Abstract
Although incarcerated adults are at elevated risk of dying from cancer, little is known about cancer screening in carceral settings. This study compared stage-specific incidence of screen-detectable cancers among incarcerated and recently released people with the general population, as a reflection of screening practices. We calculated the age- and sex-standardized incidence ratios (SIR) for early- and late-stage cancers for incarcerated and recently released adults compared to the general Connecticut population between 2005 and 2016. Our sample included 143 cancer cases among those incarcerated, 406 among those recently released, and 201 360 in the general population. The SIR for early-stage screen-detectable cancers was lower among incarcerated (SIR = 0.28, 95% CI = 0.17 to 0.43) and recently released (SIR = 0.69, 95% CI = 0.51 to 0.88) individuals than the general population. Incidence of late-stage screen-detectable cancer was lower during incarceration (SIR = 0.51, 95% CI = 0.27 to 0.88) but not after release (SIR = 1.32, 95% CI = 0.93 to 1.82). Findings suggest that underscreening and underdetection of cancer may occur in carceral settings.
Although cancer screening has played a central role in improving outcomes for several common cancer types in the United States, little is known about whether and how cancer screening is used in carceral settings. Recent work has suggested that being diagnosed with cancer while in prison or after release is associated with a higher mortality (1,2). However, whether low screening rates contribute to these disparities is not clear, as large studies that track cancer screening use in the general population, such as the National Health Interview Survey, exclude the incarcerated population. Other data on screening in carceral settings are often limited, focusing on specific cancer types or populations (3-6). Insufficient screening may contribute to the increased cancer-related mortality observed among justice-involved individuals, and understanding screening use in this population is critical for improving cancer outcomes.
In the absence of data on cancer screening, patterns of cancer diagnosis may provide some indication of screening use. Cancer diagnosis among screened populations typically occurs at an earlier stage compared to unscreened populations. Although not a direct measure of screening, cancer incidence and stage distribution have both been used to assess screening use and its impacts at the population level. A lower-than-expected incidence or proportion of early-stage diagnosis or a higher-than-expected incidence or proportion of late-stage diagnosis compared to a reference population may suggest lower screening rates. In addition, changes in early-stage incidence may reflect the use of screening and diagnostic testing on a relatively short time scale, while late-stage incidence (often defined as regional or metastatic disease) may reflect the effectiveness of screening programs in a stable population over the long term (7-11). Using this framework, we evaluated stage-specific incidence for screen-detectable cancers among incarcerated adults compared to the community. We also assessed stage-specific incidence among adults recently released from incarceration to identify whether screening may occur after release. Finally, as a comparison, we assessed the relation between carceral involvement and stage-specific incidence for non-screen-detectable cancers. We hypothesized that diagnosis of early-stage screen-detectable cancer would be less common among incarcerated adults and those recently released from incarceration compared to community-dwelling adults and that diagnosis at a later stage would be more common among those with a history of current or former incarceration.
This was a retrospective cohort study of incident cancer in 3 populations in Connecticut: incarcerated adults, adults recently released from incarceration (defined as within a year), and the general adult population, between 2005 and 2016. Cancer cases among incarcerated and recently released adults were identified by linking Department of Correction files with Connecticut Tumor Registry data (12). For the incarcerated population, we included only cases identified among people incarcerated for 1 year or more, since individuals incarcerated for less than 1 year may not have had the opportunity to be screened. For people incarcerated more than once, cancer cases were included if the diagnosis occurred during a period of incarceration that lasted 1 year or longer. Data on cancer cases for the general Connecticut population were obtained from the Surveillance, Epidemiology, and End Results (SEER) program. Although estimates of cancer incidence in the general population includes those diagnosed among people with involvement in the justice system, we estimated that between 0.5% and 1.7% of cancer cases per year occur in this group; therefore, they are unlikely to affect our estimates.
Within each population, we categorized cancers into screen-detectable (breast, colorectal, cervical, and prostate) and non-screen-detectable (all others, excluding non-melanoma skin cancers). We did not classify lung cancer as screen-detectable since lung cancer screening was not widely available during the study period. We further classified cancer diagnoses as early (stages 0-2) versus late (stages 3-4), similar to other studies (9-11). Cases of unknown stage and hematologic malignancies that do not use typical staging were excluded from analysis.
We calculated the age- and sex-standardized incidence ratio (SIR) for the incarcerated and recently released populations compared to the general Connecticut population, stratified by screen-detectable cancer type and stage (early or late). This approach uses cancer cases over a specified timeframe in the numerator and population counts in the denominator to estimate incidence, consistent with prior approaches we have used to calculate incidence in incarcerated populations and with established methods for calculating incidence in general populations (13,14). For the incarcerated group, we used the mid-year population count, adjusted to reflect the proportion of adults incarcerated 1 year or more, as the denominator. For the recently released population, we based the denominator on the number of individuals released from prison each year, accounting for recidivism within 1 year. For the general population, we used population counts from the SEER registry.
Our primary outcome measure was the standardized incidence ratio (SIR). SIRs compare incidence in one population to another. A SIR greater than 1 suggests higher than expected incidence for the population of interest compared to a reference population, whereas a SIR less than 1 suggests lower than expected incidence. Similarly, the SIRs for early- and late-stage cancer may therefore suggest higher or lower than expected incidence of early- or late-stage disease. When evaluating SIRs, we considered a P value of less than .05 to be statistically significant. Because stage-specific SIRs may reflect differences in incidence for reasons unrelated to screening, we also evaluated the stage distribution (early vs late) among patients diagnosed with cancer in each population overall and for screen-detectable and non-screen-detectable cancers, as stage distribution is independent from overall incidence.
Lastly, we compared stage distribution among incarcerated and recently released adults to that among Connecticut residents with Medicaid or who were uninsured at the time of diagnosis. We identified the insurance source from the Connecticut Tumor Registry. Insurance status is abstracted from medical records by hospital cancer registrars at the time of diagnosis. We chose these comparisons to better understand the impact of incarceration on cancer stage at diagnosis versus other social determinants of health such as poverty and racism that also disproportionately impact people with Medicaid or who are uninsured. The Yale University and Connecticut Department of Public Health Human Investigations Committees approved this research project, which used data obtained from the Connecticut Department of Public Health.
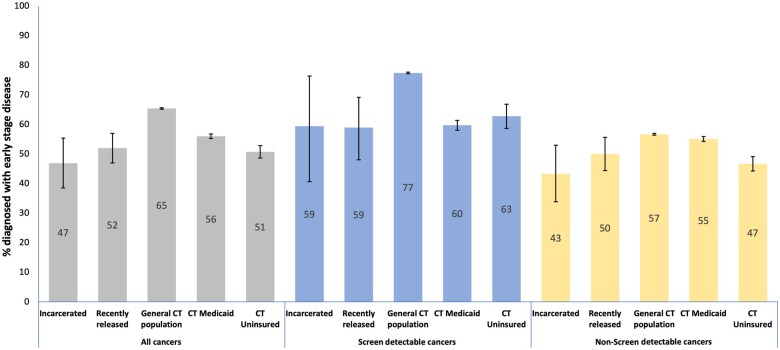
Our sample included 143 cancer cases diagnosed among those incarcerated, 406 among those recently released, and 201 360 in the general Connecticut population. The proportion of adults diagnosed with a screen-detectable cancer at an early stage was lower in the incarcerated and recently released populations compared to the general Connecticut population, but similar to that observed for adults with Medicaid or who were uninsured (Figure 1).
Figure 1.
Proportion of cancer cases diagnosed at an early stage. Figure depicts the proportion of cancers diagnosed at an early stage (0-2) within each population. CT Medicaid and CT uninsured bars depict proportion of early-stage cancers among those diagnosed in Connecticut residents with Medicaid insurance or no insurance.
The incidence of early-stage, screen-detectable cancer was lower among those incarcerated compared to the general population (SIR = 0.21, 95% CI = 0.17 to 0.43). Early-stage screen detectable cancer incidence was also lower among those recently released compared to the general population (SIR = 0.68, 95% CI = 0.51 to 0.88). Late-stage screen-detectable cancer incidence was lower during incarceration (SIR = 0.51, 95% CI = 0.27 to 0.88), although not after release (SIR = 1.32, 95% CI = 0.93 to 1.82), compared to the general population. Overall, this pattern suggests that both screening and evaluation of symptoms may be less common in carceral settings, leading to relatively low detection of both early- and late-stage screen-detectable cancers during incarceration. At the same time, estimates of late-stage disease incidence from the post-release period may indicate a shift in the time of diagnosis to the post-release period.
For non-screen-detectable cancers, the incidence of early-stage disease was lower among incarcerated adults than in the general population (SIR = 0.42, 95% CI = 0.31 to 0.56), while incidence of early-stage, non-screen-detectable disease was higher among those recently released, compared to the general population (SIR = 1.27 95% CI = 1.08 to 1.48). For those incarcerated, incidence of late-stage disease was similar (SIR = 0.91, 95% CI = 0.70 to 1.16) to the general population, but higher among those recently released (SIR = 2.17, 95% CI = 1.84 to 2.53) (Table 1). As with screen-detectable cancers, this pattern suggests a shift in diagnosis to the post-incarceration period, with a notably higher incidence of late-stage, non-screen detectable cancers among those recently released compared to the general population.
Table 1.
Cancer incidence in the incarcerated and recently released populations compared to the general Connecticut populationa
| General CT Population | Incarcerated |
Recently released |
Combined incarcerated + recently released |
||||
|---|---|---|---|---|---|---|---|
| SIR (95% CI) | P | SIR (95% CI) | P | SIR (95% CI) | P | ||
| Screen-detectable, early stage | Ref | 0.28 (0.17 to 0.43) | <.001 | 0.68 (0.51 to 0.88) | .003 | 0.49 (0.38 to 0.62) | <.001 |
| Screen-detectable, late stage | Ref | 0.51 (0.27 to 0.88) | .01 | 1.32 (0.93 to 1.82) | .12 | 0.94 (0.70 to 1.24) | .71 |
| Non-screen-detectable, early stage | Ref | 0.42 (0.31 to 0.56) | <.001 | 1.27 (1.08 to 1.48) | .004 | 0.86 (0.75 to 0.99) | .34 |
| Non-screen-detectable, late stage | Ref | 0.91 (0.70 to 1.16) | .49 | 2.17 (1.84 to 2.53) | <.001 | 1.55 (1.36 to 1.77) | <.001 |
| Overall incidence | Ref | 0.53 (0.45 to 0.63) | <.001 | 1.34 (1.21 to 1.47) | <.001 | 0.94 (0.87 to 1.03) | .18 |
Ref = reference; SIR = standardized incidence ratio.
We observed a lower incidence of early-stage, screen-detectable cancer in incarcerated and recently released populations compared to the general Connecticut population. Early-stage diagnoses also comprised a smaller proportion of all diagnosed cancers in incarcerated and recently released populations. These findings together support the hypothesis that cancer screening may be less common in carceral settings than in the general population. We also observed that the stage distribution at diagnosis among people with a history of incarceration was similar to people with Medicaid and those who are uninsured, suggesting that barriers to screening are common to those affected by a broad range of structural determinants of health including incarceration and poverty. Indeed, cancer screening rates are lower and cancer mortality rates are higher among those with Medicaid insurance or who do not have insurance compared to adults with private insurance (15,16).
We also found that the incidence of early-stage, non-screen-detectable cancers was lower than expected among incarcerated adults, whereas the incidence of early- and late-stage non-screen-detectable cancers was higher in the recently released population. This pattern suggests that there may be relative underdetection of non-screen-detectable cancers during incarceration, resulting in a delay in diagnosis until the post-release period. Indeed, timely evaluation of symptoms for many non-screen-detectable cancers, including thyroid, melanoma, and genitourinary cancers, can lead to earlier diagnosis. Underdetection may result from differences in reporting or evaluation of cancer-related symptoms, limited access to diagnostic testing while incarcerated, or impacts from transitions in care including barriers to communication between correctional and community health systems. Because we compared cancer incidence among incarcerated individuals and those in the general population, overdetection in the general population may also contribute to our findings (17).
Our study has important limitations. First, we did not directly observe screening but rather made inferences about screening based on observed patterns of diagnosis. As such, we cannot exclude other explanations for our findings, including lower cancer incidence due to differential risk factors in incarcerated populations. However, incarcerated populations are typically exposed to more risk factors for cancer, including tobacco and alcohol use, HIV, and liver disease, compared to the general population, making these explanations less likely (18,19). Second, our approach was broadly designed to draw inference about patterns of health care use, rather than quality of care per se. As such, we included all adults rather than restricting the study population to specific age groups for which screening is recommended, and we included prostate cancer as screen-detectable, even though screening is not strongly recommended for all ages. Thus, we cannot draw conclusions about quality of care, including whether screening practices are high or low value. In addition, our findings focus on cancer stage at diagnosis, an outcome that is influenced by the entire continuum of care, from patient-level factors (including the decision to decline screening even if offered), to primary care within carceral settings, to specialist evaluation provided outside of carceral settings. In particular, future research should seek to understand patient perspectives on screening in carceral settings, as many factors, including knowledge, trust, and willingness to be screened, may influence screening use, even when available and offered (4). Lastly, our data have limitations. Analyses use estimates of the sizes of the incarcerated, recently released, and general Connecticut populations as denominators to calculate incidence, which may be subject to measurement error given that individuals are moving in and out of the carceral system. We also cannot observe individuals who move out of state and thus may underestimate cancer incidence. Finally, although we use statewide data, the relatively small population size in Connecticut limits our ability to evaluate cancer stage at diagnosis by cancer type or site.
In light of known disparities in cancer mortality, our findings highlight an urgent need for further evaluation of care processes in carceral settings, including access to and use of cancer screening and follow-up, as well as appropriate diagnostic testing for those with symptoms. The Connecticut Department of Correction (DOC) has launched innovative efforts to expand cancer screening, including systematic use of fecal immunochemical-DNA testing for colorectal cancer screening, mobile mammography, and comprehensive cervical cancer screening and follow-up. The DOC is also implementing a lung cancer screening program that incorporates same-day evaluation by radiologists and pulmonologists to address the logistical complexities of lung cancer screening for people who are incarcerated. More broadly, the DOC has greatly expanded the number of health care providers and implemented electronic health records—two key system-level changes that will allow for more accessible and accountable care. Evaluating such efforts, and those undertaken in other correctional systems, will be critical for understanding what approaches are most successful in implementing screening and reducing cancer outcome disparities.
Acknowledgments
The Department of Public Health Human Investigations Committee approved this research project, which used data obtained from the Connecticut Department of Public Health. The Department of Public Health does not endorse or assume any responsibility for any analyses, interpretations, or conclusions based on the data. The authors assume full responsibility for all such analyses, interpretations, and conclusions. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R01CA230444 and K08CA248725. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A previous version of this work was presented at the Society of General Internal Medicine Annual Meeting in 2023. The funder had no role in the design, conduct, interpretation, or reporting of this work.
Contributor Information
Ilana B Richman, Cancer Outcomes, Public Policy, and Effectiveness Research, Yale School of Medicine, New Haven, CT, USA.
Pamela R Soulos, Cancer Outcomes, Public Policy, and Effectiveness Research, Yale School of Medicine, New Haven, CT, USA.
Hsiu-ju Lin, School of Social Work, University of Connecticut, Storrs, CT, USA; Connecticut Department of Mental Health and Addiction Services, Hartford, CT, USA.
Jenerius A Aminawung, Cancer Outcomes, Public Policy, and Effectiveness Research, Yale School of Medicine, New Haven, CT, USA; SEICHE Center for Health and Justice, Yale School of Medicine, New Haven, CT, USA.
Oluwadamiloa T Oladeru, Department of Radiation Oncology, Mayo Clinic, Jacksonville, FL, USA.
Lisa B Puglisi, School of Social Work, University of Connecticut, Storrs, CT, USA.
Emily A Wang, SEICHE Center for Health and Justice, Yale School of Medicine, New Haven, CT, USA.
Cary P Gross, Cancer Outcomes, Public Policy, and Effectiveness Research, Yale School of Medicine, New Haven, CT, USA.
Data availability
The data used in this study cannot be shared by the authors because it contains protected health information. Statistical code or other methodological information is available upon request.
Author contributions
Ilana Richman (Conceptualization; Methodology; Writing—original draft; Writing—review & editing), Pamela Soulos (Data curation; Formal analysis; Methodology; Writing—review & editing), Hsiu-ju Lin (Data curation; Methodology; Writing—review & editing), Jenerius Aminawung (Data curation; Methodology; Writing—review & editing), Oluwadamiloa T. Oladeru (Conceptualization; Writing—review & editing), Lisa Puglisi (Conceptualization; Writing—review & editing), Emily Wang (Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Writing—review & editing), Cary P. Gross (Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Writing—review & editing).
Funding
The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R01CA230444 (Wang and Gross) and K08CA248725 (Richman).
Conflicts of interest
The authors report the following disclosures: IBR receives salary support from the Centers for Medicare and Medicaid services to develop health care quality measures. OTO reports funding unrelated to the submitted work from Radiation Oncology Institute, NRG Oncology, Pfizer/ASCO Foundation, and Bristol Meyers Squibb Foundation. CPG has received research funding from the National Comprehensive Cancer Network (NCCN) Foundation (funds provided by AstraZeneca) and Genentech as well as funding from Johnson and Johnson to help devise and implement new approaches sharing clinical trial data. The other authors have no competing interests to disclose.
References
- 1. Manz CR, Odayar VS, Schrag D.. Disparities in cancer prevalence, incidence, and mortality for incarcerated and formerly incarcerated patients: a scoping review. Cancer Med. 2021;10(20):7277-7288. doi: 10.1002/cam4.4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oladeru OT, Aminawung JA, Lin H-J, et al. Incarceration status and cancer mortality: a population-based study. PLoS One. 2022;17(9):e0274703. doi: 10.1371/journal.pone.0274703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Cancer and Cardiovascular Health Inequities in Prison Settings: A Rapid Literature Review. https://www.who.int/europe/publications/i/item/WHO-EURO-2022-5814-45579-65357. Accessed November 27, 2023.
- 4. Binswanger IA, White MC, Pérez-Stable EJ, Goldenson J, Tulsky JP.. Cancer screening among jail inmates: frequency, knowledge, and willingness. Am J Public Health. 2005;95(10):1781-1787. doi: 10.2105/AJPH.2004.052498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valera P. Cigarette smoke and cancer health among incarcerated men in U.S. northeastern prison facilities. J Correct Health Care. 2019;25(3):265-276. doi: 10.1177/1078345819856905 [DOI] [PubMed] [Google Scholar]
- 6. Kanbergs AN, Sullivan MW, Maner M, et al. Cervical cancer screening and follow-up practices in U.S. prisons. Am J Prev Med. 2023;64(2):244-249. doi: 10.1016/j.amepre.2022.09.021 [DOI] [PubMed] [Google Scholar]
- 7. Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002-1013. doi: 10.1016/S1470-2045(21)00199-6 [DOI] [PubMed] [Google Scholar]
- 8. Welch HG, Albertsen PC.. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst. 2009;101(19):1325-1329. doi: 10.1093/jnci/djp278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155(5):1383-1391.e5. doi: 10.1053/j.gastro.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jemal A, Culp MB, Ma J, Islami F, Fedewa SA.. Prostate cancer incidence 5 years after US Preventive Services Task Force recommendations against screening. J Natl Cancer Inst. 2021;113(1):64-71. doi: 10.1093/jnci/djaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bleyer A, Welch HG.. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998-2005. doi: 10.1056/NEJMoa1206809 [DOI] [PubMed] [Google Scholar]
- 12. Puglisi L, Halberstam AA, Aminawung J, et al. Incarceration and Cancer-Related Outcomes (ICRO) study protocol: using a mixed-methods approach to investigate the role of incarceration on cancer incidence, mortality and quality of care. BMJ Open. 2021;11(5):e048863. doi: 10.1136/bmjopen-2021-048863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defining Cancer Statistics, Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/statistics/types/incidence.html. Accessed October 25, 2023.
- 14. Aminawung JA, Soulos PR, Oladeru OT, et al. Cancer incidence among incarcerated and formerly incarcerated individuals: a statewide retrospective cohort study. Cancer Med. 2023;12(14):15447-15454. doi: 10.1002/cam4.6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sabatino SA, Thompson TD, White MC, et al. Cancer screening test use—U.S., 2019. Am J Prev Med. 2022;63(3):431-439. doi: 10.1016/j.amepre.2022.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J, Han X, Nogueira L, et al. Health insurance status and cancer stage at diagnosis and survival in the United States. CA Cancer J Clin. 2022;72(6):542-560. doi: 10.3322/caac.21732 [DOI] [PubMed] [Google Scholar]
- 17. Welch HG, Kramer BS, Black WC.. Epidemiologic signatures in cancer. N Engl J Med. 2019;381(14):1378-1386. doi: 10.1056/NEJMsr1905447 [DOI] [PubMed] [Google Scholar]
- 18. Binswanger IA, Carson EA, Krueger PM, Mueller SR, Steiner JF, Sabol WJ.. Prison tobacco control policies and deaths from smoking in United States prisons: population based retrospective analysis. BMJ 2014;349(7970):g4542. doi: 10.1136/bmj.g4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazel S, Yoon IA, Hayes AJ.. Substance use disorders in prisoners: an updated systematic review and meta-regression analysis in recently incarcerated men and women. Addiction. 2017;112(10):1725-1739. doi: 10.1111/add.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study cannot be shared by the authors because it contains protected health information. Statistical code or other methodological information is available upon request.