Abstract
To evaluate the relationship between testosterone replacement therapy (TRT) and arterial and/or venous thrombosis in patients with pre-treatment total testosterone (TT) <12 nmol l−1, we performed a meta-analysis following the Population Intervention Comparison Outcome model. Population: men with TT <12 nmol l−1 or clear mention of hypogonadism in the inclusion criteria of patients; intervention: TRT; comparison: placebo or no therapy; outcomes: arterial thrombotic events (stroke, myocardial infarction [MI], upper limbs, and lower limbs), VTE (deep vein thrombosis [DVT], portal vein thrombosis, splenic thrombosis, and pulmonary embolism), and mortality. A total of 2423 abstracts were assessed for eligibility. Twenty-four studies, including 14 randomized controlled trials (RCTs), were finally included, with a total of 4027 and 310 288 hypotestosteronemic male patients, from RCTs and from observational studies, respectively. Based on RCT-derived data, TRT did not influence the risk of arterial thrombosis (odds ratio [OR] = 1.27, 95% confidence interval [CI]: 0.47–3.43, P = 0.64), stroke (OR = 1.34, 95% CI: 0.09–18.97, P = 0.83), MI (OR = 0.51, 95% CI: 0.11–2.31, P = 0.39), VTE (OR = 1.42, 95% CI: 0.22–9.03, P = 0.71), pulmonary embolism (OR = 1.38, 95% CI: 0.27–7.04, P = 0.70), and mortality (OR = 0.70, 95% CI: 0.20–2.38, P = 0.56). Meanwhile, when only observational studies are considered, a significant reduction in the risk of developing arterial thrombotic events, MI, venous thromboembolism, and mortality was observed. The risk for DVT remains uncertain, due to the paucity of RCT-based data. TRT in men with TT <12 nmol l−1 is safe from the risk of adverse cardiovascular events. Further studies specifically assessing the risk of DVT in men on TRT are needed.
Keywords: hypogonadism, testosterone, testosterone replacement therapy, thromboembolism, thrombosis, TRT
INTRODUCTION
Hypogonadism, defined as impaired spermatogenesis and/or impaired secretion of testosterone (T), is a congenital or acquired disease, divided into primary (testicular cause), secondary (hypothalamic/pituitary cause), or functional (intact hypothalamic–pituitary–testicular [HPT] axis, activity reduced in relation to extrinsic cause) forms.1 Organic hypogonadism refers to diseases of the hypothalamus, pituitary, or testes, resulting in clinical syndromes of infertility and/or androgen deficiency.1,2 Some authors described late-onset hypogonadism (LOH) as symptoms of hypogonadism and a low T concentration in older men, and its management (in the absence of organic hypogonadism) is unclear and subject to debate.3,4,5 Some authors suggested categorizing men in this manner if symptoms suggestive of hypogonadism are present and serum total T (TT) levels are <12 nmol l−1 (<3.5 ng ml−1) confirmed on at least two morning blood samples.5 When not reversible, T replacement therapy (TRT) is the primary medical intervention for the treatment of male hypogonadism, except when contraindications coexist or patients try to have a child.4,5
On January 31, 2014, the Food and Drug Administration (FDA) of the USA issued a safety warning on the risk of stroke, heart attack, and death in men taking previously FDA-approved T products. The announcement was made because two previous studies suggested an increased risk of cardiovascular events among patients on T therapy.6,7 As a result, prescriptions of T have dropped significantly8 and the scientific community has begun to further evaluate this issue. Numerous studies and meta-analyses on this topic have been published since 2014, revealing that TRT is safe when prescribed to patients with hypogonadism.9,10 When it comes to the safety of TRT, the guidelines of the European Academy of Andrology (EAA)11 made a clear distinction between cardiovascular (CV) events and venous thromboembolism (VTE). In particular, while there is no clear evidence of increased TRT-related CV risk,10 the association between endogenous T levels may be associated with VTE,12 possibly due to a pre-existing state of thrombophilia-hypofibrinolysis.13,14 Therefore, before prescribing TRT, the EAA guidelines suggest collecting personal and family history of VTE, as well as evaluating the presence of risk factors for VTE (recommendation No. 27; level 2 ⊕ 〇〇〇 [low]). Similar conclusions have been proposed very recently in a joint position statement by the Italian Society of Andrology and Medical Sexology and the Italian Society of Endocrinology.5
This evidence prompted some authors to investigate further the relationship between TRT and VTE. A meta-analysis that included 13 randomized controlled trials (RCTs) and assessed the risk of VTE in patients on TRT found no increase in the treated arm compared to the one that received placebo. However, baseline serum T levels were not fully detailed in all included studies. Furthermore, the presence of hypogonadism was not fully specified among the inclusion criteria. These two aspects represent important limitations that could likely have influenced the results of this meta-analysis.15 In contrast, a meta-analysis performed on 27 RCTs found an increased risk of VTE in patients treated with T compared to those treated with placebo, although only 14 of the 27 studies included in the meta-analysis showed a pretreatment serum T level <12 nmol l−1.16 Finally, a more recent meta-analysis investigated the occurrence of CV events and mortality in patients on TRT, suggesting the absence of an increased risk in the short and medium term.17
Therefore, the present systematic review and meta-analysis aimed to evaluate the relationship between TRT and the risk of arterial and/or venous thrombosis (including VTE) in hypotestosteronemic patients. To accomplish this, we carefully selected the studies that enrolled patients with serum TT levels <12 nmol l−1. We also evaluated the association between the endpoint measures and TT levels using a meta-regression model for all the outcomes.
MATERIALS AND METHODS
Search strategy
The present meta-analysis was performed according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines for Meta-analyses and Systematic Reviews of Observational Studies18 and the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P).19 The MOOSE and PRISMA-P checklists have been included in Supplementary Table 1 and 2, respectively.
Supplementary Table 1.
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols 2020 checklist
| PRISMA 2020 checklist section and topic | Location where item is reported | ||
|---|---|---|---|
|
| |||
| Title | |||
| Title | 1 | Identify the report as a systematic review | Page 1 |
|
| |||
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for abstracts checklist | Page 2–3 |
| Introduction | |||
|
| |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge | Page 4–5 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses | Page 5 |
|
| |||
| Methods | |||
|
| |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses | Table 1, page 6 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted | Page 5–6 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used | Page 5–6 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process | Page 5–6 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process | Page 5–6 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect | Page 5–7 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information | Page 5–7 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool (s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process | Page 7–8 |
| Effect measures | 12 | Specify for each outcome the effect measure (s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results | Page 7–8 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis [item #5]) | Page 7–8 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions | Page 7–8 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses | Page 7–8 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice (s). If meta-analysis was performed, describe the model (s), method (s) to identify the presence and extent of statistical heterogeneity, and software package (s) used | Page 7–8 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression) | Page 7–8 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results | Page 7–8 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases) | Page 7–8 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome | Page 7–8 |
|
| |||
| Results | |||
|
| |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram | Page 8–9 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded | Page 8–9, Figure 1 and Supplementary Table 3 | |
| Study characteristics | 17 | Cite each included study and present its characteristics | Page 8–10 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study | Page 11–12 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) Summary statistics for each group (where appropriate) and (b) An effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots | Page 12–16 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies | Page 12–16 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect | Page 12–16 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results | Page 12–16 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results | Page 12–16 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed | Page 12–16 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed | Page 12–16 |
|
| |||
| Discussion | |||
|
| |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence | Page 16 |
| 23b | Discuss any limitations of the evidence included in the review | Page 22 | |
| 23c | Discuss any limitations of the review processes used | Page 22 | |
| 23d | Discuss implications of the results for practice, policy, and future research | Page 22–23 | |
|
| |||
| Other information | |||
|
| |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered | - |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared | - | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol | - | |
| Support | 25 | Describe sources of financial or nonfinancial support for the review, and the role of the funders or sponsors in the review | - |
| Competing interests | 26 | Declare any competing interests of review authors | - |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review | - |
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. For more information, visit: PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols. Available from: https://www.prisma-statement.org/ (last accessed on 15 March, 2023)
Supplementary Table 2.
Meta-Analysis of Observational Studies in Epidemiology checklist for meta-analyses of observational studies
| Item number | Recommendation | Reported on page number |
|---|---|---|
|
| ||
| Reporting of background should include | ||
| 1 | Problem definition | 4 |
| 2 | Hypothesis statement | 5 |
| 3 | Description of study outcome(s) | 7 |
| 4 | Type of exposure or intervention used | 5–6 |
| 5 | Type of study designs used | 5–6 |
| 6 | Study population | 5–6 |
|
| ||
| Reporting of search strategy should include | ||
|
| ||
| 7 | Qualifications of searchers (e.g., librarians and investigators) | 5–7 |
| 8 | Search strategy, including time period included in the synthesis and key words | 5–7 |
| 9 | Effort to include all available studies, including contact with authors | 6 |
| 10 | Databases and registries searched | 5 |
| 11 | Search software used, name and version, including special features used (e.g., explosion) | 6 |
| 12 | Use of hand searching (e.g., reference lists of obtained articles) | 6 |
| 13 | List of citations located and those excluded, including justification | Supplementary Table 3 |
| 14 | Method of addressing articles published in languages other than English | 5–6 |
| 15 | Method of handling abstracts and unpublished studies | 5–6 |
| 16 | Description of any contact with authors | 5–6 |
|
| ||
| Reporting of methods should include | ||
|
| ||
| 17 | Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested | 8–16 |
| 18 | Rationale for the selection and coding of data (e.g., sound clinical principles or convenience) | 8–16 |
| 19 | Documentation of how data were classified and coded (e.g., multiple raters, blinding and interrater reliability) | 8–16 |
| 20 | Assessment of confounding (e.g., comparability of cases and controls in studies where appropriate) | 8–16 |
| 21 | Assessment of study quality, including blinding of quality assessors, stratification or regression on possible predictors of study results | 8–16 |
| 22 | Assessment of heterogeneity | 8–16 |
| 23 | Description of statistical methods (e.g., complete description of fixed or random effects models, justification of whether the chosen models account for predictors of study results, dose-response models, or cumulative meta-analysis) in sufficient detail to be replicated | 8–16 |
| 24 | Provision of appropriate tables and graphics | Figure 2–7 and Supplementary Figure 1 (93.2KB, tif) –16 (77.2KB, tif) |
|
| ||
| Reporting of results should include | ||
|
| ||
| 25 | Graphic summarizing individual study estimates and overall estimate | Figure 2–7 and Supplementary Figure 1 (93.2KB, tif) –16 (77.2KB, tif) |
| 26 | Table giving descriptive information for each study included | Table 2 |
| 27 | Results of sensitivity testing (e.g., subgroup analysis) | Supplementary Figure 1 (93.2KB, tif) –16 (77.2KB, tif) |
| 28 | Indication of statistical uncertainty of findings | 8–16 |
|
| ||
| Reporting of discussion should include | ||
|
| ||
| 29 | Quantitative assessment of bias (e.g., publication bias) | 22–23 |
| 30 | Justification for exclusion (e.g., exclusion of non-English language citations) | 22–23 |
| 31 | Assessment of quality of included studies | 22–23 |
|
| ||
| Reporting of conclusions should include | ||
|
| ||
| 32 | Consideration of alternative explanations for observed results | 17–19 |
| 33 | Generalization of the conclusions (i.e., appropriate for the data presented and within the domain of the literature review) | 22–24 |
| 34 | Guidelines for future research | 22–24 |
| 35 | Disclosure of funding source | - |
From: Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA 2000; 283: 2008–12
The following search strategy was used: “(TITLE-ABS-KEY (trt) AND TITLE-ABS-KEY (thromb*) OR TITLE-ABS-KEY (testosterone) AND TITLE-ABS-KEY (thromb*) OR TITLE-ABS-KEY (trt) AND TITLE-ABS-KEY (embol*) OR TITLE-ABS-KEY (testosterone*) AND TITLE-ABS-KEY (embol*)) ( ALL (trt) AND ALL (thromb*) OR ALL (testosterone) AND ALL (thromb*) OR ALL (trt) AND ALL (embol*) OR ALL (testosterone*) AND ALL (embol*)) (ALL (testosterone) AND ALL (thromb*) OR ALL (testosterone) AND ALL (embol*))”.
These searches were performed in the Scopus, PubMed, Cochrane, and Embase databases, from their inception through November 2022. The search was limited to original articles, studies on humans only, and without language restrictions. After the duplicates were eliminated, the identified abstracts were screened for eligibility by the researchers.
Selection criteria
Full-text articles of eligible abstracts, including non-English abstracts, were downloaded and translated into English if needed. They were assessed for eligibility using the Population, Intervention, Comparison/Comparator, Outcome, Study type (PICOS) model system20 (Supplementary Table 3). The selection of eligible studies was carried out by 3 researchers (CG, CL, and VG). For each article, the eligibility assessment was performed by two reviewers independently in a non-blinded manner. The titles and abstracts of the studies were first independently screened for inclusion. In cases of uncertainty, each researcher reviewed the full text to determine inclusion. Any disagreement between the reviewers was resolved by discussion between the two reviewers. However, if a consensus was not reached, another reviewer made the final decision (RC). The selected articles were finally subjected to data extraction.
Supplementary Table 3.
Inclusion and exclusion criteria according to the population, intervention, comparison/comparator, outcomes, study type model20
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Patients with TT levels T <12 nmol l−1 | Eugonadal patients, women |
| Intervention | TRT | Other treatments (e.g., SERMs, AIs) alone or in combination with testosterone |
| Comparison | Placebo or no therapy | Other treatments (e.g., SERMs, AIs) |
| Outcomes | Arterial thrombotic events (all districts, stroke, myocardial infarction, upper limbs, lower limbs) VTE (all districts, DVT, portal vein thrombosis, splenic thrombosis, pulmonary embolism) Mortality | / |
| Study type | Observational studies, randomized controlled studies, case-control studies | Animal studies, in vitro studies, review and meta-analyses, case reports, book chapters, editorials |
AIs: aromatase inhibitors; DVT: deep vein thrombosis; SERMs: selective estrogen receptor modulators; VTE: venous thromboembolism; TRT: testosterone replacement therapy; TT: total testosterone
Data extraction
The following data were collected: study design, the timing when the event took place, presence of thrombophilia, D-dimer levels, fibrinogen, partial thromboplastin time (PTT), international normalized ratio (INR), hematocrit, TT levels before and during treatment, levels of 17β-estradiol (E2), and presence of Klinefelter syndrome. The number of events and population size was collected for each outcome in the treated and untreated groups.
Quality assessment
The quality of evidence (QoE) of the studies was assessed by 3 investigators (CG, CL, and VG). In detail, all studies were assessed using Cambridge Quality Checklists.21 Further assessment of QoE was performed for RCTs using the Cochrane Risk of Bias scale.22
Statistical analyses
Quantitative data analysis was performed using the Comprehensive Meta-Analysis Software (version 3; Biostat Inc., Englewood, NJ, USA) and the Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, 2020; https://community.cochrane.org/organizational-info/resources/policies/policies-all-members-and-supporters/cochrane-privacy-policy#aboutus) for meta-analysis of quantitative data. The odds ratio (OR) was used as the effect size for statistical comparison between cases and controls, and P ≤ 0.05 was considered statistically significant. Cochran’s Q test and heterogeneity index (I2) were used to assess heterogeneity across pooled studies, with P < 0.10 considered statistically significant. I2 is an indicator of heterogeneity and lies between 0 and 100%, where <25% suggests low heterogeneity, 50% suggests moderate heterogeneity, and 75% suggests high heterogeneity. The pooled effect size was calculated using both the fixed effect and random effect models, depending on the level of heterogeneity. In the case of low heterogeneity, the fixed effect model was adopted, while in the case of significant heterogeneity, the random effects model was adopted. For each outcome, sub-analysis was performed based on the study design (namely, RCTs and observational studies). Cumulative analysis was performed to assess the chronological trend of statistical significance over a period. For this, the effect size and the corresponding CI were calculated after the addition of each new study in chronological order. The trend of the P-value and the statistical inference were used to draw inferences regarding the strength of the association, its vulnerability to variations, and the history of variations. The pooled effect size and the corresponding confidence interval (CI) were calculated after the exclusion of one study at a time. A study resulting in the change of inference upon its exclusion was labeled as a “sensitive study”. Publication bias was qualitatively analyzed by the asymmetry of the funnel plot, which suggested some missing studies on one side of the graph. For quantitative analysis of publication bias, we employed Egger’s intercept test, which evaluated the statistical significance of publication bias. In case of the presence of publication bias, unbiased estimates were calculated using the “trim and fill” method.23
RESULTS
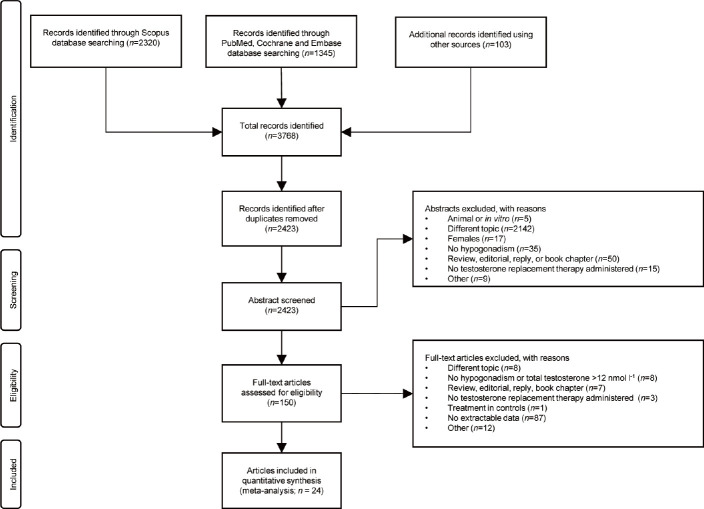
Using the aforementioned search strategy, 2320 abstracts were extracted using the Scopus database. Further research was carried out using PubMed, Cochrane and Embase (n=1345), and manually sifting the bibliographic citations of the most recent meta-analyses on the subject (n = 100). After duplicates removal, a total of 2423 records were screened, based on the main title and abstract examination. After the exclusion of 2273 abstracts, 150 full-text articles were assessed for eligibility. Of those, 126 were unsuitable for our study. Particularly, 87 full-text articles were excluded due to a lack of extractable data. Finally, 24 studies were included in the quantitative synthesis (Figure 1). A complete list of the studies excluded with reason is shown in Supplementary Table 4.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
Supplementary Table 4.
Studies excluded with reasons
| Study | Exclusion reason |
|---|---|
| Yarnell et al.64 | Females |
| Pastuszak et al.65 | Different outcomes |
| Abbe et al.66 | No original research |
| Walker et al.14 | No available data |
| Glueck et al.67 | Case report |
| Glueck et al.68 | Females |
| Li et al.69 | No numbers of events |
| Martinez et al.50 | No number of events |
| Glueck et al.70 | No available data |
| Baillargeon et al.49 | No number of events |
| Glueck et al.71 | Female |
| Haider et al.72 | Previous cardiovascular events |
| Kaufman et al.73 | No available data |
| Kalinchenko et al.74 | Different outcomes |
| Etminan et al.75 | No data available |
| Sharma et al.76 | No data available |
| Oni et al.77 | No data available |
| Baillargeon et al.78 | No hypogonadism |
| Kenny et al.79 | Total testosterone >12 nmol l−1 |
| Nair et al.80 | Total testosterone >12 nmol l−1 |
| Snyder et al.81 | Total testosterone >12 nmol l−1 |
Among the 24 studies included in this meta-analysis, we found 5 retrospective cohort studies, 4 observational studies, one prospective controlled registry study, and 14 RCTs (Table 1 and 2).
Table 1.
Main characteristics of the randomized controlled trials included in the analysis
| Study | Control group (n) | Patients for each TRT formulation (n) | Event timing (week) | Age (year), mean±s.d. | Hematocrit (%), mean±s.d. | Total testosterone (ng ml−1), mean±s.d. | Symptoms of hypoandrogenism | E2 (pg ml−1), mean±s.d. | Klinefelter among cases (yes/no) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||||||||
| Transdermal | Injective | Oral | Case | Control | Case | Control | Baseline (case) | Baseline (control) | TRT (case) | Placebo (control) | Case | Control | Case | Control | ||||
| Barnouin et al.24 | Placebo (41) | 42 | 26 | 73.6±3.7 | 72.2±3.2 | 42.0±0.5 | 40.6±0.6 | 2.1±0.1 | 2.2±0.1 | 3.1±0.2 | 0.6±0.2 | Not mention | 61.5±4.3 | 54.2±4.8 | - | - | ||
| Behre et al.42 | Placebo (179) | 183 | 24 | 61.9±6.6 | 62.1±6.3 | - | - | 3.0±0.7 | 3.1±0.7 | 5.8±4.4 | 3.1±1.0 | Yes | - | - | No | No | ||
| Brock et al.40 | Placebo (275) | 283 | 36 | 54.7±10.6 | 55.9±11.4 | - | - | 2.0±0.7 | 2.0±0.7 | - | - | Yes | - | - | No | No | ||
| Brock et al.37 | Placebo (357) | 358 | - | - | 12 | 53.9±10.8 | 55.4±11.1 | - | - | 2.0±0.7 | 2.0±0.7 | - | - | Yes | - | - | ||
| Snyder et al.25 | Placebo (394) | 394 | - | - | 52 | ≥65a | ≥65a | - | - | <2.8b | <2.8b | - | - | Yes | - | - | No | No |
| Tan et al.43 | Placebo (58) | - | 56 | - | 48 | 53.8±6.9c | 53.1±8.3c | 43.1±3.4c | 43.4±2.9c | 2.6±0.6c | 2.6±0.5c | 6.9±1.7 | 3.2±1.0 | Yes | - | - | No | No |
| Srinivas-Shankar et al.44 | Placebo (132) | 130 | - | - | 24 | 73.7±5.7 | 73.9±6.4 | 44.0±3.0 | 42.0±4.0 | 3.2±0.9 | 3.1±0.9 | 5.3±2.7 | 3.1±1.0 | Not mention | - | - | No | No |
| Basaria et al.38 | Placebo (151) | 155 | - | - | 156 | 66.9±5.0 | 68.3±5.3 | 43.7±3.7 | 43.6±3.6 | 3.1±0.6 | 3.1±0.7 | 5.7±2.5 | 3.3±1.0 | Not mention | 21.8±14.9 | 18.5±10.0 | No | No |
| Hildreth et al.26 | Placebo (47) | 96 | - | - | 52 | 66.5±5.8 | 66.5±5.2 | 46.3±3.0 | 46.7±3.0 | 3.0±0.4 | 2.9±0.4 | 5.3±2.9 | 2.9±0.7 | Not mention | - | - | No | No |
| Ng Tang Fui et al.27 | Placebo (51) | - | 49 | - | 56 | 54.3 (47.3–59.8)d | 52.8 (47.6–60.1)d | 43.0±2.0 | 44.0±2.0 | 2.4±0.7 | 2.4±0.7 | 4.1e | 2.9e | Not mention | - | - | No | No |
| Basaria et al.28 | Placebo (103) | 106 | - | - | 24 | 74.0±6.0 | 74.0±5.0 | - | - | 2.5±0.6 | 2.4±0.7 | 5.7±4.0 | 2.9±1.6 | Not mention | - | - | No | No |
| Emmelot-Vonk et al.29 | Placebo (110) | - | - | 113 | 27 | 67.1±5.0 | 67.4±4.9 | 46.0±3.0 | 45.0±2.0 | 3.2±0.5 | 3.0±0.5 | - | - | Not mention | - | - | - | - |
| Aversa et al.30 | Placebo (10) | - | 40 | - | 52 | 58.0±10.0 | 57.0±8.0 | 43.0±3.5 | 44.0±3.0 | 2.4±0.7 | 2.6±0.5 | 4.1±0.4 | 0.1±0.4 | Yes | - | - | - | - |
| Ho et al.31 | Placebo (58) | - | 56 | - | 48 | 53.4±7.4 | 53.0±8.2 | - | - | 2.6±0.6 | 2.6±0.5 | 6.8e | 3.2e | Yes | - | - | - | - |
aMinimum. bMaximum. cMedian±s.d.. dMedian (IQR). eMean. E2: 17β-estradiol; TRT: testosterone replacement therapy; s.d.: standard deviation; -: not available
Table 2.
Main characteristics of the observational studies included in the analysis
| Study | Type of study | Control group (n) | Patients for each TRT formulation (n) | Event timing (week) | Age (year), mean±s.d. | Hematocrit (%), mean±s.d. | Total testosterone (ng ml−1), mean±s.d. | Symptoms of hypoandrogenism | E2 (pg ml−1), mean±s.d. | Klinefelter among cases (yes/no) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||||||||
| Transdermal | Injective | Oral | Case | Control | Case | Control | Baseline (case) | Baseline (control) | TRT (case) | Placebo (control) | Case | Control | Case | Control | |||||
| Sharma et al.41 | Retrospective cohort study | No treatment (10 854) | 38 362 | - | 64.0±11.2 | 66.6±13.1 | - | - | - | - | - | - | Not mention | - | - | - | - | ||
| Maggi et al.32 | Retrospective study | No treatment (249) | 510 | 225 | 15 | 165 | 58.9±10.3 | 59.7±11.1 | - | - | 2.4±1.1 | 2.7±1.1 | 4.4a | 3.3a | Not mention | - | - | - | - |
| Ramasamy et al.33 | Retrospective study | No treatment (64) | 47 | 53 | - | 187 | 74.0±6.3 | 75.0±6.0 | 45.0±5.0 | 42.0±5.0 | - | - | 4.8±3.4 | 2.4±0.5 | Yes | 37.0±21.0 | 26.0±7.0 | - | - |
| Vigen et al.6 | Retrospective cohort study | No treatment (7486) | 1223 | 75 | 60.6±7.6 | 63.8±9.0 | - | - | 1.8±0.6 | 2.1±0.7 | - | - | Not mention | - | - | No | No | ||
| Cheetham et al.39 | Retrospective cohort study | No treatment (35 527) | 8808 | 132 | 58.4a | 59.8a | - | - | 3.18a | - | - | - | Not mention | - | - | No | No | ||
| Traish et al.34 | Observational study | No treatment (296) | - | 360 | - | 416 | 57.4±7.3 | 64.8±4.3 | - | - | 2.8±0.4 | 2.8±0.4 | 4.8±0.5 | 2.6±0.4 | Yes | - | - | Yes | Yes |
| Yassin et al.35 | Prospective controlled registry study | No treatment (184) | - | 321 | - | 624 | 59.0±9.5 | 66.1±7.6 | - | - | 2.2±0.6 | 2.7±0.7 | 4.6a | 2.7a | Yes | - | - | - | - |
| Muraleedharan et al.45 | Observational study | No treatment (174) | 60 | 1 | 3 | 187 | 60.9±11.8 | 58.5±10.4 | - | - | 1.9±0.7 | 2.2±0.5 | - | - | Not mention | - | - | - | - |
| Eisenberg et al.46 | Observational study | No treatment (225) | 204 | 80 | - | - | 54.1±8.7 | 54.9±11.1 | - | - | 3.3±2.0 | 3.6±1.4 | - | - | Not mention | - | - | - | - |
| Shores et al.36 | Observational cohort study | No treatment (122 302) | 43 502 | 39 053 | - | 223.6 | 61.7±10.2 | 59.8±9.4 | - | - | 2.1±1.3 | 1.7±0.7 | - | - | Not mention | - | - | - | - |
aMean. E2: 17β-estradiol; TRT: testosterone replacement therapy; s.d.: standard deviation; -: not available
Quality of evidence of included studies
All 24 included studies were assessed using the Cambridge Quality Checklist. Although this scale does not establish a precise threshold for differentiating between high- and low-quality studies, out of a total score of 15, seventeen studies scored >10, six studies scored 6 to 10, and one study scored <6. Fourteen RCT studies were also evaluated using the Cochrane risk bias for RCTs (Supplementary Table 5).
Supplementary Table 5.
Quality of evidence assessment of the included studies (results of the Cambridge Quality Checklist,21 Cochrane risk of bias for randomized controlled trials22)
| Study | Type of study | Cambridge Quality Checklists | Cochrane risk of bias for RCTs (risk of bias) | ||
|---|---|---|---|---|---|
|
| |||||
| Checklist for correlates | Checklist for risk factors | Checklist for causal risk factors | |||
| Sharma et al.41 | Retrospective cohort study | 3 | 2 | 4 | - |
| Barnouin et al.42 | RCT | 4 | 3 | 7 | High |
| Shores et al.36 | Retrospective cohort study | 3 | 2 | 4 | - |
| Maggi et al.32 | Retrospective study | 3 | 3 | 6 | - |
| Ramasamy et al.33 | Retrospective study | 0 | 2 | 4 | - |
| Behre et al.24 | RCT | 2 | 3 | 7 | High |
| Brock et al.40 | RCT | 3 | 3 | 7 | High |
| Brock et al.37 | RCT | 2 | 3 | 7 | Some concerns |
| Snyder et al.25 | RCT | 3 | 3 | 7 | High |
| Tan et al.43 | RCT | 2 | 3 | 7 | High |
| Srinivas-Shankar et al.44 | RCT | 4 | 3 | 7 | High |
| Basaria et al.38 | RCT | 4 | 3 | 7 | High |
| Hildreth et al.26 | RCT | 3 | 3 | 7 | High |
| Ng Tang Fui et al.27 | RCT | 3 | 3 | 7 | High |
| Basaria et al.28 | RCT | 3 | 3 | 7 | High |
| Vigen et al.6 | Retrospective cohort study | 2 | 2 | 5 | - |
| Cheetham et al.39 | Retrospective cohort study | 2 | 2 | 5 | - |
| Traish et al.34 | Observational study | 3 | 2 | 5 | - |
| Emmelot-Vonk et al.29 | RCT | 2 | 3 | 7 | High |
| Aversa et al.30 | RCT | 1 | 3 | 1 | High |
| Ho et al.31 | RCT | 1 | 3 | 7 | High |
| Yassin et al.35 | Prospective controlled registry study | 2 | 3 | 5 | - |
| Muraleedharan et al.45 | Observational study | 1 | 2 | 5 | - |
| Eisenberg et al.46 | Observational study | 3 | 3 | 5 | - |
RCT: randomized controlled trial
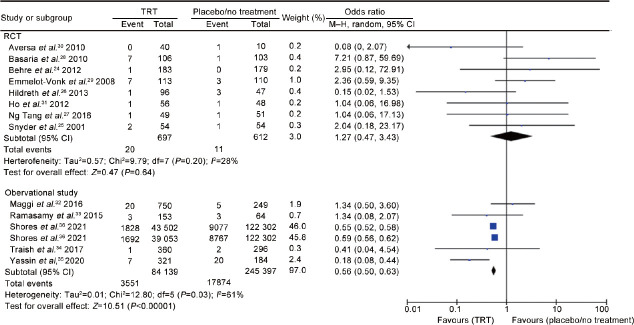
Arterial thrombotic events (all districts)
Eight RCTs24,25,26,27,28,29,30,31 including 1309 men (697 cases vs 612 controls), and 5 observational studies32,33,34,35,36 including 329 536 men (84 139 cases vs 245 397 controls) evaluated arterial thrombotic events in any district. The analysis showed the presence of inter-study heterogeneity, as demonstrated by the Q-test (Q-value = 27.6; P = 0.010) and I2 = 53%. In RCTs, TRT did not influence the risk of arterial thrombosis (OR = 1.27, 95% CI: 0.47–3.43, P = 0.64). According to the evidence coming from observational studies, the risk of arterial thrombosis was significantly reduced in patients compared to controls (OR = 0.56, 95% CI: 0.50–0.63, P < 0.00001; Figure 2).
Figure 2.
Risk of arterial thrombotic events in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. TRT: testosterone replacement therapy; RCT: randomized controlled trial; df: degree of freedom; CI: confidence interval; M–H: Mantel–Haenszel.
There was no evidence of publication bias as shown by Egger’s test (P = 0.50) and the symmetry of the funnel plots (Supplementary Figure 1a (93.2KB, tif) ) of arterial thrombotic events. No study was sensitive enough to alter the above-reported results (Supplementary Figure 1b (93.2KB, tif) ).
The cumulative analysis showed that the reduced risk of arterial thrombosis in patients with TRT remains significant with the addition of each study (Supplementary Figure 2 (73.8KB, tif) ).
Meta-regression analysis showed no significant association between the risk of developing an arterial thrombosis and TT levels (either measured before or after treatment) both in the hypotestosteronemic patients while undergoing TRT (patient group) and in the untreated hypotestosteronemic patients (control group), as shown in Supplementary Figure 3 (64.3KB, tif) .
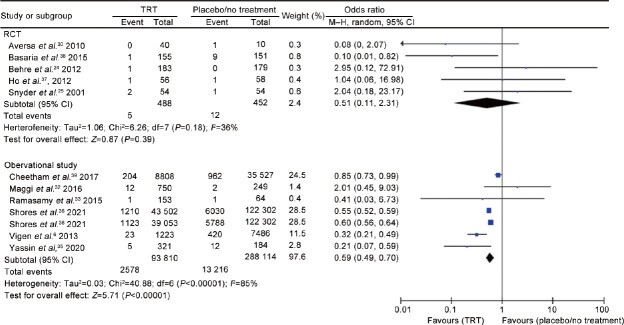
Stroke
Two RCTs37,38 including 1021 men (513 cases vs 508 controls) and 6 observational studies6,32,33,35,36,39 including 381 924 men (93 810 cases vs 288 114 controls) evaluated the risk of stroke. The analysis showed the presence of significant inter-study heterogeneity, as demonstrated by the Q-test (Q-value = 581.4; P < 0.0001) and I2 = 99%. According to the RCTs, TRT did not influence the risk of stroke (OR = 1.34, 95% CI: 0.09–18.97, P = 0.83). Similarly, considering the evidence coming only from the observational studies, the risk of stroke did not significantly differ between patients and controls (OR = 0.62, 95% CI: 0.26–1.46, P = 0.27; Figure 3).
Figure 3.
Risk of stroke in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. TRT: testosterone replacement therapy; RCT: randomized controlled trial; df: degree of freedom; CI: confidence interval; M–H: Mantel–Haenszel.
There was no evidence of publication bias as shown by Egger’s test (P = 0.86) and the symmetry of the funnel plots (Supplementary Figure 4a (76.1KB, tif) ). No study was sensitive enough to alter the above-reported results (Supplementary Figure 4b (76.1KB, tif) ). The cumulative analysis showed that the risk of stroke reached a non-significant value with the addition of the study by Shores et al.,36 to the pool and remained non-significant after that (Supplementary Figure 5 (79.9KB, tif) ). There were no enough studies to perform the meta-regression analysis.
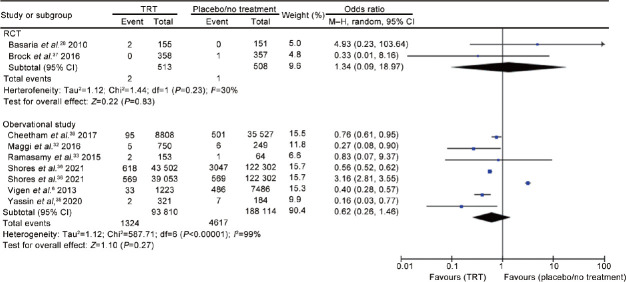
Myocardial infarction
Five RCTs24,25,30,31,38 including 940 men (488 cases vs 452 controls) and six observational studies6,32,33,35,36,39 including 381 924 men (93 810 cases vs 288 114 controls) evaluated the risk of myocardial infarction. The analysis showed the presence of inter-study heterogeneity, as demonstrated by the Q-test (Q-value = 47.1; P < 0.0001) and I2 = 77%. In the RCTs, TRT did not influence the risk of myocardial infarction (OR = 0.51, 95% CI: 0.11–2.31, P = 0.39). According to the evidence coming from the observational studies, TRT was associated with a reduced risk of myocardial infarction (OR = 0.59, 95% CI: 0.49 –0.70, P < 0.00001; Figure 4).
Figure 4.
Risk of myocardial infarction in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. TRT: testosterone replacement therapy; RCT: randomized controlled trial; df: degree of freedom; CI: confidence interval; M–H: Mantel–Haenszel.
There was no evidence of publication bias as shown by Egger’s test (P = 0.93) and the symmetry of the funnel plots (Supplementary Figure 6a (87.6KB, tif) ) of myocardial infarction. No study was sensitive enough to alter the above-reported results (Supplementary Figure 6b (87.6KB, tif) ). The cumulative analysis showed that the reduced risk of myocardial infarction in patients with TRT remains significant with the addition of each study (Supplementary Figure 7 (79.6KB, tif) ).
Meta-regression analysis showed that the risk of developing myocardial infarction in hypotestosteronemic patients was significantly correlated with serum TT levels measured during the treatment (Supplementary Figure 8 (65.8KB, tif) ).
Venous thromboembolism (all districts)
Three RCTs24,37,40 (824 cases vs 811 controls) and five observational studies32,33,35,36,41 (122 141 cases vs 255 955 controls) evaluated the risk of venous thrombosis. The analysis showed the presence of inter-study heterogeneity, as demonstrated by the Q-test (Q-value = 26.3; P = 0.001) and I2 = 70%. Evidence coming from RCTs showed no significantly different risk in patients versus controls (OR = 1.42, 95% CI: 0.22–9.03, P = 0.71). According to observational studies, the risk of venous thrombosis was significantly reduced in patients compared to controls (OR = 0.78, 95% CI: 0.61–1.00, P = 0.05; Supplementary Figure 9 (83.4KB, tif) ).
There was no evidence of publication bias as shown by Egger’s test (P = 0.125) and the symmetry of the funnel plots (Supplementary Figure 10a (79.9KB, tif) ). Three studies35,36,40 were sensitive enough to alter the above-reported results. Their removal resulted in the loss of significance (Supplementary Figure 10b (79.9KB, tif) ). The cumulative analysis showed the achievement of statistical significance after the addition of the study by Yassin et al.35 (Supplementary Figure 11 (81.9KB, tif) ). There were no enough studies to perform the meta-regression analysis.
Deep vein thrombosis (DVT)
Only one RCT24 and two observational studies32,41 (39 112 cases vs 11 103 controls) evaluated the risk of DVT. Due to the absence of heterogeneity, as demonstrated by the Q-test (Q-value = 0.2; P = 0.893) and I2 = 0, the fixed effect model was used. According the observational studies, TRT was associated with an increased risk of DVT (OR = 1.44, 95% CI: 1.04–2.00, P = 0.03). Only one RCT was included.24 which reported no difference in the DVT risk (Supplementary Figure 12 (68.8KB, tif) ).
There was no evidence of publication bias as shown by Egger’s test (P=0.095) and the symmetry of the funnel plots (Supplementary Figure 13a (69.3KB, tif) ) of DVT. The study by Sharma et al.41 was sensitive enough to alter the above-reported results (OR = 1.854, 95% CI: 0.468–7.348, P = 0.38; Supplementary Figure 13b (69.3KB, tif) ). The cumulative analysis showed no change in the results with the addition of any of the studies (Supplementary Figure 14 (66.4KB, tif) ). There were no enough studies to perform the meta-regression analysis.
Pulmonary embolism
Three RCTs37,40,42 including 683 cases and 673 controls and four observational studies32,33,35,41 including 1431 cases and 538 controls evaluated pulmonary embolism. Due to the absence of significant heterogeneity, as demonstrated by the Q-test (Q-value = 2.4; P = 0.881) and I2 = 0, the fixed effect model was used. In RCTs, TRT did not influence the risk of pulmonary embolism (OR = 1.38, 95% CI: 0.27–7.04, P = 0.70). Similarly, the evidence coming from observational studies showed no significantly different risk in patients versus controls (OR = 0.76, 95% CI: 0.35–1.75, P = 0.67; Supplementary Figure 15 (84.7KB, tif) ).
There was no evidence of publication bias as shown by Egger’s test (P = 0.41) and the symmetry of the funnel plots (Supplementary Figure 16a (77.2KB, tif) ). No study was sensitive enough to alter the above-reported results (Supplementary Figure 16b (77.2KB, tif) ). The cumulative analysis showed no change in the results with the addition of any of the studies (Supplementary Figure 17 (79.5KB, tif) ). There were no enough studies to perform the meta-regression analysis.
Mortality
Four RCTs38,40,43,44 including 624 cases and 614 controls and seven observational studies6,33,35,39,45,46 including 11 213 cases and 43 956 controls, evaluated mortality in association with TRT. The analysis showed the presence of heterogeneity among the studies, as demonstrated by the Q-test (Q-value = 27.8; P = 0.002) and I2 = 64%. In RCTs, TRT did not influence the risk of mortality (OR = 0.70, 95% CI: 0.20–2.38, P = 0.56). According to observational studies, the risk of mortality was significantly reduced in patients versus controls (OR = 0.51, 95% CI: 0.35–0.76, P = 0.0001; Supplementary Figure 18 (92KB, tif) ).
There was evidence of publication bias as shown by Egger’s test (P = 0.021) and the asymmetry of the funnel plots (Supplementary Figure 19a (80.4KB, tif) ) of mortality. No study was sensitive enough to alter the final result (Supplementary Figure 19b (80.4KB, tif) ). The cumulative analysis showed that the risk of overall mortality reached a non-significant value with the addition of two studies.28,44 It returned to reach significance after the addition of the other studies (Supplementary Figure 20 (81.5KB, tif) ). There were no enough studies to perform the meta-regression analysis.
DISCUSSION
Key findings
This systematic review and meta-analysis aimed to assess the association between TRT, arterial thrombosis, and VTE in men with TT <12 nmol l−1. This task was accomplished by selecting and analyzing data from several studies on this topic. Overall, 24 studies were selected and included. The data analyzed concerned serum TT levels before and after TRT, mean hematocrit value during treatment with T or placebo, mean serum E2 values, information on thrombophilia, and the risk of developing arterial or venous thromboembolic events. The outcomes analyzed were arterial thrombosis, stroke, myocardial infarction, VTE, DVT, pulmonary embolism, and mortality.
Data analyses showed that TRT does not influence the risk of arterial events, including stroke and pulmonary embolism, of developing arterial and venous thrombosis and myocardial infarction, and the risk of mortality. Meanwhile, when only observational studies are considered, a significant reduction in the risk of developing arterial thrombotic events, MI, venous thromboembolism and mortality was observed. The risk of developing DVT remains uncertain, due to limited RCT-derived evidence.
The sensitive analysis of three outcomes (arterial thrombosis, stroke, and venous thrombosis) showed that the study by Shores et al.36 was sensitive enough to skew the results. One possible cause could be the way T exposure was calculated. Contrary to many studies comparing patients using T with patients not receiving therapy or receiving a placebo, Shores et al.36 compared current users of T with previous ones. This was done to evaluate the association between current T exposure and cardiovascular events. In addition, the databases used in the study did not contain any information on signs or symptoms of T deficiency or indications for T treatment. Furthermore, patients were assumed to have started treatment at the date of compilation and were compliant with the treatment. Removal of this study resulted in a significant reduction in the risk of vascular events.
Importance of the topic
The value of our data lies in the presence of controversies in the literature regarding TRT, endogenous serum T levels, and CV/thromboembolic risk.
In January 2014, the FDA launched a safety warning for T-containing drugs as two studies had shown a correlation between T administration and adverse CV effects, such as heart attack and stroke. This led to a notable reduction in TRT prescriptions.8 TRT is the primary treatment of hypogonadism, a clinical syndrome resulting from the failure of the testis to produce physiological testosterone concentrations,1,2 and which, if left untreated, leads to poor quality of life causing loss of muscle mass and strength, sexual dysfunctions, osteoporosis, and, according to some studies, even an increased risk of CV events.47,48 Given the importance of treating hypogonadism, several studies have focused on the presumed association between TRT, CV events, and VTE. On the other hand, authors also investigated the relationship between endogenous T and CV/thromboembolic risk. Therefore, the present systematic review and meta-analysis aimed to probe the current literature on the association between TRT and CV/thromboembolic risk to assess whether or not this association is real in patients with hypotestosteronemia so that specialists can prescribe T with greater awareness with a benefit for patients.
Some studies reported an association between TRT and adverse CV events. Particularly, the studies that led the FDA to issue the aforementioned warning on TRT found a higher risk of death, heart attack, and stroke in patients who underwent TRT, compared to the untreated group,6 and of myocardial infarction in the 90 days after the T prescription.7 Similarly, in a parallel-group, randomized, placebo-controlled, double-blind trial conducted on 209 patients, Basaria et al.28 found more CV-related adverse events in patients receiving T than in the placebo group.
Even the association between TRT and venous thromboembolism has been investigated but contrasting results have emerged. Baillargeon et al.49 did not find any association between TRT and VTE in middle-aged and older men in their case-control study. Ayele et al.15 also found no association between TRT and VTE. Conversely, a case-control study showed an association between TRT and the risk of VTE within the first six months after prescription. After this time, the association was no longer significant.50
Regarding the association between endogenous T levels and CV/thromboembolic risk, there is contrasting evidence in the literature. According to some studies, low endogenous levels of T appear to be associated with an increased risk of CV diseases, CV death, and all-cause of death.47,48 Yeap et al.48 found that lower TT levels predict an increased incidence of stroke and transient ischemic attack (TIA). In particular, the hazard ratio (HR) for incidence of stroke or TIA was 1.39 for men with TT <8 nmol l−1, and 2.08 for men with TT ≥8 nmol l−1 and <11.7 nmol l−1, compared with men with TT ≥11.7 nmol l−1.48 Notably, the risk was not significantly higher in men with TT <8 nmol l−1, but authors attributed this to the small number of men and events in this group. Anyway, men with TT levels <11.7 nmol l−1, have shown a significantly higher risk. Khaw et al.47 found an inverse correlation between endogenous T levels and mortality due to CV diseases and all causes. In contrast, Corona et al.51 suggested hypogonadism as a protective factor in men with a high CV risk burden, since in their study, T <12 nmol l−1 were associated with a lower incidence of CV disease in patients with high CV risk. Studies have also analyzed the association between endogenous T and arterial thrombosis. For example, Lou et al.,12 based on a two-sample Mendelian randomization study, suggest that endogenous T could be considered as a modifiable risk factor for thromboembolism and heart failure since they found a positive association between endogenous T levels and thromboembolism, heart failure, and myocardial infarction in men. As for VTE, Svartberg et al.52 did not find any association between endogenous T and a 10-year risk of VTE in middle-aged and older men. Similar results emerged from the study by Holmegard et al.53 in which T levels were not associated with the risk of VTE, pulmonary embolism, and DVT.
Finally, there are contrasting data regarding both the association between endogenous T levels and CV/thromboembolic risk and also between TRT and CV/thromboembolic risk. Our purpose was to clarify the second association, using a meta-regression approach.
Comparison with previous meta-analyses
Since T approval in medical use in 1939, association studies between TRT and major CV events have been published from 2000 onward. The main meta-analyses are briefly described in Supplementary Table 6, with details on population, studies included, time of observation, and arterial or venous thrombosis outcomes. Most of them have not found an increased risk of CV events in patients treated with T, either in the venous or arterial districts. On the contrary, the single meta-analysis that showed an association between TRT and arterial CV events was published in 2014, referring specifically to the oral formulation and the authors did not find an increased risk with the cutaneous and parenteral formulation.54
Supplementary Table 6.
Main published meta-analyses on testosterone-replacing therapy and cardiovascular events
| Author | Year of publication | Issue | Studies included | Number of studies analyzed | Intervention | Number of patients, total (case; control) | Female patients (yes/no) | Klinefelter patients | Thrombophilic patients | Hypogonadism (yes/no) | Observation time (minimum–maximum), months | Result: Increased risk (yes/no) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fallara et al.82 | 2022 | Mortality, CV events (arterial) | RCT; retrospective registry based | 10 | TRT vs placebo | 179,631 (89,515; 90,116) | No | NR | NR | Yes | 6–74.4 | No |
| Hudson et al.17 | 2022 | Mortality, CV and cerebrobascular events (arterial) | Treated-placebo | 17 | TRT vs placebo | 3431 (1750; 1681) | No | NR | NR | Yes | 4–36 | No |
| Cannarella et al.83 | 2022 | Heart failure | RCT | 7 | TRT vs placebo or no treatment | 140 (71; 69) | No | NR | NR | Yes | 2–12 | No |
| Ayele et al.15 | 2021 | Venous TE | RCT | 13 | TRT vs placebo or active-comparator | 5050 (2636; 2414) | No | NR | NR | NT | 3–36 | No |
| Sansone et al.84 | 2020 | Endothelial function (flow-mediated dilation) | RCT, observational | 6 | TRT vs placebo | Random effects model (86) | No | NR | NR | Yes | 0–12 | No |
| Corona et al.10 | 2018 | Major adverse CV events | RCT, pharmaco-epidemiological studies | 93 | TRT vs placebo | 8479 (4653; 3826) | No | NR | NR | NT | 11 (mean) | No |
| Borst et al.54 | 2014 | CV events (arterial) | RCT | 35 | TRT vs placebo | 3703 (2114; 1589) | No | NR | NR | NT | 3–62 (mean: 11.9) | Yes (only oral) |
| Corona et al.85 | 2011 | CV morbidity and mortality | Cross-sectional studies, longitudinal studies, RCT | 70 | TRT vs placebo | 25,299 (total) | No | NR | NR | Yes | 3–36 | ND |
| Calof et al.56 | 2005 | CV events (arterial) | Clinical trials | 19 | TRT vs placebo | 1084 (651; 433) | No | NR | NR | NT | 3–36 | No |
RCT: randomized controlled trials; ND: not determined; NR: not reported; NT: not totally; TE: thromboembolism; TRT: testosterone replacement therapy; CV: cardiovascular
Current meta-analyses are limited by the lack of RCT-based evidence,55 and the inclusion of patients with serum TT within the normal range.5,10,15,53,56 Conversely, the present study strictly included only patient with TT <12 nmol l−1, and analyzed a well-sized population, which increases the statistical power of our study.
Limitations
This study had several limitations. The temporal range of observation is short-to-medium ranging from 12 weeks to 624 weeks, with a median of 36 weeks for RCTs and 165 weeks for observational studies. No long-term studies assessing the correlation between TRT and thromboembolism are currently available. This conclusion is also shared by another meta-analysis on adverse CV events and mortality in men during T treatment,17 where data evaluating the CV safety of T beyond a 12-month duration of administration are scarce.
Klinefelter syndrome (KS) is a frequent chromosome disorder causing male infertility and hypogonadism. Patients with KS have a predisposition to the development of thromboembolic events.57 The inclusion of these patients could be a limitation. However, in our meta-analysis, only one study included KS patients.34 No studies reviewed in this meta-analysis reported data on the presence or absence of thrombophilia in hypotestosteronemic patients treated with T. Patients with thrombophilia could potentially have been included. Nevertheless, the data support the absence of an increased thromboembolic risk in patients undergoing TRT. Our meta-analysis also considered observational studies,34,45,46 which reduce the quality of evidence. That data could not be extracted from as many as 87 studies represents an additional limitation. Finally, several endogenous factors (sex hormone binding globulin and sensitivity of androgen receptor), the different dosages and route of administration, and the timing when the blood examination was performed, can influence the serum testosterone levels.
Wider implications
The main endocrinological, andrological, and urological associations/societies have drawn up their guidelines for the treatment of hypogonadism, also addressing the issue of CV risk.
The Endocrine Society (ES), American Urological Association (AUA), EAA, European Association of Urology (EAU), American Association of Clinical Endocrinology (AACE), British Society for Sexual Medicine (BSSM), Società Italiana di Endocrinologia (SIE), Società Italiana di Andrologia e Medicina della Sessualità (SIAMS), International Consultation on Sexual Medicine (ICSM), and International Society for the Study of the Aging Male (ISSAM) substantially agree on the importance of hematocrit evaluation because of the association between elevated hematocrit and the risk of CV disease.58 In particular, they recommend against starting TRT in patients with elevated hematocrit and they also suggest its monitoring to reduce or discontinue TRT if it exceeds 54% (50% according to AACE and ICSM),1,5,11,59,60,61,62,63 as shown in Supplementary Table 7.
Supplementary Table 7.
Main society/association guidelines/consensus statement’s recommendation on the association between testosterone-replacing therapy and cardiovascular/thromboembolic risk
| Society/association | CV/thromboembolic risk | Recommendation against TRT | Prior to TRT |
|---|---|---|---|
| AUA | At this time, it cannot be stated definitively whether testosterone therapy increases or decreases the risk of CV events | Recent (3–6 months) CV event Hematocrit >54% | Hematocrit evaluation Inform patients that there is no substantive evidence of the increased risk of CV events with TRT |
| AACE | No specific recommendations on this issue are possible until further research clarifies the potential risks and benefits of therapy | Hematocrit >50% | Hematocrit evaluation |
| BSSM | Undefined | Hematocrit >54% | Hematocrit evaluation |
| EAA | When hypogonadism is properly diagnosed and managed, there is currently no consistent evidence of an increased risk of CV disease during TRT | Recent major acute CV event Severe heart failure Polycythemia Hematocrit >48%–50% | Hematocrit evaluation Obtain a detailed family and personal history of VTE and associated risk factors |
| ES | Undefined | Hematocrit<48% | Hematocrit evaluation |
| EAU | There is no substantive evidence that testosterone treatment, when replaced to the normal physiological range, is related to the development of major adverse CV events | Hematocrit >54% | Hematocrit evaluation Electrocardiogram |
| SIAMS/SIE | Evidence on the safety of TRT on the CV profile is of moderate quality | Recent CV event | Hematocrit evaluation Evaluate global CV risk and associated morbidities, including hematocrit levels Collect a detailed family, personal and clinical history of VTE, thrombophilia-hypofibrinolysis |
| ICSM | The weight of evidence indicates that TRT is not associated with increased CV risk Preliminary evidence suggests the possibility of beneficial effects of TRT on the CV function | Hematocrit >50% | Hematocrit evaluation |
| ISAAM | NR | Hematocrit >52% Untreated severe congestive heart failure | Hematocrit evaluation |
AUA: American Urological Association; CV: cardiovascular; TRT: testosterone replacement therapy; AACE: American Association of Clinical Endocrinology; BSSM: British Society for Sexual Medicine; EAA: European Academy of Andrology; ES: Endocrine Society; EAU: European Association of Urology; SIAMS: Società Italiana di Andrologia e Medicina della Sessualità; SIE: Società Italiana di Endocrinologia; ICSM: International Consultation on Sexual Medicine; NR: not reported; VTE: venous thromboembolism; ISAAM: International Society for the Study of the Aging Male
None of them places adverse CV events among those certainly related to TRT, but most of them conclude that there is no substantive evidence. Some authors suggest investigating the patients’ CV risk and risk of VTE. Others suggest performing an electrocardiogram before TRT, especially in men with previous CV disease. Some others indicate not initiating TRT in patients with recent acute CV events.
Our study contributes to increasing awareness of the association between TRT and CV/thromboembolic risk in patients with hypotestosteronemia. Based on the largest population analyzed so far, we found no association between TRT and an increased risk of arterial events, including stroke and pulmonary embolism, of developing arterial and venous thrombosis and myocardial infarction, according to both RCT- and observational study-based evidence. The risk of developing DVT remains uncertain, due to limited RCT-derived evidence, thus suggesting that further studies, specifically assessing the risk of DVT in hypotestosteronemic patients on TRT, are needed.
CONCLUSION
This systematic review and meta-analysis examined a large cohort, and provides a high level of evidence in favor of the safety of TRT, due to the absence of association between TRT and increased risk of arterial thrombotic events. The risk for DVT remains uncertain, due to the paucity of RCT-based data. Therefore, the present study reinforces the concept that TRT in hypotestosteronemic patients is safe from the risk of CV adverse events, and indicates the need for further studies that specifically assess the risk of DVT in hypotestosteronemic patients on TRT.
AUTHOR CONTRIBUTIONS
RC and AEC are the project managers and conceived the study. RC made the literature search and analyzed the data. CG, CL, and VG extracted the data. CG, CL, VG, and RC wrote the draft. FB, AC, and AEC reviewed the article. SLV and RAC supervised the study. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
(a) Funnel plot and (b) sensitivity analysis of the risk of arterial thrombotic events in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Cumulative analysis of the risk of arterial thrombotic events in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Meta-regression analysis on the effect of testosterone levels in patients (a) before and (b) after and in controls (c) before and (d) after testosterone replacement therapy (TRT) on the risk of arterial thrombotic events.
(a) Funnel plot and (b) sensitivity analysis of the risk of stroke in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Cumulative analysis of the risk of stroke in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
(a) Funnel plot and (b) sensitivity analysis of the risk of myocardial infarction in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Cumulative analysis of the risk of myocardial infarction in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Meta-regression analysis on the effect of testosterone levels in patients (a) before and (b) after and in controls (c) before and (d) after testosterone replacement therapy (TRT) on the risk of myocardial infarction.
Risk of venous thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of venous thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of venous thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Risk of deep vein thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of deep vein thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of deep vein thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Risk of pulmonary embolism in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of pulmonary embolism in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of pulmonary embolism in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Risk of mortality in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of mortality in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of mortality in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
REFERENCES
- 1.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, et al. Testosterone therapy in men with hypogonadism:an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–44. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 2.Yeap BB, Grossmann M, McLachlan RI, Handelsman DJ, Wittert GA, et al. Endocrine Society of Australia position statement on male hypogonadism (Part 1):assessment and indications for testosterone therapy. Med J Aust. 2016;205:173–8. doi: 10.5694/mja16.00393. [DOI] [PubMed] [Google Scholar]
- 3.Ross A, Bhasin S. Hypogonadism:its prevalence and diagnosis. Urol Clin North Am. 2016;43:163–76. doi: 10.1016/j.ucl.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, et al. European Association of Urology guidelines on sexual and reproductive health-2021 update:male sexual dysfunction. Eur Urol. 2021;80:333–57. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Isidori AM, Aversa A, Calogero A, Ferlin A, Francavilla F, et al. Adult- and late-onset male hypogonadism:the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE) J Endocrinol Invest. 2022;45:2385–403. doi: 10.1007/s40618-022-01859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 7.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lokeshwar SD, Bitran J, Ramasamy R. Changes in testosterone prescribing patterns after FDA warning. Transl Androl Urol. 2019;8:S287–8. doi: 10.21037/tau.2019.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, et al. Testosterone therapy in hypogonadal men:a systematic review and network meta-analysis. BMJ Open. 2017;7:e015284. doi: 10.1136/bmjopen-2016-015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, et al. Testosterone and cardiovascular risk:meta-analysis of interventional studies. J Sex Med. 2018;15:820–38. doi: 10.1016/j.jsxm.2018.04.641. [DOI] [PubMed] [Google Scholar]
- 11.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. 2020;8:970–87. doi: 10.1111/andr.12770. [DOI] [PubMed] [Google Scholar]
- 12.Luo S, Au Yeung SL, Zhao JV, Burgess S, Schooling CM. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: Mendelian randomisation study in UK Biobank. BMJ. 2019;364:l476. doi: 10.1136/bmj.l476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim W, Le Gal G, Bates SM, Righini M, Haramati LB, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism:diagnosis of venous thromboembolism. Blood Adv. 2018;2:3226–56. doi: 10.1182/bloodadvances.2018024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker RF, Zakai NA, MacLehose RF, Cowan LT, Adam TJ, et al. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern Med. 2020;180:190–7. doi: 10.1001/jamainternmed.2019.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayele HT, Brunetti VC, Renoux C, Tagalakis V, Filion KB. Testosterone replacement therapy and the risk of venous thromboembolism:a systematic review and meta-analysis of randomized controlled trials. Thromb Res. 2021;199:123–31. doi: 10.1016/j.thromres.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men:a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;18(11):108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson J, Cruickshank M, Quinton R, Aucott L, Aceves-Martins M, et al. Adverse cardiovascular events and mortality in men during testosterone treatment:an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. 2022;3:e381–93. doi: 10.1016/S2666-7568(22)00096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology:a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015:elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 20.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S, et al. PICO, PICOS and SPIDER:a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors:the Cambridge quality checklists. J Exp Criminol. 2009;5:1–23. [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, et al. RoB 2:a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. A nonparametric “trim and fill”method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 24.Behre HM, Tammela TL, Arver S, Tolrá JR, Bonifacio V, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male. 2012;15:198–207. doi: 10.3109/13685538.2012.699562. [DOI] [PubMed] [Google Scholar]
- 25.Snyder PJ, Peachey H, Berlin JA, Rader D, Usher D, et al. Effect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of age. Am J Med. 2001;111:255–60. doi: 10.1016/s0002-9343(01)00813-0. [DOI] [PubMed] [Google Scholar]
- 26.Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab. 2013;98:1891–900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet:a randomised controlled trial. BMC Med. 2016;14:153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men:a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 30.Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome:results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 31.Ho CC, Tong SF, Low WY, Ng CJ, Khoo EM, et al. A randomized, double-blind, placebo-controlled trial on the effect of long-acting testosterone treatment as assessed by the aging male symptoms scale. BJU Int. 2012;110:260–5. doi: 10.1111/j.1464-410X.2011.10755.x. [DOI] [PubMed] [Google Scholar]
- 32.Maggi M, Wu FC, Jones TH, Jackson G, Behre HM, et al. Testosterone treatment is not associated with increased risk of adverse cardiovascular events:results from the Registry of Hypogonadism in Men (RHYME) Int J Clin Pract. 2016;70:843–52. doi: 10.1111/ijcp.12876. [DOI] [PubMed] [Google Scholar]
- 33.Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, et al. Association between testosterone supplementation therapy and thrombotic events in elderly men. Urology. 2015;86:283–5. doi: 10.1016/j.urology.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism:a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–33. doi: 10.1177/1074248417691136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yassin AA, Alwani M, Talib R, Almehmadi Y, Nettleship JE, et al. Long-term testosterone therapy improves liver parameters and steatosis in hypogonadal men:a prospective controlled registry study. Aging Male. 2020;23:1553–63. doi: 10.1080/13685538.2020.1867094. [DOI] [PubMed] [Google Scholar]
- 36.Shores MM, Walsh TJ, Korpak A, Krakauer C, Forsberg CW, et al. Association between testosterone treatment and risk of incident cardiovascular events among US male veterans with low testosterone levels and multiple medical comorbidities. J Am Heart Assoc. 2021;10:e020562. doi: 10.1161/JAHA.120.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brock G, Heiselman D, Maggi M, Kim SW, Rodríguez Vallejo JM, et al. Effect of testosterone solution 2% on testosterone concentration, sex drive and energy in hypogonadal men:results of a placebo controlled study. J Urol. 2016;195:699–705. doi: 10.1016/j.juro.2015.10.083. [DOI] [PubMed] [Google Scholar]
- 38.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels:a randomized clinical trial. JAMA. 2015;314:570–81. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 39.Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, et al. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–9. doi: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 40.Brock G, Heiselman D, Knorr J, Ni X, Kinchen K. 9-month efficacy and safety study of testosterone solution 2% for sex drive and energy in hypogonadal nen. J Urol. 2016;196:1509–15. doi: 10.1016/j.juro.2016.04.065. [DOI] [PubMed] [Google Scholar]
- 41.Sharma R, Oni OA, Chen G, Sharma M, Dawn B, et al. Association between testosterone replacement therapy and the incidence of DVT and pulmonary embolism:a retrospective cohort study of the Veterans Administration Database. Chest. 2016;150:563–71. doi: 10.1016/j.chest.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Barnouin Y, Armamento-Villareal R, Celli A, Jiang B, Paudyal A, et al. Testosterone replacement therapy added to intensive lifestyle intervention in older men with obesity and hypogonadism. J Clin Endocrinol Metab. 2021;106:e1096–110. doi: 10.1210/clinem/dgaa917. [DOI] [PubMed] [Google Scholar]
- 43.Tan WS, Low WY, Ng CJ, Tan WK, Tong SF, et al. Efficacy and safety of long-acting intramuscular testosterone undecanoate in aging men:a randomised controlled study. BJU Int. 2013;111:1130–40. doi: 10.1111/bju.12037. [DOI] [PubMed] [Google Scholar]
- 44.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men:a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 45.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–33. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberg ML, Li S, Herder D, Lamb DJ, Lipshultz LI. Testosterone therapy and mortality risk. Int J Impot Res. 2015;27:46–8. doi: 10.1038/ijir.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 48.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94:2353–9. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 49.Baillargeon J, Urban RJ, Morgentaler A, Glueck CJ, Baillargeon G, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90:1038–45. doi: 10.1016/j.mayocp.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Martinez C, Suissa S, Rietbrock S, Katholing A, Freedman B, et al. Testosterone treatment and risk of venous thromboembolism:population based case-control study. BMJ. 2016;355:i5968. doi: 10.1136/bmj.i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corona G, Rastrelli G, Maseroli E, Fralassi N, Sforza A, et al. Low testosterone syndrome protects subjects with high cardiovascular risk burden from major adverse cardiovascular events. Andrology. 2014;2:741–7. doi: 10.1111/j.2047-2927.2014.00241.x. [DOI] [PubMed] [Google Scholar]
- 52.Svartberg J, Braekkan SK, Laughlin GA, Hansen JB. Endogenous sex hormone levels in men are not associated with risk of venous thromboembolism:the Tromso study. Eur J Endocrinol. 2009;160:833–8. doi: 10.1530/EJE-08-0888. [DOI] [PubMed] [Google Scholar]
- 53.Holmegard HN, Nordestgaard BG, Schnohr P, Tybjaerg-Hansen A, Benn M. Endogenous sex hormones and risk of venous thromboembolism in women and men. J Thromb Haemost. 2014;12:297–305. doi: 10.1111/jth.12484. [DOI] [PubMed] [Google Scholar]
- 54.Borst SE, Shuster JJ, Zou B, Ye F, Jia H, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration:a systematic review and meta-analysis. BMC Med. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faber T, Ravaud P, Riveros C, Perrodeau E, Dechartres A. Meta-analyses including non-randomized studies of therapeutic interventions:a methodological review. BMC Med Res Methodol. 2016;16:35. doi: 10.1186/s12874-016-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, et al. Adverse events associated with testosterone replacement in middle-aged and older men:a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 57.Sesti F, Pofi R, Pozza C, Minnetti M, Gianfrilli D, et al. Cardiovascular complications in patients with Klinefelter's syndrome. Curr Pharm Des. 2020;26:5556–63. doi: 10.2174/1381612826666201102105408. [DOI] [PubMed] [Google Scholar]
- 58.Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease –the Framingham study:a 34-year follow-up. Am Heart J. 1994;127:674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 59.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–32. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 60.Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ, et al. American Association of Clinical endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients –2002 update. Endocr Pract. 2002;8:440–56. [PubMed] [Google Scholar]
- 61.Hackett G, Kirby M, Edwards D, Jones TH, Wylie K, et al. British Society for Sexual Medicine guidelines on adult testosterone deficiency, with statements for UK practice. J Sex Med. 2017;14:1504–23. doi: 10.1016/j.jsxm.2017.10.067. [DOI] [PubMed] [Google Scholar]
- 62.Khera M, Adaikan G, Buvat J, Carrier S, El-Meliegy A, et al. Diagnosis and treatment of testosterone deficiency:recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:1787–804. doi: 10.1016/j.jsxm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Lunenfeld B, Mskhalaya G, Zitzmann M, Arver S, Kalinchenko S, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15. doi: 10.3109/13685538.2015.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yarnell CJ, Thiruchelvam D, Redelmeier DA. Risks of serious injury with testosterone treatment. Am J Med. 2021;134:84–94. doi: 10.1016/j.amjmed.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 65.Pastuszak AW, Hu Y, Freid JD. Occurrence of pulmonary oil microembolism after testosterone undecanoate injection:a postmarketing safety analysis. Sex Med. 2020;8:237–42. doi: 10.1016/j.esxm.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbe C, Roxby AC. Assessing safety in hormonal male contraception:a critical appraisal of adverse events reported in a male contraceptive trial. BMJ Sex Reprod Health. 2020;46:139–46. doi: 10.1136/bmjsrh-2018-200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glueck CJ, Lee K, Prince M, Jetty V, Shah P, et al. Four thrombotic events over 5 years, two pulmonary emboli and two deep venous thrombosis, when testosterone-hCG therapy was continued despite concurrent anticoagulation in a 55-year-old man with lupus anticoagulant. J Investig Med High Impact Case Rep. 2016;4:2324709616661833. doi: 10.1177/2324709616661833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glueck CJ, Prince M, Patel N, Patel J, Shah P, et al. Thrombophilia in 67 patients with thrombotic wvents after starting testosterone therapy. Clin Appl Thromb Hemost. 2016;22:548–53. doi: 10.1177/1076029615619486. [DOI] [PubMed] [Google Scholar]
- 69.Li H, Benoit K, Wang W, Motsko S. Association between use of exogenous testosterone therapy and risk of venous thrombotic events among exogenous testosterone treated and untreated men with hypogonadism. J Urol. 2016;195:1065–72. doi: 10.1016/j.juro.2015.10.134. [DOI] [PubMed] [Google Scholar]
- 70.Glueck CJ, Friedman J, Hafeez A, Hassan A, Wang P. Testosterone, thrombophilia, thrombosis. Blood Coagul Fibrinolysis. 2014;25:683–7. doi: 10.1097/MBC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 71.Glueck CJ, Richardson-Royer C, Schultz R, Burger T, Labitue F, et al. Testosterone, thrombophilia, and thrombosis. Clin Appl Thromb Hemost. 2014;20:22–30. doi: 10.1177/1076029613485154. [DOI] [PubMed] [Google Scholar]
- 72.Haider A, Yassin A, Haider KS, Doros G, Saad F, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy:observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;12:251–61. doi: 10.2147/VHRM.S108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaufman JM, Miller MG, Garwin JL, Fitzpatrick S, McWhirter C, et al. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med. 2011;8:2079–89. doi: 10.1111/j.1743-6109.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 74.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome:the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–12. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 75.Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction:a pharmacoepidemiologic study. Pharmacotherapy. 2015;35:72–8. doi: 10.1002/phar.1534. [DOI] [PubMed] [Google Scholar]
- 76.Sharma R, Oni OA, Gupta K, Chen G, Sharma M, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–15. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 77.Oni OA, Sharma R, Chen G, Sharma M, Gupta K, et al. Normalization of testosterone levels after testosterone replacement therapy is not associated with reduced myocardial infarction in smokers. Mayo Clin Proc Innov Qual Outcomes. 2017;1:57–66. doi: 10.1016/j.mayocpiqo.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48:1138–44. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–43. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–59. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 81.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–24. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fallara G, Pozzi E, Belladelli F, Corsini C, Boeri L, et al. Cardiovascular morbidity and mortality in men –findings from a meta-analysis on the time-related measure of risk of exogenous testosterone. J Sex Med. 2022;19:1243–54. doi: 10.1016/j.jsxm.2022.05.145. [DOI] [PubMed] [Google Scholar]
- 83.Cannarella R, Barbagallo F, Crafa A, Mongioì LM, Aversa A, et al. Testosterone replacement therapy in hypogonadal male patients with hypogonadism and heart failure:a meta-analysis of randomized controlled studies. Minerva Urol Nephrol. 2022;74:418–27. doi: 10.23736/S2724-6051.21.04307-X. [DOI] [PubMed] [Google Scholar]
- 84.Sansone A, Rastrelli G, Cignarelli A, de Rocco Ponce M, Condorelli RA, et al. Effect of treatment with testosterone on endothelial function in hypogonadal men:a systematic review and meta-analysis. Int J Impot Res. 2020;32:379–86. doi: 10.1038/s41443-019-0163-6. [DOI] [PubMed] [Google Scholar]
- 85.Corona G, Rastrelli G, Monami M, Guay A, Buvat J, et al. Hypogonadism as a risk factor for cardiovascular mortality in men:a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Funnel plot and (b) sensitivity analysis of the risk of arterial thrombotic events in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Cumulative analysis of the risk of arterial thrombotic events in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Meta-regression analysis on the effect of testosterone levels in patients (a) before and (b) after and in controls (c) before and (d) after testosterone replacement therapy (TRT) on the risk of arterial thrombotic events.
(a) Funnel plot and (b) sensitivity analysis of the risk of stroke in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Cumulative analysis of the risk of stroke in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
(a) Funnel plot and (b) sensitivity analysis of the risk of myocardial infarction in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Cumulative analysis of the risk of myocardial infarction in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment. RCTs are in the upper panel, observational studies in the lower panel.
Meta-regression analysis on the effect of testosterone levels in patients (a) before and (b) after and in controls (c) before and (d) after testosterone replacement therapy (TRT) on the risk of myocardial infarction.
Risk of venous thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of venous thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of venous thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Risk of deep vein thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of deep vein thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of deep vein thrombosis in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Risk of pulmonary embolism in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of pulmonary embolism in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of pulmonary embolism in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Risk of mortality in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
(a) Funnel plot and (b) sensitivity analysis of the risk of mortality in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.
Cumulative analysis of the risk of mortality in hypogonadal patients on testosterone replacement therapy compared to hypogonadal controls on placebo or no treatment.