Abstract
Background
Electronic health record–linked portals may improve health-care quality for patients with cancer. Barriers to portal access and use undermine interventions that rely on portals to reduce cancer care disparities. This study examined portal access and persistence of portal use and associations with patient and structural factors before the implementation of 3 portal-based interventions within the Improving the Management of symPtoms during And following Cancer Treatment (IMPACT) Consortium.
Methods
Portal use data were extracted from electronic health records for the 12 months preceding intervention implementation. Sociodemographic factors, mode of accessing portals (web vs mobile), and number of clinical encounters before intervention implementation were also extracted. Rurality was derived using rural-urban commuting area codes. Broadband access was estimated using the 2015-2019 American Community Survey. Multiple logistic regression models tested the associations of these factors with portal access (ever accessed or never accessed) and persistence of portal use (accessed the portal ≤20 weeks vs ≥21 weeks in the 35-week study period).
Results
Of 28 942 eligible patients, 10 061 (35%) never accessed the portal. Male sex, membership in a racial and ethnic minority group, rural dwelling, not working, and limited broadband access were associated with lower odds of portal access. Younger age and more clinical encounters were associated with higher odds of portal access. Of those with portal access, 25% were persistent users. Using multiple modalities for portal access, being middle-aged, and having more clinical encounters were associated with persistent portal use.
Conclusion
Patient and structural factors affect portal access and use and may exacerbate disparities in electronic health record–based cancer symptom surveillance and management.
The electronic health record (EHR) has become an essential part of health care. In the United States, the use of EHRs has proliferated thanks to the Patient Protection and Affordable Care Act, which included incentives for health-care professionals and hospitals to adopt EHR systems to improve the quality, safety, and efficiency of health care; reduce health-care disparities; and engage patients and families in their care. Importantly, EHRs offer patients information related to their care and condition and allow for scheduling and tracking health-care visits, viewing test results, managing medications, and communicating with their health-care team through patient portals (1-3).
Patient portal use has been associated with greater patient satisfaction and adherence to medical treatments (2,4). More recently, patient portals facilitate integration and collection of electronic patient-reported outcome measures, with responses and scores being directly integrated into the EHR system (5), enabling health-care teams to assess and monitor patient symptoms and functioning. Electronic patient-reported outcome measures are a critical component of delivering high-quality cancer care (6) and associated with survival (7).
Understanding characteristics associated with patient access to and use of portals is critical to inform strategies to overcome access-based disparities and improve health-care delivery across the cancer continuum. Because patient portals allow for remote access to and information about care, they may also provide opportunities to reduce cancer care disparities by facilitating patient access to medical information and enabling them to manage their care remotely at their convenience. If disproportionate numbers of patients from certain subgroups do not access patient portals or use them infrequently, however, it is possible that timely information or optimal communication about their care is disrupted, resulting in persistent or even widening cancer care disparities (8,9). Prior studies have shown that younger White patients (10), those living in urban areas (11), and those who are healthier and privately insured are more likely to be frequent portal users (12). Those less likely to use patient portals include patients from low socioeconomic backgrounds (9); patients who lack awareness about the potential benefits of portal use or have data privacy concerns (13); and patients with limited digital capacity, such as internet access only by smartphone, limited smartphone data plans, lack of broadband at home, and low technological skill or difficulties using technology (3). Primary care patients with greater health-care needs are also less likely to enroll in patient portals, but once enrolled, they become frequent users (14), suggesting that patients with more opportunities to interact with the health-care system may have more reasons to use the portal over time to manage their care. It is unclear, however, whether this pattern is also true among patients with cancer. Furthermore, the literature on portal use among oncology patients has been limited by cross-sectional studies (3,13) or studies with small sample sizes (12), underscoring the need for more longitudinal research in this area.
The current study examines associations of portal access and persistence of portal use by patient and structural factors in a large research consortium designed to capture and manage cancer symptoms. Funded by the National Cancer Institute through the Cancer Moonshot, the Improving the Management of symPtoms during And following Cancer Treatment (IMPACT) consortium is composed of 3 research centers, representing 8 US health-care systems and 1 coordinating center. IMPACT’s goal is to develop and test EHR-delivered cancer symptom surveillance and management systems designed to capture and manage cancer symptoms in pragmatic clinical trials. Symptoms are assessed and interventions coordinated through the patient portal, with clinical decision support tools and other clinical responses provided through EHRs to adults during and following cancer treatment. All trials have institutional review board approval and are registered on ClinicalTrials.gov.
Data from the IMPACT research centers provide a unique opportunity to identify potential barriers and opportunities to improve portal access and use before the implementation of the IMPACT interventions. We explored whether patient demographic and clinical factors, extracted from the EHR system, and structural factors (ie, rurality, broadband access) were associated with portal use. Additionally, we were interested in whether correlates of portal access differed from correlates of persistent portal use. We also examined the number of clinical encounters to assess the direct and indirect effects that health-care utilization had on study outcomes.
Methods
Research centers
Data were drawn from EHRs at all 3 IMPACT research centers: Enhanced, EHR-facilitated Cancer Symptom Control (E2C2) at Mayo Clinic; Northwestern University IMPACT (NU IMPACT) at Northwestern Memorial HealthCare; and Symptom Management Implementation of Patient Reported Outcomes in Oncology (SIMPRO), representing 6 distinct health systems. Details of each research center’s protocol are found elsewhere (15-18).
In brief, the E2C2 pragmatic trial tested the effectiveness of routine, periodic, EHR-delivered symptom surveillance using electronic patient-reported outcome measures captured through the patient portal, tablets in clinic waiting rooms, or by interactive voice response. Monitoring was paired with symptom care manager–led collaborative care and multicomponent cancer symptom management to improve symptom burden, physical function, and health-care utilization. Secondary objectives included identifying implementation strategies that enhance feasibility, adoption, reach and designing and adapting tools to support scaling evidence-based interventions for symptoms and impaired physical function in diverse populations with any type of cancer.
NU IMPACT tested the effectiveness and implementation of an EHR-integrated cancer symptom monitoring program that included symptom measures and supportive oncology care needs checklist items. In the embedded patient-level randomized trial, usual care was compared with an enhanced care condition to increase patient self-efficacy regarding symptom management. Elevated scores and patient-endorsed care needs prompted clinical alerts through EHR in-basket messages to designated clinicians. NU IMPACT provided tools in English and Spanish and will examine patient clinical outcomes, health-care utilization, cancer treatment delivery, and implementation outcomes.
The SIMPRO research center has developed and is evaluating its own electronic symptom surveillance and management system in medical and surgical oncology treatment settings within 6 health systems (Baptist Health System, Dana-Farber Cancer Institute, Dartmouth Hitchcock Medical Center, Lifespan Health System, Maine Medical Center, and West Virginia University). Primary endpoints included rates of emergency care for symptom management and hospital readmission following postsurgical discharge. Secondary endpoints were changes in symptom burden over time and patient experiences, with exploratory endpoints of treatment duration or delays.
Patient inclusion criteria for interventions at each research center varied by the cancer sites targeted, phase of cancer treatment or survivorship status, and cancer symptoms captured by electronic patient-reported outcome measures (19). Therefore, research centers were included as a control variable in all models to account for these differences.
Sampling and study period
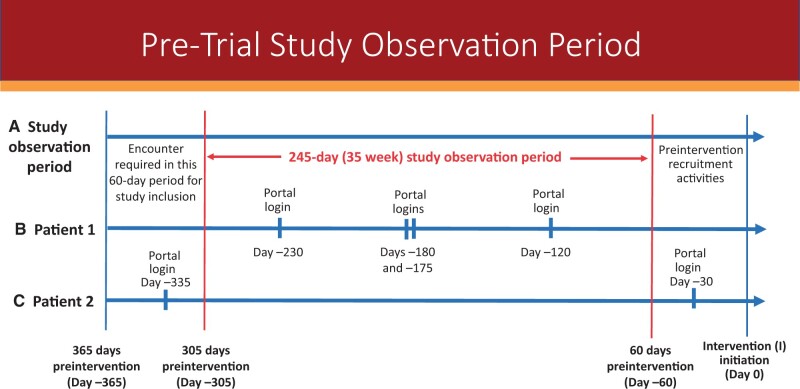
Retrospective data were from adult patients receiving cancer care at 1 of the participating health systems. The look-back period for data collection on portal activity and health encounters was 365 days before intervention implementation (Figure 1). Inclusion criteria for this study included adult cancer patients with a clinical encounter for any purpose in the health system between 365 and 305 days before the start of the intervention. This 60-day period enabled us to examine data from established patients in the health system who already had the opportunity to enroll in and use the patient portal before the start of the intervention. We fixed the end of the observation period as 60 days before the intervention started to capture patient portal access and use unrelated to start-up activities associated with the intervention’s commencement, resulting in an observation period of 245 days (35 weeks).
Figure 1.
Study observation period compared with preintervention data-collection timeline. The top line (A) shows the 245-day (35-week) study observation period (in red) compared with the 365-day preintervention timeline for which encounter data and portal logins the research centers collected for their Improving the Management of symPtoms during And following Cancer Treatment trials. The top line also shows the 60-day period when an encounter was required for study inclusion. The middle line (B) shows a patient with 4 portal logins during the observation period in 3 different weeks. This patient was counted as having 3 weeks of portal logins out of the 35-week observation period. The bottom line (C) shows a patient with 2 portal logins, but both were outside the study observation period. This patient was counted as having no portal logins out of the 35-week observation period.
Data sources and measures
The IMPACT coordinating center harmonized patient and utilization data extracted from EHRs at the research centers as of February 2022. The coordinating center derived structural-level variables for consortium-wide analyses. The dataset used for this analysis was an interim dataset created midway through the research center studies. This dataset therefore represented a subsample of the full consortium dataset, available after completion of the IMPACT interventions.
Outcome variables
Two dichotomous outcomes were assessed for this study: portal access and persistence of portal use. Portal access was defined as either no preintervention login to the portal (coded as 0) or any preintervention login to the portal (coded as 1) before implementation. Cumulative logins, defined as an aggregate number of logins, could include spurts of high-frequency use over a short period that would not indicate persistent use across the cancer continuum (20). Therefore, persistence of portal use, our second outcome, defined as the number of weeks a patient logged in to the portal for any reason during the 35-week preintervention study period, was used instead of cumulative logins; persistence was assessed only among those with any preintervention portal access. Our objective was to look at the proportion of patients who were above a certain threshold and could be considered persistent portal users. We defined infrequent users (coded as 0) as those who logged in to the portal at least once during the first 20 weeks and persistent users (coded as 1) as those who logged in at least once during weeks 21 through 35. Therefore, persistent users, on average, logged on nearly once every 2 weeks. They represented the upper quartile of the distribution.
Explanatory variables
Potential patient-level variables accounting for disparities in portal access and persistent use included age at study initiation, sex, race and ethnicity, employment, mode of access to the portal (web, mobile, both), and number of clinical encounters.
Structural variables potentially associated with differences in outcomes included adequate broadband access and rurality. Estimated using 2015-2019 American Community Survey data matched to patient zip codes, patient residence was classified as being in a community with high (≥85% of households) or low (<85% of households) broadband access, specifications consistent with eligibility criteria for federal rural broadband loans available at the time of data collection (21). Rural-urban commuting area codes were used to classify degree of rurality (ie, metropolitan, codes 1-3; micropolitan, codes 4-6; rural, codes 7-10) from the patient’s zip code. We also accounted for other patient factors potentially associated with portal access and persistent use—specifically, other health conditions under treatment or monitoring that could result in portal messages to clinicians, appointment scheduling, or viewing test results. A complete list of all health conditions for each patient in the preintervention period was not available in this dataset; therefore, number of health-care encounters was used as a proxy. The number of clinical encounters was calculated from the total number of distinct days each patient had a qualifying encounter during the study period. Qualifying encounters included emergency department visits, outpatient visits, and inpatient stays. Number of encounter days was then categorized as 0 to 5, 6 to 10, 11 to 20, 21 to 30, and more than 30.
Analysis strategy
Patient-level and structural-level variables were summarized as frequencies and percentages for categorical variables and means (SD) or medians (interquartile range) for continuous variables. We used separate multivariable logistic regression models to determine which factors were independently associated with portal access and persistence of portal use in the preintervention period. Results from the logistic regressions are presented as adjusted odds ratios (AORs) with corresponding 95% confidence intervals (CIs), with 2-sided type-3 P values for overall association. Due to the exploratory and hypothesis-generating approach of this study, no adjustment for multiple testing was applied. To minimize the chance of obtaining spurious significant findings, however, P = .01 was the a priori threshold for statistical significance.
Variables evaluated in both models were age category, gender, race, ethnicity, employment, health-care encounter days, rurality, and community broadband access (>85% or not). For persistence of portal use, mode of access to the portal was also added to the model. Sex, race, and encounter days were initially included as interaction terms to explore potential effect moderators, but none of these interactions was statistically significant (data not shown). Because research centers varied by intervention design, study populations, and time elapsed since the health system offered an EHR-linked portal, analyses controlled for research center effects. All models were adjusted by research center to control for other potential intercenter differences. Statistical analyses were conducted using SAS, version 9.4, statistical software (SAS Institute Inc, Cary, NC).
Results
Of the 53 745 patients with cancer in the IMPACT Consortium data, 28 942 met inclusion criteria (E2C2 = 22 475, SIMPRO = 4141, NU IMPACT = 2326). Those excluded could not be verified as patients in the health system during the entire preintervention period (eg, did not have an early preintervention encounter) (96.3%), did not have data that allowed for linkage to broadband access (<0.7%), or had incomplete covariate data from the EHR (<3%).
As shown in Table 1, more than half of the sample was older than 65 years of age and included more women than men. The majority of participants identified as White non-Hispanic or Latino, and more than half were retired and living in a metropolitan zip code. More than 40% of patients lived in zip codes where at least 85% of households were estimated to have broadband access.
Table 1.
Patient, structural, and research center characteristics of the study population
| Study variable | Total, No. (%) (N = 28 942) |
|---|---|
| Accessed portal during study period | |
| No | 10 061 (34.8) |
| Yes | 18 881 (65.2) |
| Persistence of portal use | |
| <21 wk active | 14 142 (74.9) |
| ≥21 wk active | 4739 (25.1) |
| IMPACT research center | |
| E2C2 | 22 475 (77.7) |
| NU IMPACT | 2326 (8.0) |
| SIMPRO | 4141 (14.3) |
| Patient-level characteristics | |
| Age, y | |
| 18 to <40 | 1490 (5.1) |
| 40-65 | 11 934 (41.2) |
| >65 | 15 518 (53.6) |
| Employment | |
| Employed | 9 854 (34.0) |
| Not working for pay or other | 4 113 (14.2) |
| Retired | 14 975 (51.8) |
| Ethnic identification | |
| Hispanic or Latino | 495 (1.7) |
| Non-Hispanic or Latino | 28 447 (98.3) |
| Sex | |
| Female | 17 503 (60.5) |
| Male | 11 439 (39.5) |
| No. of days patient had a qualifying encounter in the 35-wk study period | |
| 0-5 | 10 991 (38.0) |
| 6-10 | 5856 (20.2) |
| 11-20 | 6018 (20.8) |
| 21-30 | 2808 (9.7) |
| >30 | 3269 (11.3) |
| Racial identificationa | |
| Asian | 398 (1.4) |
| Black or African American | 691 (2.4) |
| White | 27 415 (94.7) |
| Other | 438 (1.5) |
| Structural-level characteristics | |
| Broadband access | |
| <85% of neighborhood | 17 239 (59.6) |
| ≥85% of neighborhood | 11 703 (40.4) |
| Residence | |
| Metropolitan/urban | 16 365 (56.5) |
| Micropolitan/large rural | 5127 (17.7) |
| Small town/small rural/rural | 7450 (25.7) |
Other race included American Indian, Native Hawaiian/Pacific Islander, and those who self-identified as other. E2C2 = Enhanced, EHR-facilitated Cancer Symptom Control; EHR = electronic health record; IMPACT = Improving the Management of symPtoms during And following Cancer Treatment; NU IMPACT = Northwestern University IMPACT; SIMPRO = Symptom Management Implementation of Patient Reported Outcomes in Oncology.
More than one-third (35%) of patients did not access the patient portal in the preintervention study period (Table 2). Compared with patients older than 66 years of age, patients younger than 40 years (AOR = 2.56, 95% CI = 2.21 to 2.98) and patients between 40 and 65 years (AOR = 1.81, CI = 1.68 to 1.97) had higher odds of having any portal access. Men had lower odds than women (AOR = 0.85; 95% CI = 0.80 to 0.89). Compared with White patients, Black or African American patients (AOR = 0.38; 95% CI = 0.32 to 0.44) and patients of other races (AOR = 0.55, 95% CI = 0.44 to 0.68) had lower odds of having any portal access. Similarly, those who identified as Hispanic had lower odds (AOR = 0.72, 95% CI = 0.59 to 0.90) of portal access than non-Hispanic patients. Patients not working for pay (AOR = 0.41, 95% CI = 0.38 to 0.45) and those who had retired (AOR = 0.64, 95% CI = 0.59 to 0.70) also had lower odds of any portal access than employed patients. Those living in rural areas had lower odds of any portal access than those in metropolitan areas (AOR = 0.9, 95% CI = 0.84 to 0.97), and those living in areas with lower access to broadband had lower odds of portal access than those in areas with higher access to broadband (AOR = 0.72, 95% CI = 0.67 to 0.76). Patients with more encounters also had higher odds of portal access, such that with each increased increment of days with encounters during the preintervention period, the odds of portal access increased monotonically. For example, compared with patients with 0 to 5 days of encounters, those with 6 to 10 encounters had approximately 70% higher odds of portal access.
Table 2.
Comparison of patient, structural, and research center characteristics by portal access and associated adjusted odds ratios for accessing the portal at least once during the 35-week preimplementation study perioda
| Patient, structural, and research center factors (N = 28 942) | Did not access portal, No. (%) (n = 10 061) | Accessed portal at least once, No. (%) (n = 18 881) | Overall Pb | Adjusted odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Patient-level characteristics | ||||
| Age, y | <.0001 | |||
| 18 to <40 | 331 (3.3) | 1159 (6.1) | 2.56 (2.21 to 2.98)f | |
| 40-65 | 3276 (32.6) | 8658 (45.9) | 1.81 (1.68 to 1.97)f | |
| >65 | 6454 (64.1) | 9064 (48.0) | (Referent) | |
| Employment | <.0001 | |||
| Employed | 2312 (23.0) | 7542 (39.9) | (Referent) | |
| Not working for pay or other | 1607 (16.0) | 2506 (13.3) | 0.41 (0.38 to 0.45)f | |
| Retired | 6142 (61.0) | 8833 (46.8) | 0.64 (0.59 to 0.7)f | |
| Ethnic identification | .003 | |||
| Hispanic or Latino | 196 (1.9) | 299 (1.6) | 0.72 (0.59 to 0.9)d | |
| Non-Hispanic or Latino | 9865 (98.1) | 18 582 (98.4) | (Referent) | |
| Sex | <.0001 | |||
| Female | 5781 (57.5) | 11 722 (62.1) | (Referent) | |
| Male | 4290 (42.5) | 7159 (37.9) | 0.85 (0.8 to 0.89)f | |
| No. of days with qualifying encounters in 35-wk study period | <.0001 | |||
| 0-5 | 4581 (45.5) | 6410 (33.9) | (Referent) | |
| 6-10 | 1873 (18.6) | 3963 (21.1) | 1.69 (1.58 to 1.81)f | |
| 11-20 | 1869 (18.6) | 4149 (22.0) | 1.88 (1.75 to 2.02)f | |
| 21-30 | 831 (8.3) | 1977 (10.5) | 2.09 (1.91 to 2.3)f | |
| >30 | 907 (9.0) | 2362 (12.5) | 2.33 (2.13 to 2.55)f | |
| Racial identificationc | <.0001 | |||
| Asian | 128 (1.3) | 270 (1.4) | 0.82 (0.65 to 1.03) | |
| Black or African American | 332 (3.3) | 359 (1.9) | 0.38 (0.32 to 0.44)f | |
| White | 9403 (93.5) | 18 012 (95.4) | (Referent) | |
| Other | 198 (2.0) | 240 (1.3) | 0.55 (0.44 to 0.68)f | |
| Structural characteristics | ||||
| Broadband access | <.0001 | |||
| <85% of neighborhood | 6843 (68.0) | 10 396 (55.1) | 0.72 (0.67 to 0.76)f | |
| >85% of neighborhood | 3218 (32.0) | 8485 (44.9) | (Referent) | |
| Residence | .01 | |||
| Metropolitan/urban | 4984 (49.5) | 11 381 (60.3) | (Referent) | |
| Micropolitan/large rural | 1989 (19.8) | 3138 (16.6) | 0.97 (0.9 to 1.04) | |
| Small town/small rural/rural | 3088 (30.7) | 4362 (23.1) | 0.9 (0.84 to 0.97)d |
All models were adjusted for research center.
Threshold for statistical significance was P < .01.
Other race included Native American, Native Hawaiian/Pacific Islander, and those who self-identified as other.
P < .01.
P < .001.
P < .0001.
Persistent portal users represented 25% (n = 4739) of those who logged in to the portal (n = 18 881) during the preintervention period (Table 3). In adjusted models, the odds of persistent use were lower among those who used only mobile phones to access the portal than among web-only users (AOR = 0.82, 95% CI = 0.69 to 0.96) and higher among those who used both modes of access (AOR = 1.87, 95% CI = 1.72 to 1.03). Middle age was also associated with higher odds of persistent use than older age (AOR = 1.27, 95% CI = 1.13 to 1.43). Much like the findings with portal access, patients with more encounters had a dose-response relationship with persistent portal use. For example, compared with those with 0 to 5 encounters, those with 6 to 10 encounters had more than 300% higher odds of persistent portal use.
Table 3.
Comparison of patient, structural, and research center characteristics by persistence of portal use (accessed the portal ≤20 weeks vs ≥21 weeks) and associated adjusted odds ratios for persistent portal use during the 35-week preimplementation study perioda
| Patient, structural, and research center factors (N = 18 881) | No. of weeks ≤20, No. (%) (n = 14 142) | No. of weeks ≥21, No. (%) (n = 4739) | Overall Pb | Adjusted odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Patient-level characteristics | ||||
| Age, y | .0002 | |||
| 18 to <40 | 879 (6.2) | 280 (5.9) | 1.21 (0.99 to 1.46) | |
| 40-65 | 6390 (45.2) | 2268 (47.9) | 1.27 (1.13 to 1.43)f | |
| >65 | 6873 (48.6) | 2191 (46.2) | (Referent) | |
| Employment | .1 | |||
| Employed | 5740 (40.6) | 1802 (38.0) | (Referent) | |
| Not working for pay or other | 1737 (12.3) | 769 (16.2) | 0.91 (0.8 to 1.02) | |
| Retired | 6665 (47.1) | 2168 (45.7) | 1.05 (0.93 to 1.18) | |
| Ethnicity | .6 | |||
| Hispanic or Latino | 205 (1.4) | 94 (2.0) | 1.07 (0.78 to 1.47) | |
| Non-Hispanic or Latino | 13 937 (98.6) | 4645 (98.0) | (Referent) | |
| Sex | .2 | |||
| Female | 8806 (62.3) | 2916 (61.5) | (Referent) | |
| Male | 5336 (37.7) | 1823 (38.5) | 1.05 (0.97 to 1.13) | |
| No. of days with qualifying encounter | <.0001 | |||
| 0-5 | 6001 (42.4) | 408 (8.6) | (Referent) | |
| 6-10 | 3241 (22.9) | 742 (15.7) | 3.75 (3.28 to 4.29)f | |
| 11-20 | 2727 (19.3) | 422 (30.0) | 9.36 (8.25 to 10.64)f | |
| 21-30 | 1085 (7.7) | 892 (18.8) | 16.5 (14.27 to 19.11)f | |
| >30 | 1088 (7.7) | 1274 (26.9) | 24.86 (21.6 to 28.68)f | |
| Race | .1 | |||
| Asian | 199 (1.4) | 71 (1.5) | 0.95 (0.69 to 1.31) | |
| Black or African American | 242 (1.7) | 117 (2.5) | 0.73 (0.56 to 0.95) | |
| White | 13 538 (95.7) | 4474 (94.4) | (Referent) | |
| Otherc | 163 (1.2) | 77 (1.6) | 1.11 (0.78 to 1.58) | |
| Structural characteristics | ||||
| Broadband access | .03 | |||
| <85% of neighborhood | 8059 (57.0) | 2337 (49.3) | 0.9 (0.82 to 0.98) | |
| >85% of neighborhood | 6083 (43.0) | 2402 (50.7) | (Referent) | |
| Mode of access | <.0001 | |||
| Both | 5130 (36.3) | 2468 (52.1) | 1.87 (1.72 to 2.03)f | |
| Mobile | 1579 (11.2) | 243 (5.1) | 0.82 (0.69 to 0.96)d | |
| Web | 7433 (52.6) | 2028 (42.8) | (Referent) | |
| Residence | .7 | |||
| Metropolitan/urban | 8235 (58.2) | 3146 (66.4) | (Referent) | |
| Micropolitan/large rural | 2458 (17.4) | 680 (14.3) | 1.02 (0.91 to 1.14) | |
| Small town/small rural/rural | 3449 (24.4) | 913 (19.3) | 0.97 (0.87 to 1.09) |
All models were adjusted for research center.
Threshold for statistical significance was P < .01.
Other race included Native American, Native Hawaiian/Pacific Islander, and those who self-identified as other.
P < .01.
P < .001.
P < .0001.
Discussion
Investigating factors associated with portal access and persistent portal use can help identify target patient populations for interventions to achieve consistent portal use among adult patients with cancer. In addition to the communication, scheduling, and monitoring benefits associated with portals, portal access and increased portal use can improve the likelihood that patients answer electronic patient-reported outcome measures, which may improve cancer symptom surveillance by triggering timely intervention strategies. Findings from this large, multisite sample of patients with cancer before the launch of a routine cancer symptom surveillance and management intervention indicated that patient-level and structural-level characteristics are associated with patient portal access and persistence of portal use. Our data suggest opportunities for more directed approaches based on underlying characteristics to support and improve patient engagement in EHR-linked portals.
We observed that older age, male sex, membership in a racial or ethnic minority group, rural dwelling, not working for pay or being retired, and living in an area with limited broadband access were statistically significantly associated with lower odds of portal access. Our findings support those of previous studies on disparities in patient portal enrollment and provide additional evidence that subgroups are more likely to benefit from support to register and use the patient portal. For example, previous studies have shown that young White patients with cancer are more likely to enroll in the patient portal (10,22), while patients who are older, Black or African American, Hispanic or Latino, not living with a partner, and male are less likely to enroll (23). We revealed that the number of patient encounters increased the odds of portal access. Patients with more medical encounters may have more opportunities to enroll in and use the portal and be more likely to use the portal to review test results or send questions to clinicians related to their recent encounter.
Providing training on portal use and information about portal security features as well as allaying concerns about potential costs that the health-care systems charge for requesting services through the portal may improve portal activity (24). In previous studies, these types of efforts have led to portal enrollment and persistent use (9). Tailoring efforts to encourage portal use not solely for self-management but also to provide patient education about the benefits relative to other options (eg, secure messaging vs calling) (24) may help initiate and increase portal use.
In contrast to the significant associations between patient-level and structural-level variables and portal access, male sex, racial minority status, Hispanic or Latino ethnicity, employment status, and rurality were not statistically associated with persistence of portal use, suggesting that once these patients are established and initiate portal access, they have fewer barriers to continued engagement. Consistent with the literature, we also found that patients using multiple modes (web-based and mobile-based platforms) to access the portal had statistically greater odds of persistent portal use (25). As patient portals increasingly become a ubiquitous part of cancer care delivery (2,5), the differences observed in digital access may exacerbate disparities between patients who are more digitally connected and have greater comfort using technology and those without access or access only with mobile technology, which makes navigating online patient information more difficult (26,27). Therefore, EHR-enabled interventions such as those the IMPACT research centers deployed should continue to provide alternative modes of data collection, such as in-clinic tablets, paper-based forms, and interactive voice response systems, as stop-gap measures for those less likely to use the patient portal.
This study also has limitations. Information about marital status, educational attainment, English language proficiency, insurance status, complete lists of comorbidities, income, digital literacy, patient attitudes about the portal, reasons for portal use, portal registration, individual-level access to broadband, and caregiver or proxy use of the patient portal were not available. Clinical variables, such as cancer type, phase, and stage during the preintervention period, for those whose cancer was diagnosed before the interventions, were not fully available. Similarly, symptom severity and functional status impairments were not available in this cohort; future research should investigate whether repeated portal engagement is associated with improved symptom severity or patient satisfaction. Finally, despite large sample sizes, our study population was predominately White and non-Hispanic; therefore, even with a sample size large enough to test interactions, results may not be generalizable.
This study has several notable strengths, as well. Our diverse sample was drawn from 8 health-care systems in major metropolitan, suburban, and rural areas in the United States. In contrast to other studies, our large, well-characterized sample enabled us to examine the effects of employment status, area-level broadband access, and portal access mode and provided the statistical power to test interactions. Our study addressed gaps in the previous literature that have focused on portal enrollment rather than portal access (23) and used EHR data to assess outcomes rather than patient self-report, which is subject to recall bias (3,13). Finally, because the aggregate number of portal logins to define portal use has been criticized as an unreliable indicator of technology adoption (28), persistent portal use may provide a more robust indicator of whether the technology is integrated into patient self-management.
Increasing patient portal access and facilitating persistent, routine portal use are critical to enhancing patient-centered care as patient portals continue to become an integral aspect of cancer care delivery. We found significant patient-level and structural-level factors associated with portal access and persistence of portal use. Efforts to increase the uptake of routine EHR-based symptom surveillance may benefit from efforts that focus on targeting and tailoring engagement efforts to those sociodemographic groups least likely to enroll or be active in patient portals and sustaining engagement over time.
Acknowledgements
Consortium members: Our IMPACT Consortium group includes the following authors: David Cella, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Andrea Cheville, MD, MSCE (Mayo Clinic, Rochester, MN); Michael J. Hassett, MD, MPH (Dana-Farber Cancer Institute, Boston, MA); Raymond U. Osarogiagbon, MBBS, FACP (Baptist Memorial Hospital, Memphis, TN); Deborah Schrag, MD, MPH (Memorial Sloan Kettering Cancer Institute, New York, NY); Sandra L. Wong, MD (Dartmouth Hitchcock Medical Center, Lebanon, NH); Barbara L. Kroner, PhD, MPH (RTI International, Research Triangle Park, NC); Ashley Wilder Smith, PhD, MPH (National Cancer Institute, Bethesda, MD); Lisa DiMartino, PhD (RTI International, Research Triangle Park, NC); Sofia Garcia, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Joan Griffin, PhD (Mayo Clinic, Rochester, MN); Roxanne Jensen, PhD (National Cancer Institute, Bethesda, MD); Sandra Mitchell, PhD, CRNP (National Cancer Institute, Bethesda, MD); Kathryn Ruddy, MD, MPH (Mayo Clinic, Rochester, MN); Justin D. Smith, PhD (University of Utah Spencer Fox Eccles School of Medicine, Salt Lake City, UT); Betina Yanez, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Jessica J. Bian, MD (Maine Medical Center, Portland, ME); Don S. Dizon, MD, FACP (Lifespan Cancer Institute, Providence, RI); Hannah W. Hazard-Jenkins, MD, FACS (West Virginia University Cancer Institute, Morgantown, WV); Mary-Anne Ardini (RTI International, Research Triangle Park, NC); Paige Ahrens, MS (Maine Medical Center, Portland, ME); Jessica Austin, PhD (Mayo Clinic, Scottsdale, AZ); Fiona Barrett (Dana-Farber Cancer Institute, Boston, MA); Michael Bass, MS (Northwestern University Feinberg School of Medicine, Chicago, IL); Megan Begnoche, RN, MSN (Lifespan Cancer Institute, Providence, RI); September Cahue, MPH (Northwestern University Feinberg School of Medicine, Chicago, IL); Kimberly Caron, RN, BSN, CCRC (Maine Medical Center, Portland, ME); Linda Chlan, PhD, RN (Mayo Clinic, Rochester, MN); Ava Coughlin, MAEd (Northwestern University Feinberg School of Medicine, Chicago, IL); Christine Cronin (Dana-Farber Cancer Institute, Boston, MA); Samira Dias, MPH (Dana-Farber Cancer Institute, Boston, MA); Nicolas Faris, MDiv (Baptist Memorial Hospital, Memphis, TN); Ann Marie Flores, PT, PhD, CLT (Northwestern University Feinberg School of Medicine, Chicago, IL); Martha Garcia (Northwestern University Feinberg School of Medicine, Chicago, IL); Karla Hemming, PhD (University of Birmingham, Edgbaston, Birmingham, UK); Jeph Herrin, PhD, MS (Yale University School of Medicine, New Haven, CT); Christine Hodgdon, MS (GRASP, Baltimore, MD); Sheetal Kircher, MD (Northwestern University Feinberg School of Medicine, Chicago, IL); Kurt Kroenke, MD, MAC (Indiana University, Indianapolis, IN); Veronica Lam (Mayo Clinic, Rochester, MN); Nicola Lancki, MPH (Northwestern University Feinberg School of Medicine, Chicago, IL); Quan H. Mai, MS (Northwestern University Feinberg School of Medicine, Chicago, IL); Jennifer Mallow, PhD, FNP-BC (West Virginia University Cancer Institute, Morgantown, WV); Nadine J. McCleary, MD, MPH (Dana-Farber Cancer Institute, Boston, MA); Wynne Norton, PhD (National Cancer Institute, Bethesda, MD); Mary O’Connor, MS (Northwestern University Feinberg School of Medicine, Chicago, IL); Deirdre Pachman, MD (Mayo Clinic, Rochester, MN); Loretta Pearson, MPhil, CCRC (Dartmouth Hitchcock Medical Center, Lebanon, NH); Frank Penedo, PhD (University of Miami, Miami, FL); Jewel Podratz, MBA (Mayo Clinic, Rochester, MN); Jennifer Popovic, DVM, MA (RTI International, Research Triangle Park, NC); Liliana Preiss, MSE (RTI International, Research Triangle Park, NC); Parvez Rahman, MHI (Mayo Clinic, Rochester, MN); Sarah Redmond, PhD, MA (Mayo Clinic, Rochester, MN); James Reich, PMP (Maine Medical Center, Portland, ME); Joshua Richardson, PhD (RTI International, Research Triangle Park, NC); Kimberly Richardson, MA (Black Cancer Collaborative, Chicago, IL); Jennifer Ridgeway, PhD (Mayo Clinic, Rochester, MN); Lila Rutten, PhD (Mayo Clinic, Rochester, MN); Karen Schaepe, PhD (Mayo Clinic, Rochester, MN); Denise Scholtens, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Tiana Poirier-Shelton, MPH (Baptist Memorial Hospital, Memphis, TN); Philip Silberman, MA (Northwestern University Feinberg School of Medicine, Chicago, IL); Jaclyn Simpson, MBA (Baptist Memorial Hospital, Memphis, TN); Laura Tasker, BS, RT(N) (West Virginia University Cancer Institute, Morgantown, WV); Nathan Tesch, MS (Mayo Clinic, Rochester, MN); Cindy Tofthagen, PhD (Mayo Clinic, Jacksonville, FL); Angela Tramontano, MPH (Dana-Farber Cancer Institute, Boston, MA); Benjamin D. Tyndall, PhD (RTI International, Research Triangle Park, NC); Hajime Uno, PhD (Dana-Farber Cancer Institute, Boston, MA); Firas Wehbe, MD, PhD (Northwestern University Feinberg School of Medicine, Chicago, IL); Bryan Weiner, PhD, MA (University of Washington, Seattle, WA).
This research was funded by cooperative agreement grants, a funding mechanism in which National Institutes of Health staff co-authors have permissible involvement (see https://grants.nih.gov/grants/funding/funding_program.htm). None of the authors were involved in grant funding decisions or evaluation of progress; those decisions are made by a separate National Cancer Institute program officer. The authors are solely responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The paper’s content is solely the responsibility of the authors, and the opinions expressed by the authors are their own. This material should not be interpreted as representing the official viewpoint of the US Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
An earlier version of this paper was presented as a poster at the 2022 American Society of Clinical Oncology Quality Care Symposium.
Contributor Information
Joan M Griffin, Division of Health Care Delivery Research, Mayo Clinic, Rochester, MN, USA; Robert E. and Patricia D. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN, USA.
Barbara L Kroner, Center for Clinical Research, RTI International, Research Triangle Park, NC, USA.
Sandra L Wong, Department of Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA.
Liliana Preiss, Center for Clinical Research, RTI International, Research Triangle Park, NC, USA.
Ashley Wilder Smith, Outcomes Research Branch, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Andrea L Cheville, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, MN, USA.
Sandra A Mitchell, Outcomes Research Branch, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Nicola Lancki, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Michael J Hassett, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Deborah Schrag, Department of Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Raymond U Osarogiagbon, Multidisciplinary Thoracic Oncology Program, Baptist Cancer Center, Memphis, TN, USA.
Jennifer L Ridgeway, Division of Health Care Delivery Research, Mayo Clinic, Rochester, MN, USA; Robert E. and Patricia D. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN, USA.
David Cella, Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA.
Roxanne E Jensen, Outcomes Research Branch, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Ann Marie Flores, Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA; Department of Physical Therapy and Human Movement Science, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Jessica D Austin, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, USA.
Betina Yanez, Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Data availability
The data used in this article will be available through Dataverse, a public repository.
Author contributions
Joan Griffin, PhD (Conceptualization; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing), Ann Marie Flores, PhD (Writing—review & editing), Roxanne E. Jensen, PhD (Writing—review & editing), David Cella, PhD (Funding acquisition; Project administration; Writing—review & editing), Jennifer L. Ridgeway, PhD (Conceptualization; Methodology; Writing—review & editing), Raymond U. Osarogiagbon, MBBS (Funding acquisition; Project administration; Writing—review & editing), Deborah Schrag, MD, MPH (Funding acquisition; Project administration; Writing—review & editing), Jessica D. Austin, PhD (Writing—review & editing), Michael Hassett, MD, MPH (Funding acquisition; Project administration; Writing—review & editing), Sandra A. Mitchell, PhD (Funding acquisition; Writing—review & editing), Andrea L. Cheville, MD, MS (Conceptualization; Funding acquisition; Methodology; Writing—review & editing), Ashley Wilder Smith, PhD (Funding acquisition; Writing—review & editing), Liliana Preiss, PhD (Formal analysis; Methodology; Validation; Writing—review & editing), Sandra Wong, MD (Conceptualization; Methodology; Writing—original draft; Writing—review & editing), Barbara Kroner, PhD (Conceptualization; Methodology; Writing—original draft; Writing—review & editing), Nicola Lancki, PhD (Data curation; Formal analysis; Project administration; Writing—review & editing), Betina Yanez, PhD (Conceptualization; Validation; Writing—original draft; Writing—review & editing).
Funding
The IMPACT Consortium is a Cancer Moonshot Research Initiative under the authorization of the 2016 21st Century Cures Act. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award No. UM1CA233033 (Mayo Clinic, Rochester, MN), UM1CA233035 (Northwestern University, Chicago, IL), UM1CA233080 (Baptist Health System, Memphis, TN; Dana-Farber Cancer Institute, Boston, MA; Dartmouth Hitchcock Medical Center, Lebanon, NH; Lifespan Health System, Providence, RI; Maine Medical Center, Portland, ME; and West Virginia University, Morgantown, WV), and U24CA232980 (RTI International, Research Triangle Park, NC).
Conflicts of interest
Joan Griffin has a contract with Exact Sciences, Inc, paid to her institution. Deborah Schrag has a contract with Grail, paid to her institution, and personal fees for editorial services from the Journal of the American Medical Association. No other authors report any conflicts of interest.
References
- 1. Centers for Medicare and Medicaid. Promoting Interoperability Programs.https://www.cms.gov/regulations-and-guidance/legislation/ehrincentiveprograms?redirect=/ehrincentiveprograms. Accessed June 6, 2023.
- 2. Kruse CS, Bolton K, Freriks G.. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res .2015;17(2):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner K, Clary A, Hong YR, Alishahi Tabriz A, Shea CM.. Patient portal barriers and group differences: cross-sectional national survey study. J Med Internet Res .2020;22(9):e18870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis Giardina T, Menon S, Parrish DE, Sittig DF, Singh H.. Patient access to medical records and healthcare outcomes: a systematic review. J Am Med Inform Assoc .2014;21(4):737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basch E, Barbera L, Kerrigan CL, Velikova G.. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book .2018;38:122-134. [DOI] [PubMed] [Google Scholar]
- 6. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol .2016;34(6):557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Habouch J, Mane A.. When oncology care goes digital: a closer look at disparities. J Clin Oncol .2021;39(suppl 28):119-119. [Google Scholar]
- 9. Phelps RG, Taylor J, Simpson K, Samuel J, Turner AN.. Patients’ continuing use of an online health record: a quantitative evaluation of 14,000 patient years of access data. J Med Internet Res .2014;16(10):e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerber DE, Laccetti AL, Chen B, et al. Predictors and intensity of online access to electronic medical records among patients with cancer. J Oncol Pract .2014;10(5):e307-e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luoh RP, Tevaarwerk AJ, Chandereng T, et al. Patterns and predictors of cancer-specific patient health portal usage among patients with cancer: results from the UWCCC Survivorship Program. Cancer Med .2021;10(20):7373-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grossman LV, Masterson Creber RM, Ancker JS, et al. Technology access, technical assistance, and disparities in inpatient portal use. Appl Clin Inform .2019;10(1):40-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCleary NJ, Greenberg TL, Barysauskas CM, et al. Oncology patient portal enrollment at a comprehensive cancer center: a quality improvement initiative. J Oncol Pract .2018;14(8):e451-e461. [DOI] [PubMed] [Google Scholar]
- 14. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res .2020;22(2):e14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finney Rutten LJ, Ruddy KJ, Chlan LL, et al. Pragmatic cluster randomized trial to evaluate effectiveness and implementation of enhanced EHR-facilitated cancer symptom control (E2C2). Trials. 2020;21(1):480-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrin J, Finney Rutten LJ, Ruddy KJ, Kroenke K, Cheville AL.. Pragmatic cluster randomized trial to evaluate effectiveness and implementation of EHR-facilitated collaborative symptom control in cancer (E2C2): addendum. Trials. 2023;24(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hassett MJ, Wong S, Osarogiagbon RU, et al. ; SIMPRO Co-Investigators. Implementation of patient-reported outcomes for symptom management in oncology practice through the SIMPRO research consortium: a protocol for a pragmatic type II hybrid effectiveness-implementation multi-center cluster-randomized stepped wedge trial. Trials. 2022;23(1):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cella D, Garcia SF, Cahue S, et al. Implementation and evaluation of an expanded electronic health record-integrated bilingual electronic symptom management program across a multi-site Comprehensive Cancer Center: the NU IMPACT protocol. Contemp Clin Trials .2023;128:107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilder Smith A, DiMartino LD, Garcia SF, et al. Systematic symptom management in the IMPACT consortium: rationale and design for three effectiveness-implementation trials. JNCI Cancer Spectrum. 2023;7(6):pkad073. doi: 10.1093/jncics/pkad073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beal LL, Kolman JM, Jones SL, Khleif A, Menser T.. Quantifying patient portal use: systematic review of utilization metrics. J Med Internet Res .2021;23(2):e23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Department of Agriculture. Telecommunications Infrastructure Loands and Loan Guarantees. https://www.rd.usda.gov/programs-services/telecommunications-programs/rural-broadband-loans-loangrant-combinations-and-loan-guarantees#overview. Accessed June 6, 2023.
- 22. Halbert CH, Jefferson M, Allen CG, et al. Racial differences in patient portal activation and research enrollment among patients with prostate cancer. J Clin Oncol Clin Cancer Inform .2021;5:768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinha S, Garriga M, Naik N, et al. Disparities in electronic health record patient portal enrollment among oncology patients. JAMA Oncol .2021;7(6):935-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. Paper presented at: AMIA Annual Symposium Proceedings; 2017. [PMC free article] [PubMed]
- 25. Cronin C, Tramontano A, Schrag D, et al. Evaluating the use of web versus mobile devices for ePRO reporting and severe symptom responses at 6 cancer centers. J Clin Oncol. 2022;40(28_suppl): 241. [Google Scholar]
- 26. Rodriguez JA, Lipsitz SR, Lyles CR, Samal L.. Association between patient portal use and broadband access: a national evaluation. J Gen Intern Med .2020;35(12):3719-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perzynski AT, Roach MJ, Shick S, et al. Patient portals and broadband internet inequality. J Am Med Inform Assoc .2017;24(5):927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gheorghiu B, Hagens S.. Measuring interoperable EHR adoption and maturity: a Canadian example. BMC Med Inform Decis Mak .2016;16(1):8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this article will be available through Dataverse, a public repository.