Abstract
The low treatment rate, low treatment success rate and high mortality rate of patients with pre-extensively drug-resistant tuberculosis (pre-XDR-TB) and extensively drug-resistant TB (XDR-TB) need serious attention. The aim of this study was to describe the profiles of patients with pre-XDR-TB and XDR-TB cases and to determine associated risk factors of their incidence in Indonesia. A retrospective case-control study was conducted at H. Adam Malik General Hospital in Medan, North Sumatra of which all sensitive-drug TB (SD-TB), pre-XDR-TB, and XDR-TB patients aged 18 years or older treated between October 2019 to June 2022 were included. Chi-squared test or Kruskal Wallis test and multiple logistic regression were used to determine the risk factors associated with pre-XDR-TB and XDR-TB incidence. A total 16 patients of case group (15 pre-XDR-TB and one XDR-TB) and 116 SD-TB patients (control group) were included in the final analysis. Out of total patients within case group, 62.5% were male, 43.8% aged between 56–65 years, 62.5% graduated from high school or equivalent, and 25% were unemployed. The majority of patients had no comorbid (62.5%), had history of anti-TB treatment (93.8%), and had secondary resistance (93.8%). Multivariate analysis indicated that age (OR: 10.01; 95%CI: 1.49– 66.91, p=0.018) and previous history of anti-TB treatment (OR: 216.25; 95%CI: 18.62– 2511.60, p<0.001) were significantly associated with incidence of pre-XDR-TB and XDR-TB. This study highlights that having previous history of anti-TB treatment and older age are the predictors of the incidence of pre-XDR-TB and XDR-TB.
Keywords: Tuberculosis, pre-XDR-TB, XDR-TB, drug resistant-tuberculosis, risk factor
Introduction
Multidrug resistant tuberculosis (MDR-TB), TB that caused by Mycobacterium tuberculosis strains that are resistant to at least isoniazid and rifampicin, is a global concern. In the recent years, pre-extensively drug-resistant tuberculosis (pre-XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are emerged [1]. Pre-XDR-TB is defined as the MDR-TB which resistant to any fluoroquinolone while XDR-TB is defined as MDR-TB which also resistant to any fluoroquinolone and at least one additional Group A drugs (levofloxacin, moxifloxacin, bedaquiline or linezolid) [1]. The global incidence of pre-XDR-TB and XDR-TB in 2021 was 25,100 cases, and around 22,600 cases started treatment [2]. The number of confirmed pre-XDR-TB and XDR-TB cases in Southeast Asia is around 11,700 of which 10,500 cases have started treatment [2]. In Indonesia, laboratory-confirmed pre-XDR-TB and XDR-TB cases in 2021 were 392 patients, and 417 patients started treatment [2].
According to data from a multicenter study conducted in seven countries with high TB burden, the mortality rate of pre-XDR-TB and XDR-TB was 39% [3]. Based on a study from a tertiary hospital in India, the average cost of treatment for XDR-TB was $8,401 which was the most expensive compared to other types of TB such as MDR-TB ($5,609) or extrapulmonary TB ($5,609) [4].
A study from Russia found that the majority age of XDR-TB patients aged between 25 and 44 years old with a male predominance (80.9%) [5]. The study also reported that the majority of the patients were unemployed (75.6%) and 57% of patients had suffered from the disease for more than three years and third of them (31.8%) had HIV co-infection with HIV. The resistance to all first-line and second-line anti-TB drugs was 10.1% [5]. A retrospective study in France with 168 pre-XDR-TB and 62 XDR-TB patients found that the factors independently associated with pre-XDR-TB were male gender, history of birth in Europe, and resistance to ethambutol and predictor for XDR-TB were history of foreign birth (born outside Europe), previous TB treatment, positive smear result, and resistance to ethambutol [6]. These studies indicated that some risk factors are associated with the incidence of pre-XDR-TB and XDR-TB. However, studies in Indonesia assessing the risk factors of incidence if pre-XDR-TB and XDR-TB are limited. The aim of this study was to assess the profile of the patients and risk factors of pre-XDR-TB and XDR-TB incidence.
Methods
Study design
A retrospective case-control study was conducted at H. Adam Malik General Hospital, Medan, Indonesia. The hospital is the national reference hospital for Sumatra region. All TB patients above 18 years old were included. The case group was patients with pre-XDR-TB or XDR-TB while the control was sensitive-drug TB (SD-TB). The data was collected from October 2019 until June 2022. The inclusion criteria for pre-XDR-TB and XDR-TB were patients age above 18 years, diagnosed bacteriologically with rapid molecular testing and followed by drug sensitivity test and who were treated with all oral TB regiment. Inclusion criteria of the SD-TB were patients age above 18 years and diagnosed bacteriologically with rapid molecular testing.
Study variables
Some potential risk factors of the pre-XDR-TB and XDR-TB were collected and assessed such as sociodemographic characteristics (age, sex, educational level, occupation), comorbid, history of anti-TB treatment (ATT), history of previous TB treatment outcomes, suspected DR-TB criteria, resistance type and current TB treatment outcome.
History of ATT was the history of whether the patients have received ATT before the diagnosis of pre-XDR-TB and XDR-TB was made, broadly divided into having received ATT and never received ATT before. Subjects who had previously received ATT were divided into having been treated once with drug-sensitive ATT, having been treated more than once with drug-sensitive ATT, and having been treated with drug-resistant ATT. History of previous TB treatment outcomes was the outcome of TB treatment before the diagnosis of pre-XDR-TB and XDR-TB was made and divided in to ever loss to follow up, failed, cured and never. Loss to follow up case was a patient who consumed anti-TB drugs for one month or more and then stop consuming for more than two months in a row while failed case was defined as TB patients with positive acidfast bacilli (AFB) or sputum culture results in the fifth month or the end of treatment [7]. Cured case was TB patient with positive bacteriological confirmation at the start of treatment and negative sputum AFB or negative culture at the end of treatment and had negative test results in one of the previous examinations [7].
Suspected DR-TB criteria was criteria of patients who have TB symptoms that have a history of one or more condition listed in the National Guideline for Tuberculosis Treatment [7]. In this study, categories included in suspected DR-TB criteria were loss to follow up, 1st ATT category failure, 2nd ATT category failure, relapse case, and none.
Type of resistance was divided in to primary resistance (resistance to ATT in patients who have never received TB treatment before or have ATT for less than one month) and secondary resistance (resistance among patients who have been treated with ATT ≥1 month, including patients with failed case, relapse case or loss to follow up case) [8]. Resistance pattern to anti-TB drugs was collected from information system of the TB website sumatera.sitb.id/sitb/app.
The current treatment outcome was the treatment outcome of the pre-XDR-TB and XDR-TB patients and divided into four categories: drop out, cured, dead, and ongoing treatment.
Statistical analysis
The data was presented by table and graph using descriptive analysis. Chi-squared test or Kruskal Wallis test were used to determine the risk factors associated with pre-XDR-TB and XDR-TB incidence in univariate analyses. In multivariate analysis, multiple logistic regression test was used determine the associated risk factors of pre-XDR-TB and XDR-TB.
Results
Characteristics of patients
There were 16 pre-XDR/XDR-TB and 116 SD-TB patients included in this study. The characteristics of patients with pre-XDR-TB and XDR-TB are presented in Table 1. Most pre-XDR/XDR-TB patients were male, with a total of 10 patients (62.5%), and most were in the 56-65 years age group, with a total of seven people (43.8%). The majority of patients (62.5%) had high school education levels. Three patients (20%) worked as civil servants, housewives, and farmers. Most patients (62.5%) had no comorbidity, while six (37.5%) with diabetes mellitus. Most patients (60.1%) had history of sensitive-drug ATT once. A total of six (37.5%) had history of cured from TB. Based on current DR-TB treatment, it was found that six patients (37.5%) cured from current TB treatment.
Table 1. Characteristics of pre-XDR-TB and XDR-TB patients included in the study (n=16).
| Characteristics | Frequency | Percentage |
|---|---|---|
| Gender | ||
| Male | 10 | 62.5 |
| Female | 6 | 37-5 |
| Age (year) | ||
| 17-25 | 0 | 0.0 |
| 26-35 | 1 | 6.2 |
| 36-45 | 3 | 18.8 |
| 46-55 | 5 | 31.2 |
| 56-65 | 7 | 43.8 |
| >65 | 0 | 0.0 |
| Education | ||
| Elementary | 1 | 6.2 |
| Junior high school | 3 | 18.8 |
| Senior high school | 9 | 62.5 |
| University | 2 | 12.5 |
| Occupation | ||
| Civil servant | 3 | 18.8 |
| Housewife | 3 | 18.8 |
| Army or police | 1 | 6.1 |
| Farmer | 3 | 18.8 |
| Entrepreneur | 2 | 12.5 |
| Unemployed | 4 | 25.0 |
| Comorbid | ||
| Diabetes melitus | 6 | 37.5 |
| No comorbid | 10 | 62.5 |
| History of anti-TB treatment | ||
| Once drug-sensitive | 10 | 62.5 |
| More than once drug-sensitive | 1 | 6.2 |
| Once drug-resistant | 4 | 25.0 |
| Never | 1 | 6.2 |
| History of previous TB treatment outcome | ||
| Ever loss to follow up | 5 | 31.2 |
| Ever failed | 4 | 25.0 |
| Ever cured | 6 | 37.5 |
| Never | 1 | 6.2 |
| Suspected DR-TB criteria | ||
| Loss to follow-up | 5 | 31.2 |
| 1st anti-TB treatment category failure | 2 | 12.5 |
| 2nd anti-TB treatment category failure | 2 | 12.5 |
| Relapse case | 6 | 37.5 |
| None | 1 | 6.2 |
| Resistance type | ||
| Primary | 1 | 6.2 |
| Secondary | 15 | 93.8 |
| Current treatment outcome | ||
| Drop out | 3 | 18.8 |
| Cured | 6 | 37.5 |
| Dead | 3 | 18.8 |
| Ongoing | 4 | 25.0 |
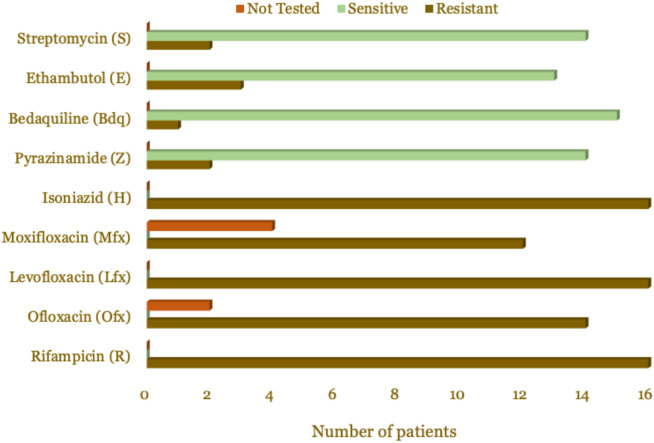
Based on the criteria for suspected DR-TB, most patients (37.5%) were relapse cases. Based on the type of resistance, almost all patients (93.8%) were secondary resistance type, while one patient (6.2%) was primary resistance. The characteristics of the patients are presented in Table 1. Based on the resistance pattern, all patients were resistant to rifampicin, isoniazid, and levofloxacin, as presented in Table 2 and Figure 1.
Table 2. Resistance patterns of pre-XDR-TB and XDR-TB patients.
| Drug | Resistant | Sensitive | Drug sensitivity test was not done | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| Rifampicin (R) | 16 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Ofloxacin (Ofx) | 14 | 87.5 | 0 | 0.0 | 2 | 12.5 |
| Levofloxacin (Lfx) | 16 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Moxifloxacin (Mfx) | 12 | 75.0 | 0 | 0.0 | 4 | 25 |
| Isoniazid (H) | 16 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Pyrazinamide (Z) | 2 | 33.3 | 14 | 66.7 | 0 | 0.0 |
| Bedaquiline (Bdq) | 1 | 6.2 | 15 | 93.8 | 0 | 0.0 |
| Ethambutol (E) | 3 | 18.7 | 13 | 81.3 | 0 | 0.0 |
| Streptomycin (S) | 2 | 12.5 | 14 | 87.5 | 0 | 0.0 |
Figure 1. Resistance pattern visualization of pre-XDR-TB and XDR-TB patients.
Factors associated with pre-XDR-TB and XDR-TB
The univariate analysis using the Chi-squared and Fischer’s Exact test tests showed a significant relationship between age (p=0.044), and previous anti-TB treatment history (p<0.001) with the incidence of pre-XDR-TB and XDR-TB (Table 3). Our data indicated that sex, education level, occupation, and comorbidity was not associated with pre-XDR-TB and XDR-TB.
Table 3. Univariate analysis of showing factors associated with pre-XDR-TB and XDR-TB incidence.
| Characteristic | Pre-XDR/XDR- | DS-TB | Odds ratio (95%CI) | p-value |
|---|---|---|---|---|
| TB (n=16) | (n=116) | |||
| n (%) | n (%) | |||
| Sex | 0.383a | |||
| Male | 10 (62.5) | 85 (73.3) | 0.64 (0.25-1.65) | |
| Female | 6 (37.5) | 31 (26.7) | ||
| Age | ||||
| >50 years | 10 (62.5) | 42 (36.2) | 2.56 (0.99-6.63) | 0.044a |
| <50 years | 6 (37.5) | 74 (63.8) | ||
| Educational level | 0.086b | |||
| Primary and elementary school | 4 (25) | 11 (9.5) | 2.60 (0.96-7.03) | |
| High school and college | 12 (75) | 105 (90.5) | ||
| Occupation | ||||
| Unemployed | 4 (25) | 21 (18.1) | 1.42 (0.50-4.05) | 0.504b |
| Employed | 12 (75) | 95 (81.9) | ||
| Comorbidity | ||||
| Any | 6 (37.5) | 51 (44.0) | 0.78 (0.30-2.04) | 0.625a |
| No comorbidity | 10 (62.5) | 65 (56.0) | ||
| Previous ATT history | ||||
| Any | 15 (93.8) | 13 (11.2) | 55.71 (7.68-403.83) | <0.001b |
| No ATT history | 1 (6.2) | 103 (88.8) |
Analyzed using Chi-squared test
Analyzed using Fischer’s exact test
The type of multivariate analysis used is multiple logistic regression because the dependent variable in this study is categorical. The variables included in the multivariate analysis are independent variables with a p<0.25 from the bivariate analysis results. The independent variables that met the requirements were age (p=0.044), educational level (p=0.086), and previous ATT history (p<0.001) (Table 4).
Table 4. Multivariate analysis showing factors associated with pre-XDR-TB and XDR-TB incidence.
| Associated factor | Odds ratio | 95%CI | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Selection 1 | ||||
| Age | 9.82 | 1.42 | 67.51 | 0.020 |
| Educational level | 1.13 | 0.12 | 10.03 | 0.911 |
| Previous history of anti-TB treatment | 215.07 | 18.50 | 2499.52 | <0.001 |
| Selection 2 | ||||
| Age | 10.01 | 1.49 | 66.91 | 0.018 |
| Previous history of anti-TB treatment | 216.25 | 18.62 | 2511.60 | <0.001 |
The most dominant variable influencing the occurrence of pre-XDR-TB and XDR-TB was history of previous ATT (OR: 216.25; 95%CI: 18.62–2511.60, p<0.001) indicating that TB patients with history of previous TB treatment tend to be 216 times more likely to develop pre-XDR-TB or XDR-TB compared to TB patients who have never received ATT before. Our data also indicated that TB patients aged >50 years had the chance of 10 times to have pre-XDR-TB and XDR-TB compared to TB patients aged ≤50 years (OR: 10.01; 95%CI: 1.49–66.91, p=0.018).
Discussion
We did the study to have better understanding of the characteristics of the patients with pre-XDR-TB and XDR-TB with the main goal to determine the risk factors of the incidence of pre-XDR-TB and XDR-TB in a national reference hospital for Sumatra region of Indonesia. Demographics data of our patients are comparable with previous studies in Brazil [9], South Africa [10], Russia [5], Peru [10], and India [12].
Out of total pre-XDR-TB and XDR-TB patients, most of them (62.5%) had a history of consuming drug-sensitive ATT, while 25% had previously taken drug-resistant ATT, it means that many pre-XDR-TB or XDR-TB patients were ex TB patients. It can be assumed that the previous treatment was not effective against the bacteria. As many as 37.5% of patients had cured previously, in contrast to the study from Sao Paulo, in which 78.3% of pre-XDR-TB and 81.3% of XDR-TB patients had a history of treatment failure [9]. Our data indicated that 37.5% were relapse cases and 31.2% were loss to follow-up cases. A study concluded that AFB smear grade was independently associated with TB relapse after successful treatment [13]. In this study, 93.8% of patients were secondary pre-XDR-TB and XDR-TB indicating that most of the patients had history of pulmonary TB before. A small number of mutations that occurred in MDR-TB patients initiation and during treatment can result conversion to XDR-TB and precise adjustment of regimens were required for treatment success [14].
Our data indicated that the age groups >50 years significantly associated with the incidence of pre-XDR-TB and XDR-TB. This is quite different from a retrospective cohort study in Pakistan which stated that the age group ≤38 years, student employment status, previous history of ATT, and cavities on chest X-rays were independent risk factors for XDR-TB [15]. The possible reason why elderly is associated with the incidence of pre-XDR-TB and XDR-TB is elderly patients often experience memory loss which causes patients to forget to take the medication [8]. Old age is associated with cognitive and memory decline, so extra work is needed to improve treatment adherence. Drug ingestion supervisors (DIS) should remain empowered because they can improve treatment compliance [16]. According to a study, TB patients supervised by DIS cured five times greater than those without DIS, and the most effective DIS was the patients’ family [21,22]. Another method can be done with medication event and reminder monitor (MERM), a tool such as a medicine box with an alarm for reminders to take ATT that is effective in improving treatment adherence [19]. In addition, more side effects in elderly patients are also likely to result in low adherence to treatment [20]. Severe gastrointestinal side effects of pyrazinamide in elderly patients can result in non-adherence and treatment failure, so it is necessary to detect the symptoms in early treatment [21]. Support from medical staff and family is essential for the successful treatment in elderly patients.
Our data also found a significant association between previous ATT history and the incidence of pre-XDR-TB and XDR-TB. This is similar to the previous study that found that the most common medical risk factor of XDR-TB was a history of previous TB treatment withdrawal [22]. In addition, non-adherence in TB treatment was associated with the evolution of drug-resistant M. tuberculosis variants, and may become pre-XDR/XDR-TB [23]. Based on a study from China, patients with poor adherence were elderly [24]. Patients with a history of ATT are predictors of treatment failure and are at risk of becoming drug-resistant TB [25]. More intensive and consistent education is needed to make patient compliant with treatment. Even, counseling by a pharmacist can increase knowledge and compliance with TB patients and improve therapy outcomes [26]. Information technology-based education can be quite effective, such as using the mobile health application, reminders method to take medications, regular visit schedules, education, and social support [27]. Audiovisual psychoeducation based on implementation intervention can increase intention and treatment adherence in TB patients [28]. In India, TB patients monitoring with isoniazid urine tests during home visits are more effective in increasing treatment adherence than patients monitoring with 99DOTS, a mobile phone-based program [29].
This study has some limitations. It was a single-center retrospective study. The sample size was relatively small because the data were collected from a short period. Further study with more patients, centers, and variables is needed for better results.
Conclusion
The prevalence of pre-XDR/XDR-TB at H. Adam Malik General Hospital, Medan, Indonesia from October 2019 until June 2022 was 7.8%. The majority of patients were pre-XDR-TB, male, age group between 56–65 years, had high school graduate or equivalent, unemployed, and had previous ATT. Age and previous history of ATT were significantly associated with the incidence of pre-XDR-TB and XDR-TB.
Acknowledgments
Author would like to thank all doctors, nurses and administration staff at DOTS Clinic of H. Adam Malik General Hospital, Medan, Indonesia for the assistance and help during data collection process.
Ethics approval
This research has obtained an ethical code from the Health Research Ethics Committee of the Universitas Sumatera Utara, Medan, Indonesia (No. 583/KEPK/USU/2022; dated 7 July 2022).
Competing interests
The authors declare that there is no conflict of interest.
Funding
This study received no external funding.
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Sinulingga HE, Sinaga BYM, Siagian P, et al. Profile and risk factors of pre-XDR-TB and XDR-TB patients in a national reference hospital for Sumatra region of Indonesia. Narra J 2023; 3 (3): e407 - http://doi.org/10.52225/narra.v3i3.407.
References
- 1.World Health Organization. WHO announces updated definitions of extensively drug-resistant tuberculosis. Available from: https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resista nt-tuberculosis. Accessed: 13 September 2021.
- 2.World Health Organization. Global tuberculosis report 2021. Geneva. 2021. [Google Scholar]
- 3.Zürcher K, Reichmuth ML, Ballif M, et al. Mortality from drug-resistant tuberculosis in high-burden countries comparing routine drug susceptibility testing with whole-genome sequencing: A multicentre cohort study. Lancet Microbe 2021;2(7):e320–e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyckendorf J, Van-Leth F, Avsar K, et al. Treatment responses in multidrug-resistant tuberculosis in Germany. Int J Tuberc Lung Dis 2018;22(4):399–406. [DOI] [PubMed] [Google Scholar]
- 5.Pasechnik OA, Zimoglyad AA, Yarusova IV, et al. Prevalence of extensively drug-resistant tuberculosis: A descriptive study. Epidemiol Vaccinal Prev 2018;17(4):13–19. [Google Scholar]
- 6.Guglielmetti L, Veziris N, Aubry A, et al. Risk factors for extensive drug resistance in multidrug-resistant tuberculosis cases: A case-case study. Int J Tuberc Lung Dis 2018;22(1):54–59. [DOI] [PubMed] [Google Scholar]
- 7.Kementerian Kesehatan Republik Indonesia. Pedoman Nasional pelayanan tata laksana tuberkulosis. Available from: https://repository.kemkes.go.id/book/124. Accessed: 13 September 2021.
- 8.Direktorat Jenderal Pencegahan dan Pengendalian Penyakit. Petunjuk Teknis Penatalaksanaan Tuberkulosis Resistan Obat di Indonesia. Kementerian Kesehatan Republik Indonesia. Jakarta. 2020. [Google Scholar]
- 9.Gallo JF, Pinhata JMW, Simonsen V, et al. Prevalence, associated factors, outcomes and transmission of extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in Sao Paulo, Brazil : A cross-sectional study. Clin Microbiol Infect 2018;24(8):889–895. [DOI] [PubMed] [Google Scholar]
- 10.Peterson ML, Gandhi NR, Clennon J, et al. Extensively drug-resistant tuberculosis “hotspots” and sociodemographic associations in Durban, South Africa. Int J Tuberc Lung Dis 2019;23(6):720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alarcón V, Alarcón-Arrascue E, Mendoza-Ticona A, et al. Programmatic management of patients with pre-extensively drug-resistant tuberculosis in Peru, 2011-2014. Int J Tuberc Lung Dis 2018;22(10):1220–1226. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni GS, Telkhade AJ, Dugad S.. Drug resistance patterns among XDR-TB patients visiting a TB centre at a tertiary health care facility. MVP J Med Sci 2020;7(1):53–59. [Google Scholar]
- 13.Cudahy PGT, Wilson D, Cohen T.. Risk factors for recurrent tuberculosis after successful treatment in a high burden setting: A cohort study. BMC Infect Dis 2020;20(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Toole RF. Antibiotic resistance acquisition versus primary transmission in the presentation of extensively drug-resistant tuberculosis ronan. Int J Mycobacteriology 2022;11(4):343–348. [DOI] [PubMed] [Google Scholar]
- 15.Saifullah A, Mallhi TH, Khan YH, et al. Evaluation of risk factors associated with the development of MDR-and XDR-TB in a tertiary care hospital: A retrospective cohort study. PeerJ 2021;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahmi N, Medison I, Suryadi I.. Hubungan tingkat kepatuhan penderita tuberkulosis paru dengan perilaku kesehatan, efek samping OAT dan peran PMO pada pengobatan fase intensif di puskesmas seberang padang September 2012 - Januari 2013. J Kesehat Andalas 2017;6(2):345. [Google Scholar]
- 17.Soesilowati R, Haitamy MN. Perbedaan antara kesembuhan pasien TB paru dengan pengawas minum obat (PMO) dan tanpa PMO di RSUD. Prof. Dr. Margono Soekarjo. Sainteks 2016;13(1):50–60. [Google Scholar]
- 18.Fitriani D, Ayuningtyas G.. Hubungan antara peran keluarga sebagai pengawas minum obat (PMO) dengan tingkat kepatuhan pasien TB paru terhadap program pengobatan di wilayah Puskesmas Serpong 1 Kota Tangerang Selatan. Edu Dharma J 2019;3(2):17–23. [Google Scholar]
- 19.Liu X, Blaschke T, Thomas B, et al. Usability of a medication event reminder monitor system (MERM) by providers and patients to improve adherence in the management of tuberculosis. Int J Environ Res Public Health 2017;14(10):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caraux-Paz P, Diamantis S, de Wazières B, et al. Tuberculosis in the elderly. J Clin Med 2021;10(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon BS, Kim Y, Lee SH, et al. The high incidence of severe adverse events due to pyrazinamide in elderly patients with tuberculosis. PLoS One 2020;15(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parolina LE, Otpuschennykova O, Kazimirova N, et al. Social determinants and co-morbidities of patients with extensively drug resistant tuberculosis. Eur Respir J 2020;56 :497. [Google Scholar]
- 23.Kurnianingsih W, Tamtomo DG, Murti B.. The effect of non-compliance with medication on multidrug resistant of tuberculosis. J Epidemiol Public Heal 2020;5(4):442. [Google Scholar]
- 24.Gong X, Li Y, Wang J, et al. Treatment adherence among sputum smear-positive pulmonary tuberculosis patients in Xinjiang, China: A prospective study. RSC Adv 2018;8(16):8983–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejeta E, Beyene G, Balay G, et al. Factors associated with unsuccessful treatment outcome in tuberculosis patients among refugees and their surrounding communities in Gambella Regional State, Ethiopia. PLoS One 2018;13(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sriwijaya RA, Kumala S, Keban SA. Pengaruh edukasi farmasis terhadap outcome terapi pasien TB paru fase intensif di RSUP Persahabatan periode Maret-Juli 2015. J Penelit Sains 2018;20(3):86–91. [Google Scholar]
- 27.Pampalia N, Waluyo A.. Mhealth untuk kepatuhan pasien TB dalam pengobatan: Sebuah tinjauan literatur. J Wacana Kesehat 2019;4(1):404–410. [Google Scholar]
- 28.Anggraini ADSHL. Pengaruh psikoedukasi audio visual berbaris implementation intention terhadap niat dan perilaku kepatuhan minum obat pasien tuberkulosis (TB). J Penelit Kesehat Suara Forikes 2019;10(1):299–304. [Google Scholar]
- 29.Thomas BE, Kumar JV, Chiranjeevi M, et al. Evaluation of the accuracy of 99DOTS, a novel cellphone-based strategy for monitoring adherence to tuberculosis medications: Comparison of digitaladherence data with urine isoniazid testing. Clin Infect Dis 2020;71(9):E513–E516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on request.