical role during gastrulation in the formation of axial mesodermal (node and notochord) and paraxial mesodermal (somite) structures (29). Development and posterior elongation of the notochord from the node is severely impaired, delayed, or incomplete. In severely affected Shp-2−/− embryos, there is not even a distinguishable node. The well-developed animals display varying degrees of posterior truncation and lateral projection of the notochord. The development of somites is also blocked or severely reduced. In the present study, we observed a corresponding abnormal phenotype at later stages of development in Shp-2−/−–WT chimeric animals. Consistent with the defects in the notochord in Shp-2−/− embryos, the Shp-2 deficiency caused split lumbar vertebra structures with abnormal cell accumulation in one vertebral body in chimeras. In addition, most chimeric mice displayed truncations of terminal structures, as evidenced by short legs or aberrant digit formation. These results are consistent with those of a previous study showing that interference with Shp-2 function by injecting a catalytically inactive Shp-2 mutant mRNA into Xenopus embryos caused severe posterior truncations (34). Limb bud initiation and subsequent patterning in development is derived from immediate mesoderm (10). In this process, fibroblast growth factors (FGFs) play a central role and stimulate the outgrowth of cells located in the limb bud. FGFs are highly expressed in this area and are closely linked to the determination of cell fate in limb development. Biochemical evidence indicates that Shp-2 is a positive signal transducer downstream of FGF receptors in mediating the activation of Erk kinases (29, 34). A similar defect in axial mesodermal patterning and early postimplantation development was also observed in FGFR-1 knockout mice (3, 40). Therefore, defective FGF signaling caused by the Shp-2 mutation might explain in part the abnormalities in terminal and skeletal structures in Shp-2−/−–WT chimeric animals. In addition, we have observed that Shp-2−/− fibroblasts, like focal adhesion kinase (FAK)-deficient cells (9), are impaired in their ability to migrate and spread on fibronectin and display an increased number of focal adhesion sites (41). Interestingly, FAK−/− embryos die at midgestation with a phenotype similar to that of Shp-2 mutant embryos, having severe defects in mesodermal patterning and posterior truncations (7, 9). These results suggest that Shp-2 might work in concert with FAK in mediating the formation of posterior structures during mouse development.
In summary, we provide genetic evidence that Shp-2, a widely expressed phosphatase, participates in a variety of intracellular signaling pathways and functions at different developmental stages. In hematopoiesis, Shp-2 might be required in the commitment and differentiation of hematopoietic stem and/or progenitor cells.
ACKNOWLEDGMENTS
We thank Andras Nagy, Stuart H. Orkin, Fong-Ying Tsai, Phillippe Soriano, and Mark Kaplan as well as other members of our laboratory for helpful discussion and critical reading of the manuscript.
This work was partially supported by grants from the National Institutes of Health (R29GM53660), the American Heart Association-Indiana Affiliate Inc., and the Indiana University Cancer Center to G.-S.F. and by NIH R01 grant HL56416 to H.E.B. G.-S.F. had a career development award from the American Diabetes Association.
REFERENCES
- 1.Bash R O, Hall S, Timmons C F, Crist W M, Amylon M, Smith R G, Baer R. Does activation of the TAL1 gene occur in a majority of patients with T-cell acute lymphoblastic leukemia? A pediatric oncology group study. Blood. 1995;86:666–676. [PubMed] [Google Scholar]
- 2.Cooper, S., and H. E. Broxmeyer. 1996. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin and the other potent co-stimulating cytokines steel factor and Flt-3 ligand. Curr. Protocols Immunol. 18(Suppl.):6.4.1-6.4.12. [DOI] [PubMed]
- 3.Deng C X, Wynshaw-Boris A, Shen M M, Daugherty C, Ornitz D M, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 4.de Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 5.Feng G S, Hui C C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 6.Feng G S, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 7.Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 8.Georgopoulos K, Bigby M, Wang J H, Molnar A, Wu P, Winandy S, Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 9.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 10.Johnson R L, Tabin C J. Molecular models for vertebrate limb development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- 11.Keller G M. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 12.Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- 13.Matthews W, Jordan C T, Wiegand G W, Pardoll D, Lemischka I R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65:1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 14.Milarski K L, Saltiel A R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 15.Mortensen R M, Conner D A, Chao S, Geisterfer-Lowrance A A T, Seidman J G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mucenski M L, McLain K, Kier A B, Swerdlow S H, Schreiner C M, Miller T A, Pietryga D W, Scott W J, Jr, Potter S S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 17.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh W, Stern L. Respiratory diseases of the newborn. In: Stern L, Vert P, editors. Neonatal medicine. New York, N.Y: Masson Publishing USA, Inc.; 1987. p. 395. [Google Scholar]
- 21.Okuda T, van Deursen J, Hiebert S W, Grosveld G, Downing J R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 22.Orkin S H. Hematopoiesis: how does it happen? Curr Opin Cell Biol. 1995;7:870–877. doi: 10.1016/0955-0674(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 23.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D’Agati V, Orkin S H, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 24.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt F W, Orkin S H. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 25.Qu C K, Feng G S. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–440. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- 26.Qu C-K, Shi Z-Q, Shen R, Tsai F-Y, Orkin S H, Feng G-S. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robb L, Elwood N J, Elefanty A G, Kontgen F, Li R, Barnett L D, Begley C G. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 1996;15:4123–4129. [PMC free article] [PubMed] [Google Scholar]
- 28.Roche S, McGlade J, Jones M, Gish G D, Pawson T, Courtneidge S A. Requirement of phospholipase C gamma, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for the existence of Ras-dependent and Ras-independent pathways. EMBO J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- 29.Saxton T M, Henkemeyer M, Gasca S, Shen R, Shalaby F, Feng G S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott E W, Simon M C, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 31.Shalaby F, Ho J, Stanford W L, Fischer K D, Schuh A C, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 32.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X F, Breitman M L, Schuh A C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 33.Shi Z Q, Lu W, Feng G S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4892–4896. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 34.Tang T L, Freeman R, Jr, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 35.Tauchi T, Feng G S, Marshall M S, Shen R, Mantel C, Pawson T, Broxmeyer H E. The ubiquitously expressed Syp phosphatase interacts with c-kit and Grb2 in hematopoietic cells. J Biol Chem. 1994;269:25206–25211. [PubMed] [Google Scholar]
- 36.Tauchi T, Feng G S, Shen R, Hoatlin M, Bagby G, Jr, Kabat D, Lu L, Broxmeyer H E. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J Biol Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 37.Tsai F Y, Keller G, Kuo F C, Weiss M, Chen J, Rosenblatt M, Alt F W, Orkin S H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 38.Weiss M J, Keller G, Orkin S H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 39.Weiss M J, Orkin S H. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci USA. 1995;92:9623–9627. doi: 10.1073/pnas.92.21.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi T P, Harpal K, Henkemeyer M, Rossant J. FGFR-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 41.Yu D H, Qu C K, Henegariu O, Lu X, Feng G S. Protein tyrosine phosphatase Shp-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]