Abstract
Background
Few studies have evaluated mental health disorders comprehensively among patients with prostate cancer on long-term follow-up. The primary aim of our study was to assess the incidence of mental health disorders among patients with prostate cancer compared with a general population cohort. A secondary aim was to investigate potential risk factors for mental health disorders among patients with prostate cancer.
Methods
Cohorts of 18 134 patients with prostate adenocarcinomas diagnosed between 2004 and 2017 and 73470 men without cancer matched on age, birth state, and follow-up time were identified. Mental health diagnoses were identified from electronic health records and statewide health-care facilities data. Cox proportional hazard models were used to estimate hazard ratios. All statistical tests were 2-sided.
Results
The hazard ratios for mood disorders, including depression, among prostate cancer survivors increased for all follow-up periods compared with the general population. The hazard ratios for any mental illness increased with Hispanic, Black, or multiple races; people who were underweight or obese; those with advanced prostate cancer; and those undergoing their first course cancer treatment. We also observed statistically significantly increased hazard ratios for mental health disorders among patients with lower socioeconomic status (P < .0001) and increasing duration of androgen-deprivation therapy (P = .0348). Prostate cancer survivors had a 61% increased hazard ratio for death with a depression diagnosis.
Conclusion
Prostate cancer diagnosis was associated with a higher risk of mental health disorders compared with the general population, which was observed as long as 10-16 years after cancer diagnosis. Providing long-term mental health support may be beneficial to increasing life expectancy for patients with prostate cancer.
More than 3.1 million people in the United States had prostate cancer as of 2022 (1). Over the past few decades, the 10-year survival rate for prostate cancer exceeded 98%, and the 15-year survival rate was 95% (1). Thus, the goal for prostate cancer treatment is transitioning from increasing survival rates to improving quality of life, such as the common use of active surveillance for patients with early-stage prostate cancer (2).
Based on fears of disease progression and cancer recurrence, a prostate cancer diagnosis can cause mood disturbances and mental health disorders among patients (3). A few studies have investigated the adverse effects of cancer treatment and clinical characteristics on mental health disorders, but the participants in these studies were mostly older (≥65 years old) with advanced-stage cancer, and the studies had limited follow-up periods (4-9). Furthermore, socioeconomic status must be considered with regard to mental health among patients with prostate cancer (10,11). More recently, the development of new drugs, such as abiraterone and enzalutamide, has provided alternative treatment options for castration-sensitive prostate cancer (12,13). To our knowledge, no study has evaluated dose-response relationships between prostate cancer treatment and mental health disorders.
The goal of this study was to estimate the risk of mental health disorders among patients with prostate cancer compared with a general-population cohort. A secondary aim was to assess potential risk factors, such as socioeconomic status, tumor characteristics, and treatment doses, for mental health disorders among patients with prostate cancer.
Methods
The eligibility criteria were men who were diagnosed with an invasive first primary prostate adenocarcinoma at 18 years of age or older between 2004 and 2017 in Utah (Surveillance, Epidemiology, and End Results [SEER] International Classification of Diseases for Oncology, third edition code C61). An initial cohort of 18 871 prostate cancer survivors was identified from the statewide SEER Utah Cancer Registry. We started the cohort in 2004 because Gleason and prostate-specific antigen scores are available from 2004 for SEER data. A total of 737 patients with prostate cancer were excluded because 459 were missing cancer stage information, 28 could not be matched to a general-population individual, 21 did not have Utah residence for more than 1 year. Each prostate cancer survivor was matched with up to 5 men by birth state, birth year, and follow-up time. The cancer-free cohort was identified by selecting individuals from the Utah Population Database who had not been diagnosed with any type of cancer throughout the follow-up period. The final study sample consisted of 18 134 prostate cancer survivors and 73 470 cancer-free individuals. This study was approved by the University of Utah Resource for Genetic and Epidemiologic Research (the oversight committee for the Utah Population Database) and the University of Utah Institutional Review Board.
Outcome data used for this study included statewide ambulatory and inpatient data from the Utah Department of Health and electronic health record (EHR) data (inpatient and outpatient) from Intermountain Healthcare and the University of Utah Health, which covers a large proportion of the Utah population, with a 90.5% linkage rate for Intermountain Healthcare and 95.5% linkage rate for University of Utah Health, available from 1996. In addition, Utah is known for having a low percentage of residents seeking health care outside the state, and only about 2.9% of Utahns left the state, resulting in a relatively low out-migration rate (14). Data from the Utah Population Database included records from the Utah Cancer Registry, Utah driver’s licenses, vital records, and the Utah Department of Health. Mental illnesses and short-term side effects were identified by International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes in Supplementary Table 1 (available online). Further detailed methods are available in the Supplementary Methods (available online) (15,16-18).
Statistical analysis
We used χ2 tests to compare baseline characteristics between the prostate cancer survivor and general-population cohorts. Cox proportional hazards models were used to calculate hazard ratios (HRs) for long-term mental illness outcomes and risk factors. Cox proportional hazards models were adjusted for matching factors (age and birth state), baseline body mass index (BMI), baseline Charlson Comorbidity Index, and race and ethnicity. Kaplan-Meier survival curves were used to estimate survival among patients with and without overall mental illness/depression.
All statistical tests were 2-sided, and a P < .05 was considered statistically significant. All analyses were performed using SAS, version 9.4, statistical software (SAS Institute Inc, Cary, NC), except the Spline Flex model, which was performed using Stata, version 17, software (StataCorp, College Station, TX).
Results
The median follow-up time for patients with prostate cancer was 7.4 years. Approximately 22.8% of patients with prostate cancer were younger than 60 years of age at cancer diagnosis, and 41.0% had a Gleason score below 7 (Table 1). Radical prostatectomy alone was performed in 39.4% of cases, and radiation therapy (RT) alone was administered to 19.4% of patients within 1 year of cancer diagnosis.
Table 1.
Demographic and clinical characteristics of patients with prostate cancer and the general population
| No. (%) |
|||||
|---|---|---|---|---|---|
| Prostate cancer(n = 18 134) | General population(n = 73 470) | P for χ2 | |||
| Ethnicity | <.001 | ||||
| Hispanic | 1588 | (8.8) | 5032 | (6.9) | |
| Non-Hispanic | 16 546 | (91.2) | 68 438 | (93.2) | |
| Race | <.001 | ||||
| Asian | 106 | (0.6) | 559 | (0.8) | |
| Black | 130 | (0.7) | 220 | (0.3) | |
| Multiple races | 625 | (3.5) | 2133 | (2.9) | |
| Othera | 19 | (0.1) | 309 | (0.4) | |
| Missing | 112 | (0.6) | 1443 | (2.0) | |
| Baseline body mass indexb | <.001 | ||||
| <18.5 | 41 | (0.2) | 233 | (0.3) | |
| 18.5-24.9 | 4183 | (23.1) | 3210 | (23.7) | |
| 25.0-29.9 | 8704 | (48.0) | 17421 | (46.2) | |
| ≥30 | 4166 | (23.0) | 33904 | (25.5) | |
| Missing | 1040 | (5.7) | 18 702 | (4.4) | |
| Baseline Charlson Comorbidity Index | .003 | ||||
| 0 | 9283 | (51.2) | 36 898 | (50.2) | |
| ≥1 | 8851 | (48.8) | 36 572 | (49.8) | |
| Educationb | .001 | ||||
| High school or less | 8217 | (45.3) | 34 224 | (46.6) | |
| College | 5039 | (27.8) | 19 418 | (26.4) | |
| Post-college | 4878 | (26.9) | 19 828 | (27.0) | |
| Baseline marital status | .004 | ||||
| Married | 16 012 | (88.3) | 65 071 | (88.6) | |
| Widowed | 1368 | (7.5) | 5102 | (6.9) | |
| Unmarriedc | 754 | (4.2) | 3297 | (4.5) | |
| Baseline health insurance status | <.001 | ||||
| Medicare | 6793 | (37.5) | 22 462 | (30.6) | |
| Other government supportd | 381 | (2.1) | 1624 | (2.2) | |
| Private health insurance | 6782 | (37.4) | 27 840 | (37.9) | |
| Blue Cross/Blue Shield | 1584 | (8.7) | 6015 | (8.2) | |
| Othere | 597 | (3.3) | 3699 | (5.0) | |
| Missing | 1997 | (11.0) | 11 830 | (16.1) | |
| No. (%) |
|
|---|---|
| Prostate cancer | |
| (n = 18 134) | |
| Residency | |
| Urban | 15 391 (84.9) |
| Rural | 2743 (15.1) |
| US Census Bureau tract-level median household income | |
| ≤$32 047 | 444 (2.5) |
| $32 048-$53 412 | 6053 (33.4) |
| $53 413-$106 826 | 11 035 (60.9) |
| $106 827-$373 894 | 595 (3.3) |
| Missing | —f |
| Yost Socioeconomic Indexg | |
| Quintile 1 | 3094 (17.1) |
| Quintile 2 | 3330 (18.4) |
| Quintile 3 | 3643 (20.1) |
| Quintile 4 | 3394 (18.7) |
| Quintile 5 | 4531 (25.0) |
| Unknown | 143 (0.8) |
| Age at diagnosis, y | |
| <50 | 421 (2.3) |
| 50-59 | 3720 (20.5) |
| 60-69 | 7391 (40.8) |
| 70-79 | 5140 (28.3) |
| ≥80 | 1462 (8.1) |
| Follow-up time, median (IQR), y | 7.43 (3.79-10.39) |
| PSA at diagnosis, median (IQR), ng/mL | 6.6 (4.8-10.4) |
| PSA value, ng/mL | |
| <4.0 | 1875 (10.3) |
| 4.0-6.9 | 6570 (36.2) |
| 7.0-10.0 | 3167 (17.5) |
| 10.1-20.0 | 2454 (13.5) |
| >20.0 | 1691 (9.3) |
| Missing | 2377 (13.1) |
| Gleason Grade Grouph | |
| 3 + 3 or less | 7435 (41.0) |
| 3 + 4 | 5271 (29.1) |
| 4 + 3 | 2394 (13.2) |
| 4 + 4 | 1476 (8.1) |
| 9 or 10 | 1558 (8.6) |
| Modified risk groupi | |
| Very low | 1956 (10.8) |
| Low | 2977 (16.4) |
| Favorable intermediate | 5079 (28.0) |
| Unfavorable intermediate | 3225 (17.8) |
| High | 2293 (12.6) |
| Very high | 2604 (14.4) |
| American Joint Committee on Cancer staging | |
| I | 4933 (27.2) |
| II | 9095 (50.2) |
| III | 3174 (17.5) |
| IV | 932 (5.1) |
| First course of treatment | |
| Surgery only | 7137 (39.4) |
| Radiation only | 3510 (19.4) |
| Hormone only | 831 (4.6) |
| Surgery + other treatmentj | 501 (2.8) |
| Radiation + hormone | 1804 (10.0) |
| Chemotherapy + other treatmentk | 363 (2.0) |
| Conservative treatmentl | 3988 (22.0) |
Included American Indian, Alaska Native, Native Hawaiian, and Other Pacific Islander. IQR = interquartile range; PSA = prostate-specific antigen.
Variables were imputed for missing values.
Includes Never married, Unmarried, and Unmarried and both legal parents living together.
Includes Medicaid, Other government support, and Department of Corrections and Rehabilitation institutions.
Includes Out of pocket and Miscellaneous.
Cells with <11 were suppressed to protect patient identity.
The index is constructed using a factor analysis of 7 variables of socioeconomic status.
Variables were created based on imputed Gleason pattern and PSA value.
Defined by the National Comprehensive Cancer Network.
Includes the combination of surgery and androgen-deprivation therapy and surgery and radiation therapy.
Includes the combination of chemotherapy, surgery, and radiation therapy; surgery and chemotherapy; radiation therapy and chemotherapy; and radiation therapy, hormone therapy, and chemotherapy.
Includes active surveillance, watchful waiting, and no treatment.
Depression and anxiety disorders were the most common mental health conditions among both patients with prostate cancer and the general population. Incidence rates of depression at 7.3%, 6.4%, and 6.4% in patients with prostate cancer compared with 5.0%, 5.4%, and 5.2% in the general population, whereas anxiety incidence rates were 7.0%, 7.4%, and 9.9% for patients with prostate cancer and 4.9%, 6.4%, and 8.4% for the general population across the follow-up periods of 0-2 years, 2-5 years, and 5-10 years, respectively. In all follow-up periods, patients with prostate cancer had increased hazard ratios for anxiety and mood disorders, especially depressive disorders (Table 2). The event frequencies for these estimates are shown in Supplementary Table 2 (available online). The hazard ratios for anxiety disorder in patients with prostate cancer were 1.37-fold at 0-2 years (95% confidence interval [CI] = 1.25 to 1.49), 1.20-fold at 2-10 years (95% CI = 1.12 to 1.29), and 1.29-fold at 10-16 years (95% CI = 1.14 to 1.45). Similarly, the hazard ratios for mood disorders were 1.38-fold at 0-2 years (95% CI = 1.28 to 1.50), approximately 1.20-fold at 2-10 years (95% CI = 1.13 to 1.29), and 1.25-fold at 10-16 years (95% CI = 1.12-1.38). Increased hazard ratios for depressive disorders were also observed in overall follow-up periods. We compared clinic visit frequencies between patients with prostate cancer and the general population (Supplementary Table 3, A, available online) and observed that patients had a higher number of visits, as expected. In addition, we did not observe significant changes after adjusting for prediagnosis clinic visit frequency (Supplementary Table 3, B, available online). We also stratified mental illness risks by prostate cancer risk groups and identified higher hazard ratios for mental illness in the high-risk group (Supplementary Table 4, available online).
Table 2.
Risks of mental health disorders among prostate cancer survivors compared with the general populationa
| HRs for mental health disorders |
Estimatesb |
|||
|---|---|---|---|---|
| Diagnosis | No. at risk, prostate cancer | No. at risk, general population | HR (95% CI) | |
| Mental illness |
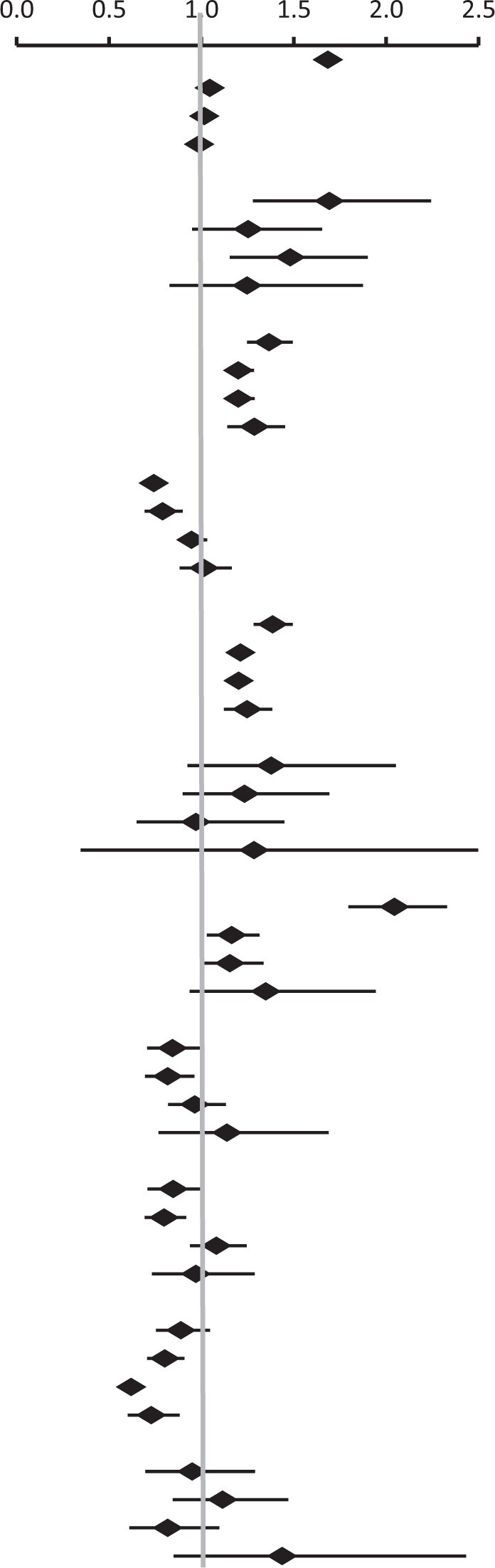
|
|||
| 0-2 yc | 11 854 | 46 602 | 1.68 (1.61 to 1.76) | |
| 2-5 y | 9158 | 40 266 | 1.05 (1.01 to 1.08) | |
| 5-10 y | 6202 | 27 253 | 1.02 (0.98 to 1.06) | |
| 10-16 y | 3084 | 12 860 | 0.99 (0.93 to 1.05) | |
| Adjustment disorder | ||||
| 0-2 y | 18 010 | 72 803 | 1.69 (1.28 to 2.24) | |
| 2-5 y | 17 876 | 72 266 | 1.25 (0.95 to 1.65) | |
| 5-10 y | 12 130 | 46 829 | 1.48 (1.15 to 1.90) | |
| 10-16 y | 5395 | 19 787 | 1.25 (0.83 to 1.88) | |
| Anxiety disorder | ||||
| 0-2 y | 16 318 | 65 615 | 1.37 (1.25 to 1.49) | |
| 2-5 yc | 15 978 | 63 649 | 1.20 (1.12 to 1.28) | |
| 5-10 y | 10 370 | 40 955 | 1.20 (1.12 to 1.29) | |
| 10-16 y | 4758 | 17 814 | 1.29 (1.14 to 1.45) | |
| Delirium, dementia, and cognitive disorders | ||||
| 0-2 yc | 17 625 | 70 582 | 0.74 (0.67 to 0.82) | |
| 2-5 y | 11 854 | 46 602 | 0.79 (0.69 to 0.90) | |
| 5-10 y | 11 604 | 44 997 | 0.95 (0.87 to 1.03) | |
| 10-16 y | 5266 | 19 422 | 1.01 (0.88 to 1.16) | |
| Mood disorders | ||||
| 0-2 yc | 15 881 | 63 227 | 1.38 (1.28 to 1.50) | |
| 2-5 y | 16 108 | 63 871 | 1.21 (1.14 to 1.29) | |
| 5-10 y | 9989 | 39 606 | 1.20 (1.13 to 1.28) | |
| 10-16 y | 4621 | 17 367 | 1.25 (1.12 to 1.38) | |
| Bipolar disorders | ||||
| 0-2 yc | 17 876 | 72 266 | 1.38 (0.92 to 2.05) | |
| 2-5 y | 16 443 | 65 603 | 1.23 (0.90 to 1.69) | |
| 5-10 y | 11 994 | 46 464 | 0.97 (0.65 to 1.45) | |
| 10-16 y | 5357 | 19 670 | 1.28 (0.35 to 4.78) | |
| Depressive disorder | ||||
| 0-2 yc | 15 978 | 63 649 | 2.05 (1.80 to 2.33) | |
| 2-5 y | 14 217 | 57 253 | 1.16 (1.03 to 1.31) | |
| 5-10 y | 10 147 | 40 181 | 1.15 (1.00 to 1.34) | |
| 10-16 y | 4637 | 17 437 | 1.35 (0.94 to 1.94) | |
| Schizophrenia and other psychotic disorders | ||||
| 0-2 y | 17 863 | 71 823 | 0.84 (0.71 to 1.00) | |
| 2-5 y | 16 739 | 66 707 | 0.82 (0.70 to 0.96) | |
| 5-10 y | 11 932 | 46 023 | 0.96 (0.82 to 1.13) | |
| 10-16 y | 5349 | 19 620 | 1.14 (0.77 to 1.69) | |
| Alcohol-related disorders | ||||
| 0-2 yc | 17 646 | 70 546 | 0.85 (0.71 to 1.01) | |
| 2-5 y | 16 746 | 66 721 | 0.80 (0.69 to 0.92) | |
| 5-10 y | 11 825 | 45 351 | 1.08 (0.94 to 1.25) | |
| 10-16 y | 5303 | 19 351 | 0.97 (0.73 to 1.29) | |
| Substance-related disorders | ||||
| 0-2 yc | 17 805 | 71 236 | 0.89 (0.75 to 1.05) | |
| 2-5 y | 14 028 | 56 599 | 0.80 (0.71 to 0.91) | |
| 5-10 y | 11 900 | 45 511 | 0.62 (0.55 to 0.70) | |
| 10-16 y | 5362 | 19 566 | 0.73 (0.60 to 0.88) | |
| Suicide and intentional self-inflicted injury | ||||
| 0-2 y | 18 032 | 72 804 | 0.95 (0.70 to 1.29) | |
| 2-5 y | 16 704 | 66 473 | 1.12 (0.85 to 1.47) | |
| 5-10 y | 12 159 | 46 852 | 0.82 (0.61 to 1.10) | |
| 10-16 y | 5413 | 19 816 | 1.44 (0.85 to 2.43) | |
All hazard ratios were adjusted for age, birth state, baseline body mass index, baseline Carlson Comorbidity Index, and race and ethnicity. CI = confidence interval; HR = hazard ratio.
Bold values indicate statistical significance (P < .05).
Proportional hazards assumption not met; flexible spline model used.
Hispanic, Black, and multiple races and ethnicities were associated with increased hazard ratios for any mental health disorder compared with non-Hispanic White patients during the first 10 years after cancer diagnosis among prostate cancer survivors (Table 3). Underweight patients with prostate cancer had increased hazard ratios in almost all time periods, whereas obese patients with prostate cancer had increased risks 5+ years after cancer diagnosis. In addition, a baseline Charlson Comorbidity Index score of 1+ and being unmarried at cancer diagnosis were potential risks factors for any mental health disorder during the overall follow-up period. Furthermore, lower socioeconomic status and lower household income were associated with increased hazard ratios for mental disorders after prostate cancer diagnosis. Risk factors for specific mental illness outcomes are shown in Supplementary Table 5 (available online).
Table 3.
Demographic risk factors for mental illness among prostate cancer survivors
| 0-2 y After cancer diagnosis |
2-5 y After cancer diagnosis |
5-10 y After cancer diagnosis |
10- y After cancer diagnosis |
|
|---|---|---|---|---|
| Mental illnessa |
Mental illnessa |
Mental illnessa |
Mental illnessa |
|
| Factor | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Ethnicity | ||||
| Non-Hispanic | (Referent) | (Referent) | (Referent) | (Referent) |
| Hispanic | 1.24 (1.14 to 1.36) | 1.09 (0.99 to 1.21) | 1.15 (1.04 to 1.28) | 1.10 (0.92 to 1.32) |
| Race | ||||
| Asian | 0.69 (0.46 to 1.05) | 0.63 (0.40 to 1.00) | 1.06 (0.72 to 1.56) | 0.67 (0.32 to 1.41) |
| Black | 1.49 (1.14 to 1.96) | 1.38 (1.00 to 1.90) | 1.18 (0.82 to 1.69) | 1.50 (0.85 to 2.64) |
| White | (Referent) | (Referent) | (Referent) | (Referent) |
| Multiple races | 1.16 (1.01 to 1.33) | 1.24 (1.07 to 1.44) | 1.27 (1.10 to 1.47) | 1.16 (0.93 to 1.46) |
| Other | 1.61 (0.80 to 3.22) | 1.28 (0.53 to 3.07) | 1.47 (0.55 to 3.92) | — |
| Baseline BMIb | ||||
| <18.5 | 1.53 (1.03 to 2.26) | 1.71 (1.12 to 2.62) | 1.64 (1.01 to 2.66) | 1.54 (0.73 to 3.27) |
| 18.5-24.9 | (Referent) | (Referent) | (Referent) | (Referent) |
| 25.0-29.9 | 0.96 (0.90 to 1.03) | 0.96 (0.89 to 1.03) | 1.02 (0.95 to 1.10) | 1.03 (0.92 to 1.14) |
| ≥30 | 0.98 (0.91 to 1.06) | 1.05 (0.97 to 1.14) | 1.20 (1.10 to 1.31) | 1.23 (1.08 to 1.39) |
| Baseline Charlson Comorbidity Indexb | ||||
| 0 | (Referent) | (Referent) | (Referent) | (Referent) |
| ≥1 | 1.69 (1.60 to 1.78) | 1.95 (1.83 to 2.07) | 1.79 (1.68 to 1.90) | 1.61 (1.47 to 1.76) |
| Educationc | ||||
| High school or less | (Referent) | (Referent) | (Referent) | (Referent) |
| College | 1.02 (0.95 to 1.08) | 1.00 (0.93 to 1.07) | 1.02 (0.95 to 1.10) | 1.08 (0.97 to 1.20) |
| Post-college | 1.02 (0.95 to 1.08) | 1.06 (0.99 to 1.14) | 1.11 (1.03 to 1.19) | 1.02 (0.92 to 1.14) |
| Baseline marital statusc | ||||
| Married | (Referent) | (Referent) | (Referent) | (Referent) |
| Widowed | 1.05 (0.95 to 1.17) | 0.96 (0.86 to 1.08) | 1.14 (1.02 to 1.27) | 1.25 (1.07 to 1.47) |
| Unmarried | 1.74 (1.55 to 1.95) | 1.53 (1.33 to 1.75) | 1.40 (1.20 to 1.62) | 1.32 (1.02 to 1.72) |
| Residencyc | ||||
| Urban | (Referent) | (Referent) | (Referent) | (Referent) |
| Rural | 1.00 (0.93 to 1.08) | 0.98 (0.90 to 1.06) | 1.00 (0.92 to 1.08) | 0.92 (0.81 to 1.04) |
| US Census Bureau tract-level median household incomed | ||||
| ≤$32 047 | (Referent) | (Referent) | (Referent) | (Referent) |
| $32 048-$53 412 | 0.79 (0.68 to 0.93) | 0.88 (0.73 to 1.05) | 1.04 (0.86 to 1.26) | 0.98 (0.73 to 1.31) |
| $53 413-$106 826 | 0.70 (0.60 to 0.82) | 0.80 (0.67 to 0.95) | 0.95 (0.78 to 1.14) | 0.95 (0.71 to 1.27) |
| $106 827-$373 894 | 0.76 (0.62 to 0.94) | 0.96 (0.76 to 1.21) | 0.93 (0.71 to 1.21) | 1.24 (0.84 to 1.85) |
| P for trend | <.0001 | .0099 | .0055 | .9205 |
| Yost Socioeconomic Indexd | ||||
| Quintile 1 | (Referent) | (Referent) | (Referent) | (Referent) |
| Quintile 2 | 0.82 (0.75 to 0.89) | 0.86 (0.78 to 0.95) | 0.87 (0.79 to 0.96) | 0.77 (0.66 to 0.90) |
| Quintile 3 | 0.86 (0.79 to 0.94) | 0.89 (0.81 to 0.97) | 0.99 (0.90 to 1.09) | 1.01 (0.88 to 1.17) |
| Quintile 4 | 0.80 (0.73 to 0.87) | 0.79 (0.72 to 0.88) | 0.90 (0.82 to 1.00) | 0.92 (0.80 to 1.07) |
| Quintile 5 | 0.74 (0.68 to 0.80) | 0.82 (0.75 to 0.90) | 0.85 (0.77 to 0.93) | 0.96 (0.84 to 1.11) |
| P for trend | <.0001 | <.0001 | .002 | .5632 |
| Baseline health insurance statusd | ||||
| Medicare | (Referent) | (Referent) | (Referent) | (Referent) |
| Other government support | 1.32 (1.11 to 1.56) | 1.23 (1.02 to 1.49) | 1.11 (0.90 to 1.38) | 1.32 (0.93 to 1.87) |
| Private health insurance | 0.83 (0.76 to 0.89) | 0.72 (0.66 to 0.79) | 0.81 (0.74 to 0.89) | 1.04 (0.91 to 1.19) |
| Blue Cross/Blue Shield | 0.84 (0.75 to 0.94) | 0.72 (0.64 to 0.82) | 0.83 (0.73 to 0.94) | 0.86 (0.71 to 1.05) |
| Other | 1.02 (0.87 to 1.18) | 0.97 (0.82 to 1.15) | 0.98 (0.83 to 1.17) | 1.10 (0.84 to 1.45) |
Bold value indicates statistical significance (P < .05). BMI = body mass index; CI = confidence interval; HR = hazard ratio.
Adjusted for race and ethnicity, diagnosis age, and education.
Adjusted for race and ethnicity, and diagnosis age.
Adjusted for race and ethnicity, marital status, baseline Charlson Comorbidity Index, baseline body mass index, age at diagnosis, and diagnosis year.
Later diagnosis year and older age at diagnosis (>69 years old) were associated with higher hazard ratios for any mental health disorder among patients with prostate cancer (Table 4). Tumor characteristics were associated with increased hazard ratios for any mental health disorder, such as prostate-specific antigen level (>20 ng/mL) and Gleason score (≥7). Patients with prostate cancer in the higher-grade risk group and American Joint Committee on Cancer staging had increased hazard ratios for any mental health disorder. Compared with surgery as the first course of treatment, increased hazard ratios for any mental health disorder were observed with hormone therapy, active surveillance, watchful waiting, and other types of treatment less than 10 years after cancer diagnosis. Clinical risk factors for specific mental illness outcomes are shown in Supplementary Table 6 (available online).
Table 4.
Clinical risk factors for mental illness among prostate cancer survivors
| 0-2 y After cancer diagnosis |
2-5 y After cancer diagnosis |
5-10 y After cancer diagnosis |
10- y After cancer diagnosis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mental illnessa |
Mental illnessa |
Mental illnessa |
Mental illnessa |
|||||||||||||
| Factor | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | ||||||||
| Diagnosis yearb | ||||||||||||||||
| 2004-2008 | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| 2009-2012 | 1.08 | (1.02 | to | 1.16) | 1.09 | (1.01 | to | 1.17) | 1.23 | (1.16 | to | 1.31) | 3.18 | (2.69 | to | 3.76) |
| 2013-2017 | 1.21 | (1.13 | to | 1.29) | 1.53 | (1.42 | to | 1.64) | 2.36 | (2.11 | to | 2.63) | — | |||
| Age at diagnosis,d y | ||||||||||||||||
| <50 | 1.18 | (0.99 | to | 1.40) | 1.01 | (0.83 | to | 1.23) | 0.84 | (0.69 | to | 1.02) | 0.58 | (0.42 | to | 0.79) |
| 50-59 | 1.08 | (1.01 | to | 1.16) | 0.91 | (0.84 | to | 0.99) | 0.82 | (0.76 | to | 0.89) | 0.85 | (0.76 | to | 0.95) |
| 60-69 | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| 70-79 | 0.91 | (0.85 | to | 0.97) | 1.16 | (1.08 | to | 1.24) | 1.17 | (1.09 | to | 1.26) | 1.21 | (1.09 | to | 1.34) |
| ≥80 | 0.96 | (0.87 | to | 1.07) | 1.37 | (1.22 | to | 1.53) | 1.36 | (1.19 | to | 1.55) | 1.01 | (0.76 | to | 1.34) |
| PSA, ng/mLb | ||||||||||||||||
| <4.0 | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| 4.0-6.9 | 0.91 | (0.84 | to | 1.00) | 1.02 | (0.95 | to | 1.09) | 0.94 | (0.86 | to | 1.03) | 0.94 | (0.82 | to | 1.07) |
| 7.0-10.0 | 0.94 | (0.85 | to | 1.03) | 1.11 | (1.02 | to | 1.22) | 0.95 | (0.85 | to | 1.05) | 0.84 | (0.72 | to | 0.98) |
| 10.1-20.0 | 0.98 | (0.88 | to | 1.08) | 1.18 | (1.06 | to | 1.32) | 0.94 | (0.84 | to | 1.05) | 0.83 | (0.70 | to | 0.98) |
| >20.0 | 1.26 | (1.13 | to | 1.40) | 1.39 | (1.24 | to | 1.56) | 1.15 | (1.01 | to | 1.32) | 1.02 | (0.81 | to | 1.27) |
| Gleason Grade Groupb | ||||||||||||||||
| 3 + 3 or less | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| 3 + 4 | 1.17 | (1.09 | to | 1.25) | 1.02 | (0.95 | to | 1.09) | 1.08 | (1.01 | to | 1.16) | 0.99 | (0.90 | to | 1.10) |
| 4 + 3 | 1.29 | (1.18 | to | 1.40) | 1.11 | (1.02 | to | 1.22) | 1.09 | (0.99 | to | 1.20) | 1.09 | (0.93 | to | 1.27) |
| 4 + 4 | 1.31 | (1.19 | to | 1.45) | 1.18 | (1.06 | to | 1.32) | 1.09 | (0.97 | to | 1.23) | 1.08 | (0.88 | to | 1.33) |
| 9 or 10 | 1.55 | (1.41 | to | 1.71) | 1.39 | (1.24 | to | 1.56) | 1.30 | (1.13 | to | 1.49) | 0.84 | (0.63 | to | 1.12) |
| Modified risk groupb | ||||||||||||||||
| Very low | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| Low | 1.10 | (0.98 | to | 1.23) | 1.12 | (1.00 | to | 1.26) | 1.01 | (0.91 | to | 1.13) | 1.05 | (0.89 | to | 1.23) |
| Favorable intermediate | 1.23 | (1.11 | to | 1.36) | 1.07 | (0.96 | to | 1.19) | 1.04 | (0.94 | to | 1.15) | 1.13 | (0.97 | to | 1.31) |
| Unfavorable intermediate | 1.33 | (1.19 | to | 1.48) | 1.07 | (0.95 | to | 1.20) | 1.00 | (0.90 | to | 1.12) | 1.04 | (0.88 | to | 1.23) |
| High | 1.46 | (1.31 | to | 1.64) | 1.30 | (1.15 | to | 1.46) | 1.09 | (0.97 | to | 1.23) | 0.95 | (0.79 | to | 1.15) |
| Very high | 1.68 | (1.50 | to | 1.87) | 1.37 | (1.22 | to | 1.55) | 1.17 | (1.03 | to | 1.32) | 0.93 | (0.75 | to | 1.16) |
| American Joint Committee on Cancer staginge | ||||||||||||||||
| I | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| II | 1.21 | (1.13 | to | 1.29) | 1.01 | (0.94 | to | 1.08) | 1.02 | (0.95 | to | 1.09) | 1.06 | (0.97 | to | 1.17) |
| III | 1.40 | (1.29 | to | 1.52) | 1.16 | (1.06 | to | 1.27) | 1.07 | (0.97 | to | 1.17) | 0.87 | (0.75 | to | 1.01) |
| IV | 2.04 | (1.82 | to | 2.29) | 1.90 | (1.66 | to | 2.19) | 1.74 | (1.45 | to | 2.08) | 1.15 | (0.79 | to | 1.67) |
| First course of treatmentf | ||||||||||||||||
| Surgery only | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| Radiation only | 0.82 | (0.76 | to | 0.89) | 0.98 | (0.89 | to | 1.07) | 1.04 | (0.95 | to | 1.13) | 0.93 | (0.81 | to | 1.06) |
| Hormone only | 0.90 | (0.79 | to | 1.03) | 1.51 | (1.31 | to | 1.74) | 1.48 | (1.25 | to | 1.75) | 0.80 | (0.54 | to | 1.19) |
| Surgery + radiation | 0.97 | (0.76 | to | 1.24) | 1.20 | (0.92 | to | 1.56) | 1.13 | (0.84 | to | 1.51) | 0.84 | (0.54 | to | 1.30) |
| Surgery + hormone | 1.09 | (0.90 | to | 1.31) | 1.15 | (0.91 | to | 1.45) | 1.12 | (0.88 | to | 1.43) | 1.28 | (0.91 | to | 1.80) |
| Radiation + hormone | 0.82 | (0.75 | to | 0.91) | 0.99 | (0.89 | to | 1.11) | 1.04 | (0.93 | to | 1.15) | 0.99 | (0.85 | to | 1.17) |
| Chemotherapy + Otherg | 0.95 | (0.79 | to | 1.13) | 1.39 | (1.12 | to | 1.71) | 1.33 | (1.03 | to | 1.71) | 0.91 | (0.57 | to | 1.47) |
| Conservative treatment | 0.69 | (0.63 | to | 0.75) | 1.19 | (1.09 | to | 1.30) | 1.17 | (1.07 | to | 1.28) | 1.08 | (0.93 | to | 1.24) |
| Quality of life side effecth | ||||||||||||||||
| Sexual dysfunctioni | — | — | — | — | 1.32 | (1.25 | to | 1.39) | — | — | — | — | — | — | — | — |
| Urinary dysfunction | — | — | — | — | 1.54 | (1.47 | to | 1.60) | — | — | — | — | — | — | — | — |
| Bowel dysfunction | — | — | — | — | 1.46 | (1.37 | to | 1.55) | — | — | — | — | — | — | — | — |
| None | — | (Referent) | — | — | ||||||||||||
Bold values indicate statistical significance (P < .05). CI = confidence interval; HR = hazard ratio.
Adjusted for race and ethnicity and diagnosis age.
Adjusted for baseline body mass index and baseline Charlson Comorbidity Index.
Adjusted for race and ethnicity and American Joint Committee on Cancer staging.
Adjusted for race and ethnicity, baseline body mass index, diagnosis year, age at diagnosis, and education.
Adjusted for race and ethnicity, baseline body mass index, baseline Charlson Comorbidity Index, risk group, age at diagnosis, and education.
Other included surgery + radiation + chemotherapy, surgery + chemotherapy, radiation therapy + chemotherapy, and radiation therapy + androgen-deprivation therapy + chemotherapy.
The evaluation of quality of life side effects was conducted for the entire follow-up period, considering their enduring and recurring characteristics. Adjusted for race and ethnicity, first course of treatment, baseline body mass index, baseline Charlson Comorbidity Index, risk group, age at diagnosis, and education.
Sexual bothersomeness is excluded in sexual dysfunction because of the overlapping issue with mental illness.
Of the 5629 patients with prostate cancer diagnosed from 2013 to 2017, 1455 (25.8%) received RT and 1101 (19.6%) received androgen-deprivation therapy (ADT). The distribution of RT dose by RT type is shown in Supplementary Figure 1 (available online). Brachytherapy was associated with fewer increased hazard ratios for mental health disorders compared with external beam RT. Compared with patients managed with RT at a dosage of 27.6-80.9 Gy, the higher the radiation dose they received, the greater the hazard ratios for any mental health disorder (Table 5). Among patients undergoing medical castration, increased hazard ratios for any mental health disorder were associated with the use of antiandrogens alone and the prescription of a CYP17 inhibitor (abiraterone) or new-generation antiandrogen (enzalutamide) compared with the use of traditional luteinizing hormone–releasing hormone agonists or antagonists. Furthermore, patients with prostate cancer had a 4% increased hazard ratio for mental health disorders for every additional 6 months of ADT (HR = 1.04, 95% CI = 1.00 to 1.08). The hazard ratios for any mental health disorder were nearly 2-fold among patients with prostate cancer on continuous ADT (HR = 1.94, 95% CI = 1.11 to 3.38) compared with those on intermittent ADT. We also investigated the impact of the 4 common types of ADT regimens but did not observe any associations. The increased hazard ratios for mental health disorders were associated with increased radiation dose (P = .0004 for trend) and every additional 6 months of ADT (P = .0348 for trend). In addition, the increased hazard ratios for mood disorders were associated with increased radiation dose (P < .0001 for trend) and every additional 6 months of ADT (P = .061 for trend). Dose-response relations adjusted further for clinical risk factors and socioeconomic status are shown in Supplementary Table 7 (available online); the observed associations were similar. More analyses for antidepressant prescriptions, psychiatry/psychology consultations, and comparison with the prostate cancer–free general population are shown in Supplementary Tables 8-10 (available online).
Table 5.
Risk factors related to radiation therapy and androgen-deprivation therapy for mental illness among prostate cancer survivors, 2013-2017
| Patients, No. (%) | Mental illnessa |
Depressiona |
Mood disordera |
Anxietya |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |||||||||||
| Radiation typeb | ||||||||||||||||||
| External beam RT | 764 | (52.5) | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| Brachytherapy | 462 | (31.8) | 0.77 | (0.63 | to | 0.94) | 1.68 | (0.46 | to | 6.15) | 0.67 | (0.47 | to | 0.95) | 0.84 | (0.57 | to | 1.24) |
| External beam RT + brachytherapy | 229 | (15.7) | 0.84 | (0.67 | to | 1.05) | NA | 0.76 | (0.52 | to | 1.13) | 0.71 | (0.44 | to | 1.14) | |||
| Radiation dose, Gy (categorical)c | ||||||||||||||||||
| 6.5-27.5 (incomplete RT regimen) | 365 | (25.1) | 1.34 | (1.09 | to | 1.64) | 3.14 | (0.84 | to | 11.74) | 1.38 | (0.99 | to | 1.92) | 1.226 | (0.85 | to | 1.78) |
| 27.6-80.9 | 608 | (41.8) | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| 81.0-124.9 | 241 | (16.6) | 2.17 | (1.58 | to | 3.00) | 9.46 | (1.76 | to | 50.84) | 3.19 | (1.86 | to | 5.46) | 2.74 | (1.53 | to | 4.90) |
| ≥125.0 | 241 | (16.6) | 2.85 | (1.46 | to | 5.58) | 36.31 | (2.76 | to | 478.46) | 5.31 | (2.26 | to | 12.52) | 1.58 | (0.39 | to | 6.37) |
| P for trendd | .0004 | .1025 | <.0001 | .1837 | ||||||||||||||
| Radiation dose,c 10 Gy (continuous) | — | — | 1.00 | (1.00 | to | 1.01) | 1.09 | (0.97 | to | 1.23) | 1.00 | (0.99 | to | 1.01) | 1.00 | (0.99 | to | 1.01) |
| ADT typeb | ||||||||||||||||||
| Orchiectomy | 11 | (1.0) | 0.91 | (0.40 | to | 2.05) | NA | 1.29 | (0.41 | to | 4.10) | 0.98 | (0.24 | to | 4.04) | |||
| Medical castration | 1077 | (97.8) | (Referent) | — | (Referent) | (Referent) | ||||||||||||
| Surgical and medical castration | 13 | (1.2) | 0.77 | (0.36 | to | 1.64) | 2.04 | (0.26 | to | 15.91) | 0.91 | (0.29 | to | 2.89) | 0.88 | (0.22 | to | 3.60) |
| Medical castration | ||||||||||||||||||
| LHRH agonist/antagonist alone | 344 | (31.6) | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| LHRH agonist/antagonist + antiandrogen | 370 | (33.9) | 1.21 | (0.98 | to | 1.49) | 1.73 | (0.62 | to | 4.79) | 1.35 | (0.95 | to | 1.91) | 1.05 | (0.70 | to | 1.58) |
| Antiandrogen alone | 278 | (25.5) | 1.30 | (1.03 | to | 1.63) | 1.39 | (0.38 | to | 5.06) | 1.22 | (0.82 | to | 1.81) | 1.18 | (0.77 | to | 1.82) |
| Abiraterone or enzalutamidee | 98 | (9.0) | 1.25 | (1.06 | to | 1.48) | 1.70 | (0.45 | to | 6.40) | 1.77 | (1.10 | to | 2.86) | 2.52 | (1.53 | to | 4.14) |
| ADT administrationf (metastatic prostate cancer only) | ||||||||||||||||||
| Intermittent | 41 | (22.0) | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| Continuous | 145 | (78.0) | 1.94 | (1.11 | to | 3.38) | 1.83 | (0.34 | to | 9.93) | 0.96 | (0.45 | to | 2.05) | 1.14 | (0.49 | to | 2.62) |
| ADT duration,f mo (categorical) | ||||||||||||||||||
| 1-3 | 208 | (19.1) | 1.00 | (0.78 | to | 1.29) | 1.34 | (0.39 | to | 4.61) | 1.43 | (0.96 | to | 2.14) | 1.32 | (0.85 | to | 2.06) |
| 4-6 | 400 | (36.7) | (Referent) | (Referent) | (Referent) | (Referent) | ||||||||||||
| 7-17 | 242 | (22.2) | 1.13 | (0.89 | to | 1.42) | 1.11 | (0.30 | to | 4.04) | 1.22 | (0.81 | to | 1.84) | 1.25 | (0.80 | to | 1.95) |
| 18-28 | 118 | (10.8) | 0.99 | (0.74 | to | 1.34) | 3.55 | (1.16 | to | 10.89) | 1.49 | (0.94 | to | 2.36) | 1.41 | (0.84 | to | 2.39) |
| ≥29 | 122 | (11.2) | 1.15 | (0.87 | to | 1.51) | 0.45 | (0.11 | to | 1.93) | 1.34 | (0.86 | to | 2.09) | 1.33 | (0.79 | to | 2.23) |
| P for trend | .3844 | .6238 | .5509 | .5209 | ||||||||||||||
| ADT duration,f 6 mo (continuous) | — | — | 1.04 | (1.00 | to | 1.08) | 1.00 | (0.87 | to | 1.15) | 1.05 | (1.00 | to | 1.11) | 1.04 | (0.97 | to | 1.11) |
| P | .0348 | .9557 | .0610 | .2455 | ||||||||||||||
Bold values indicate statistical significance (P < .05). ADT = androgen-deprivation therapy; CI = confidence interval; HR = hazard ratio; LHRH = luteinizing hormone–releasing hormone; NA = not applicable; RT = radiation therapy.
Adjusted for race and ethnicity, baseline body mass index, baseline Charlson Comorbidity Index, risk group, age at diagnosis, and diagnosis year.
Adjusted for race and ethnicity, RT type, baseline body mass index, baseline Charlson Comorbidity Index, risk group, age at diagnosis, and diagnosis year.
P for trend was tested for trend and did not include the incomplete RT regimen category (6.5-27.5 Gy).
Including LHRH agonist/antagonist + abiraterone, orchiectomy + abiraterone, LHRH agonist/antagonist + enzalutamide, orchiectomy + enzalutamide, abiraterone alone, enzalutamide alone, and other complicated ADT combination.
Adjusted for race and ethnicity, ADT type, baseline body mass index, baseline Charlson Comorbidity Index, risk group, age at diagnosis, and diagnosis year.
Prostate cancer survivors had a 27% increased hazard ratio for death associated with a mental illness diagnosis (HR = 1.27, 95% CI = 1.20 to 1.34) and an 86% increased hazard ratio for death with a depression diagnosis (HR = 1.86, 95% CI = 1.73 to 2.00), after adjusting for age at diagnosis, baseline BMI, and baseline Charlson Comorbidity Index.
Discussion
We evaluated the associations between cancer diagnosis and new mental health disorder diagnoses among patients with prostate cancer and assessed potential risk factors, including socioeconomic status and clinical characteristics. Notably higher hazard ratios for various mental health disorders, including anxiety and mood disorders, were identified in the long term (10-16 years) after cancer diagnosis. To our knowledge, this is the first study to examine both socioeconomic status and clinical risk factors for mental health disorders among patients with prostate cancer. This study was also the first to evaluate dose-response relationships among prostate cancer treatment, socioeconomic status, and hazard ratios for mental health disorders. Our results suggest that patients with prostate cancer may have higher hazard ratios for anxiety and depression for more than 10-16 years after the cancer diagnosis than the general population. This finding could be attributed to the patients’ fear of cancer progression and recurrence and long-lasting treatment toxicity, including persistent pain and fatigue, bowel problems, sexual and urinary dysfunction, and neurocognitive disorders (15-19). Although patients who receive short-term ADT can recover after discontinuing treatment, testosterone levels may not fully return to pretreatment levels (20). Moreover, side effects of treatment may disappear only slowly or incompletely (20-22). Our findings provide further evidence that any mental health disorder and depression are associated with an increased hazard ratio for death. These findings suggest that health professionals can implement a multidisciplinary approach to help patients with prostate cancer during long-term follow-up.
We observed a higher incidence of anxiety disorder (7.0%-7.4%) and depressive disorders (5.0%-5.4%) among patients with prostate cancer than in the general population. Our findings showed much lower prevalence rates than self-reported studies, which reported anxiety and depression prevalence ranging from 28.6% to 34.3% and 10.7% to 22.8%, respectively, and varied by treatment group (23-26). Self-reported measures may be subject to reporting bias, however, and may not accurately reflect the true prevalence of serious mental illness, whereas EHRs may miss diagnoses if patients do not seek medical attention. Therefore, our study findings may address some of these limitations but does have other limitations—specifically, missing less severe mental illnesses that did not require medical care.
In addition to the outcomes supported by previous studies (27,28), we observed that the hazard ratios for mental health disorders may be associated with race and ethnicity, BMI, and socioeconomic status among prostate cancer survivors. We observed that Black and multiple races were potential risk factors, which was consistent with lower satisfaction with prostate cancer treatment outcomes among Black patients in a previous study (28). Our findings also indicated that Hispanic patients with prostate cancer had a higher hazard ratio for mental health disorders during treatment (0-2 years) and cancer-free periods (≥5 years) but did not have a higher risk at 2-5 years (29). In our study, Hispanic patients with prostate cancer were more likely to have advanced stage and poor socioeconomic status (30). Schupp et al. indicated that prostate cancer survival outcomes in Hispanic patients were paradoxically better than outcomes of non-Hispanic patients with prostate cancer (30), which may explain our findings. Unhealthy weight was associated with worse outcomes, such as the exacerbation of side effects after treatment (28,31) and a higher recurrence rate (32,33), which may lead to poor mental health status. The socioeconomic status finding was supported by a previous study that showed that socioeconomic status affected the development of mental illness directly among the general population (34).
Similar to our previous findings for ovarian cancer (35), we also found that clinical characteristics related to disease progression correlated with mental distress among patients with prostate cancer. These findings may be explained by the widespread use of ADT, severe treatment side effects, poor treatment response, and poor survival rate at advanced cancer stage (19). Our findings are aligned with multiple previous studies that found that short-term side effects of prostate cancer treatment played an important role in mental health disorders in the overall follow-up period (28,36). Previous patient self-reported studies indicated that the risk of depression increased up to 2.8 times among patients with prostate cancer who experienced sexual, urinary, and bowel dysfunction as side effects within 5 years (37,38), which was higher than our findings. The difference may be explained by potential inaccuracies in identifying the presence or severity of side effects because of underreporting or lack of specific coding in our study as well as the short-term follow-up and reporting bias in previous studies. Furthermore, compared with radical prostatectomy, RT, active surveillance, and watchful waiting were associated with decreased risks of mental illness within 2 years, which can be explained by serious side effects of prostatectomy in the first 2 years (36). Unexpectedly, conservative treatment and ADT were associated with increased risks of mental illness 2-10 years after cancer diagnosis compared with radical prostatectomy, possibly because patients on active surveillance could receive radical treatment because of cancer progression more than 2 years after cancer diagnosis (36) and ADT has long-term side effects (36,39-41).
Our findings suggested that brachytherapy was less likely to be associated with mental illness and mood disorders than with external beam RT. Possible explanations for this are a lower incidence of rectal symptoms, lower-grade tumors, and a shorter treatment duration among patients who received external beam RT (42,43). Furthermore, clear dose-response trends were observed for the association between radiation duration and mood disorders and between radiation duration and any mental health disorder. Lack of RT is associated with cancer recurrence and other negative outcomes (44), which may explain the increased risk of mental illnesses with incomplete RT regimens.
Compared with the use of luteinizing hormone–releasing hormone agonists or antagonists alone, patients with prostate cancer had a higher hazard ratio for mental health disorders when they received first-generation antiandrogen monotherapy or any traditional ADT plus abiraterone or enzalutamide. Numerous randomized trials indicated that first-generation antiandrogen monotherapy was associated with a decreased 5-year mortality (45,46), but the underlying reasons for the association between treatment and mental health burden, including clinical outcomes, medication cost, and quality of life (47), have not been assessed. In addition, the new-generation antiandrogen enzalutamide and CYP17 inhibitor abiraterone are more likely to cause seizures and cardiovascular disease, which may increase the psychological burden among patients with prostate cancer (48,49) compared with traditional ADT.
Our findings also showed that patients with metastatic prostate cancer who received continuous ADT had a higher hazard ratio for mental health disorders than patients who received intermittent ADT. This association was expected because intermittent therapy is less likely to cause ADT-related symptoms, such as hot flashes, loss of desire for sexual activity, and urinary incontinence (50). We showed that ADT duration was an essential risk factor for any mental health disorder among patients with prostate cancer, which was supported by a previous study (51). A previous clinical trial (52) reported that the short-term ADT group had better quality of life and similar survival compared with the long-term ADT group. The long-term use of ADT was closely related to an increase in side effects, such as prolonged bed or chair stay, fatigue, and concentration problems (52), which may explain the ADT dose-response relationship found in our study.
Our study had several limitations. First, using EHR data may have led to coding errors, which could misrepresent the true impact of mental illnesses among prostate cancer survivors. Because this study is based on diagnosis of mental illness, we do not expect to capture milder forms of mental illness for which an individual may not seek medical care. Surveillance bias is expected because patients with prostate cancer will have more hospital visits as part of their follow-up care than an individual without cancer, but we observed some associations more than 5 years after cancer diagnosis, a time period we expected to be subject to less surveillance bias. We calculated missing BMI data in some participants. We conducted sensitivity analyses by comparing adjustment of BMI with and without imputations to ensure that the imputed BMI values did not influence inferences. Treatment information was limited to claims data and EHR data, which may not fully capture the patients’ complete treatment regimens. We have no reason to suspect differential misclassification, however, and any existing bias may lead our results toward null. Moreover, we used broad categories and continuous dosage to limit the potential influence of any misclassification. Prostate cancer survivors may receive different treatment regimens on the basis of various unrecorded factors, which may introduce confounders and bias. Therefore, we adjusted for a broad range of risk factors on the basis of a comprehensive evaluation of confounding variables to minimize the impact of the potential bias.
In conclusion, patients with prostate cancer had higher hazard ratios for a range of mental health disorders during long-term follow-up after cancer diagnosis than the general population. We identified several potential risk factors for mental health disorders among these patients and observed a dose-response association between the risk of mental health disorders and the use of ADT and RT in prostate cancer treatment. Our findings suggest that psychological perspectives should be considered in the prostate cancer treatment decision-making process.
Supplementary Material
Acknowledgements
This work was presented at the 2022 American Association for Cancer Research annual meeting.
The funder had no role in the design of the study, collection, analysis, interpretation of the data, writing of the manuscript, or the decision to submit the manuscript.
Contributor Information
Siqi Hu, Huntsman Cancer Institute, Salt Lake City, UT, USA; Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
Chun-Pin Chang, Huntsman Cancer Institute, Salt Lake City, UT, USA; Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
John Snyder, Intermountain Healthcare, Salt Lake City, UT, USA.
Vikrant Deshmukh, University of Utah Health Sciences Center, Salt Lake City, UT, USA.
Michael Newman, University of Utah Health Sciences Center, Salt Lake City, UT, USA.
Ankita Date, Pedigree and Population Resource, Population Sciences, Huntsman Cancer Institute, Salt Lake City, UT, USA.
Carlos Galvao, Pedigree and Population Resource, Population Sciences, Huntsman Cancer Institute, Salt Lake City, UT, USA.
Benjamin Haaland, Huntsman Cancer Institute, Salt Lake City, UT, USA; Department of Population Health Sciences, University of Utah School of Medicine, Salt Lake City, UT, USA.
Christina A Porucznik, Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
Lisa H Gren, Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
Alejandro Sanchez, Division of Urology, Department of Surgery, University of Utah School of Medicine, Salt Lake City, UT, USA.
Shane Lloyd, Department of Radiation Oncology, University of Utah School of Medicine, Salt Lake City, UT, USA.
Brock O’Neil, Division of Urology, Department of Surgery, University of Utah School of Medicine, Salt Lake City, UT, USA.
Mia Hashibe, Huntsman Cancer Institute, Salt Lake City, UT, USA; Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
Data availability
The data for this study was the Utah Population Database (https://uofuhealth.utah.edu/huntsman/utah-population-database), which can be accessed with Institutional Review Board and Utah Resource for Genetic and Epidemiologic Research (oversight committee) approval.
Author contributions
Siqi Hu, MD, PhD (Conceptualization; Formal analysis; Investigation; Methodology; Software; Visualization; Writing—original draft), Chun-Pin Chang, PhD (Data curation; Methodology; Resources), John Snyder, PhD (Data curation; Methodology), Vikrant Deshmukh, PhD (Data curation; Methodology), Michael Newman, PhD (Data curation; Methodology), Ankita Date, MS (Data curation; Methodology), Carlos Galvao, BS (Data curation; Methodology), Benjamin Haaland, PhD (Writing—review & editing), Christina A. Porucznik, PhD (Writing—review & editing), Lisa H. Gren, PhD (Writing—review & editing), Alejandro Sanchez, MD (Writing—review & editing), Shane Lloyd, MD (Writing—review & editing), Brock O’Neil, MD (Writing—review & editing), Mia Hashibe, PhD (Conceptualization; Funding acquisition; Supervision; Writing—review & editing).
Funding
This work was supported by grants from the National Institutes of Health (R01 CA244326, R21 CA185811, R03 CA159357, M.H., principal investigator), the Huntsman Cancer Institute and the Cancer Control and Population Sciences Program (Huntsman Cancer Institute Cancer Center Support Grant P30CA042014). This research was supported by the Utah Cancer Registry, which is funded by the National Cancer Institute’s SEER Program, Contract No. HHSN261201800016I, the US Centers for Disease Control and Prevention’s National Program of Cancer Registries, Cooperative Agreement No. NU58DP0063200-01, with additional support from the University of Utah and Huntsman Cancer Foundation. Partial support for all datasets within the Utah Population Database was provided by the University of Utah, Huntsman Cancer Institute, and the Huntsman Cancer Institute Cancer Center Support grant No. P30 CA42014 from the National Cancer Institute.
Conflicts of interest
The authors declare no potential conflicts of interest.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2. Skolarus TA, Wolf AMD, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249. doi: 10.3322/caac.21234. [DOI] [PubMed] [Google Scholar]
- 3. Meissner VH, Olze L, Schiele S, et al. Fear of cancer recurrence and disease progression in long-term prostate cancer survivors after radical prostatectomy: a longitudinal study. Cancer. 2021;127(22):4287-4295. doi: 10.1002/cncr.33836. [DOI] [PubMed] [Google Scholar]
- 4. Fervaha G, Izard JP, Tripp DA, Rajan S, Leong DP, Siemens DR.. Depression and prostate cancer: a focused review for the clinician. Urol Oncol. 2019;37(4):282-288. doi: 10.1016/j.urolonc.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 5. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901. doi: 10.1136/bmjopen-2013-003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alwhaibi M, Madhavan S, Bias T, Kelly K, Walkup J, Sambamoorthi U.. Depression treatment among elderly Medicare beneficiaries with incident cases of cancer and newly diagnosed depression. Psychiatr Serv. 2017;68(5):482-489. doi: 10.1176/appi.ps.201600190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharpley CF, Christie DR, Bitsika V, Miller BJ.. Trajectories of total depression and depressive symptoms in prostate cancer patients receiving six months of hormone therapy. Psychooncology. 2017;26(1):60-66. doi: 10.1002/pon.4100. [DOI] [PubMed] [Google Scholar]
- 8. Brunckhorst O, Hashemi S, Martin A, et al. Depression, anxiety, and suicidality in patients with prostate cancer: a systematic review and meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2021;24(2):281-289. doi: 10.1038/s41391-020-00286-0. [DOI] [PubMed] [Google Scholar]
- 9. Dinh KT, Yang DD, Nead KT, Reznor G, Trinh QD, Nguyen PL.. Association between androgen deprivation therapy and anxiety among 78 000 patients with localized prostate cancer. Int J Urol. 2017;24(10):743-748. doi: 10.1111/iju.13409. [DOI] [PubMed] [Google Scholar]
- 10. Paller CJ, Wang L, Brawley OW.. Racial inequality in prostate cancer outcomes—socioeconomics, not biology. JAMA Oncol. 2019;5(7):983-984. doi: 10.1001/jamaoncol.2019.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aarts MJ, Mols F, Thong MS, Louwman MW, Coebergh JW, van de Poll-Franse LV.. Long-term prostate cancer survivors with low socioeconomic status reported worse mental health-related quality of life in a population-based study. Urology. 2010;76(5):1224-1230. doi: 10.1016/j.urology.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 12. Kishan AU, Sun Y, Hartman H, et al. ; MARCAP Consortium Group. Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: an individual patient data meta-analysis. Lancet Oncol. 2022;23(2):304-316. doi: 10.1016/s1470-2045(21)00705-1. [DOI] [PubMed] [Google Scholar]
- 13. Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107-118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 14. Frost CJ, Morgan NJ, Allkhenfr H, et al. Determining physical and mental health conditions present in older adult refugees: a mini-review. Gerontology. 2019;65(3):209-215. doi: 10.1159/000491695. [DOI] [PubMed] [Google Scholar]
- 15. Yeoh EK, Holloway RH, Fraser RJ, Botten RJ, Di Matteo AC, Butters J.. Pathophysiology and natural history of anorectal sequelae following radiation therapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2012;84(5):e593-e599. doi: 10.1016/j.ijrobp.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 16. Matta R, Chapple CR, Fisch M, et al. Pelvic complications after prostate cancer radiation therapy and their management: an international collaborative narrative review. Eur Urol. 2019;75(3):464-476. doi: 10.1016/j.eururo.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 17. Nelson CJ, Lee JS, Gamboa MC, Roth AJ.. Cognitive effects of hormone therapy in men with prostate cancer. Cancer. 2008;113(5):1097-1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharifi N, Gulley JL, Dahut WL.. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238-244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 19. NCC Network. NCCN Guideline. Prostate Cancer (Version: 4.2022). 2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459. Accessed September 22, 2022.
- 20. Nascimento B, Miranda EP, Jenkins LC, Benfante N, Schofield EA, Mulhall JP.. Testosterone recovery profiles after cessation of androgen deprivation therapy for prostate cancer. J Sex Med. 2019;16(6):872-879. doi: 10.1016/j.jsxm.2019.03.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol. 1997;157(2):439-444. doi: 10.1016/S0022-5347(01)65165-6. [DOI] [PubMed] [Google Scholar]
- 22. Nishiyama T. Serum testosterone levels after medical or surgical androgen deprivation: a comprehensive review of the literature. Urol Oncol. 2014;32(1):38.e17-38.e28. doi: 10.1016/j.urolonc.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 23. Linscott JA, Rutan MC, Han PKJ, et al. Receipt of survivorship care plans and self-reported health status among patients with genitourinary malignancy. J Urol. 2020;204(3):564-569. doi: 10.1097/ju.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 24. Kerleau C, Guizard AV, Daubisse-Marliac L, et al. ; French Network of Cancer Registries (FRANCIM). Long-term quality of life among localised prostate cancer survivors: QALIPRO population-based study. Eur J Cancer. 2016;63:143-153. doi: 10.1016/j.ejca.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 25. Hoffman KE, Penson DF, Zhao Z, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2020;323(2):149-163. doi: 10.1001/jama.2019.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Downing A, Wright P, Hounsome L, et al. Quality of life in men living with advanced and localised prostate cancer in the UK: a population-based study. Lancet Oncol. 2019;20(3):436-447. doi: 10.1016/s1470-2045(18)30780-0. [DOI] [PubMed] [Google Scholar]
- 27. Ravi P, Karakiewicz PI, Roghmann F, et al. Mental health outcomes in elderly men with prostate cancer1Equal contribution. Urol Oncol. 2014;32(8):1333-1340. doi: 10.1016/j.urolonc.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 28. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 29. NCI. Understanding Cancer Prognosis. Updated June 17, 2019. https://www.cancer.gov/about-cancer/diagnosis-staging/prognosis. Accessed May 13, 2022.
- 30. Schupp CW, Press DJ, Gomez SL.. Immigration factors and prostate cancer survival among Hispanic men in California: does neighborhood matter? Cancer. 2014;120(9):1401-1408. doi: 10.1002/cncr.28587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156(5):1707-1713. [PubMed] [Google Scholar]
- 32. Chalfin HJ, Lee SB, Jeong BC, et al. Obesity and long-term survival after radical prostatectomy. J Urol. 2014;192(4):1100-1104. doi: 10.1016/j.juro.2014.04.086. [DOI] [PubMed] [Google Scholar]
- 33. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862-871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 34. Hudson CG. Socioeconomic status and mental illness: tests of the social causation and selection hypotheses. Am J Orthopsychiatry. 2005;75(1):3-18. doi: 10.1037/0002-9432.75.1.3. [DOI] [PubMed] [Google Scholar]
- 35. Hu S, Baraghoshi D, Chang C-P, et al. Mental health disorders among ovarian cancer survivors in a population-based cohort. Cancer Med. 2023;12(2):1801-1812. doi: 10.1002/cam4.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Resnick MJ, Koyama T, Fan K-H, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roth AJ, Weinberger MI, Nelson CJ.. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568. doi: 10.2217/14796694.4.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Odeo S, Degu A.. Factors affecting health-related quality of life among prostate cancer patients: a systematic review. J Oncol Pharm Pract. 2020;26(8):1997-2010. doi: 10.1177/1078155220959414. [DOI] [PubMed] [Google Scholar]
- 39. Venderbos LDF, van den Bergh RCN, Roobol MJ, et al. A longitudinal study on the impact of active surveillance for prostate cancer on anxiety and distress levels. Psychooncology. 2015;24(3):348-354. doi: 10.1002/pon.3657. [DOI] [PubMed] [Google Scholar]
- 40. Ross RW, Beer TM, Jacobus S, et al. ; Prostate Cancer Clinical Trials Consortium. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer. 2008;112(3):521-526. doi: 10.1002/cncr.23195. [DOI] [PubMed] [Google Scholar]
- 41. Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017;35(28):3189-3197. doi: 10.1200/jco.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 42. Baskar R, Lee KA, Yeo R, Yeoh KW.. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193-199. doi: 10.7150/ijms.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scardino PT, Linehan M, Zelefsky M, Vogelzang N.. Comprehensive Textbook of Genitourinary Oncology. 4th ed. Chicago: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011:xvii, 914 pages. [Google Scholar]
- 44. Ohri N, Rapkin BD, Guha C, Kalnicki S, Garg M.. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95(2):563-570. doi: 10.1016/j.ijrobp.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 45. Rashid M, Shamshavali K, Chhabra M.. Efficacy and safety of nilutamide in patients with metastatic prostate cancer who underwent orchiectomy: a systematic review and metaanalysis. Curr Clin Pharmacol. 2019;14(2):108-115. doi: 10.2174/1574884714666190112151202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355(9214):1491-1498. [PubMed] [Google Scholar]
- 47. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355(9214):1491-1498. doi: 10.1016/S0140-6736(00)02163-2. [DOI] [PubMed] [Google Scholar]
- 48. Stockler MR, Martin AJ, Davis ID, et al. ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP). Health-related quality of life in metastatic, hormone-sensitive prostate cancer: ENZAMET (ANZUP 1304), an international, randomized phase III trial led by ANZUP. J Clin Oncol. 2022;40(8):837-846. doi: 10.1200/jco.21.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. doi: 10.1016/s1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 50. Crook JM, O'Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. New Engl J Med. 2012;367(10):895-903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nead KT, Gaskin G, Chester C, et al. Androgen deprivation therapy and future Alzheimer's disease risk. J Clin Oncol. 2016;34(6):566-571. doi: 10.1200/jco.2015.63.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nabid A, Carrier N, Martin AG, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur Urol. 2018;74(4):432-441. doi: 10.1016/j.eururo.2018.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study was the Utah Population Database (https://uofuhealth.utah.edu/huntsman/utah-population-database), which can be accessed with Institutional Review Board and Utah Resource for Genetic and Epidemiologic Research (oversight committee) approval.


