Abstract
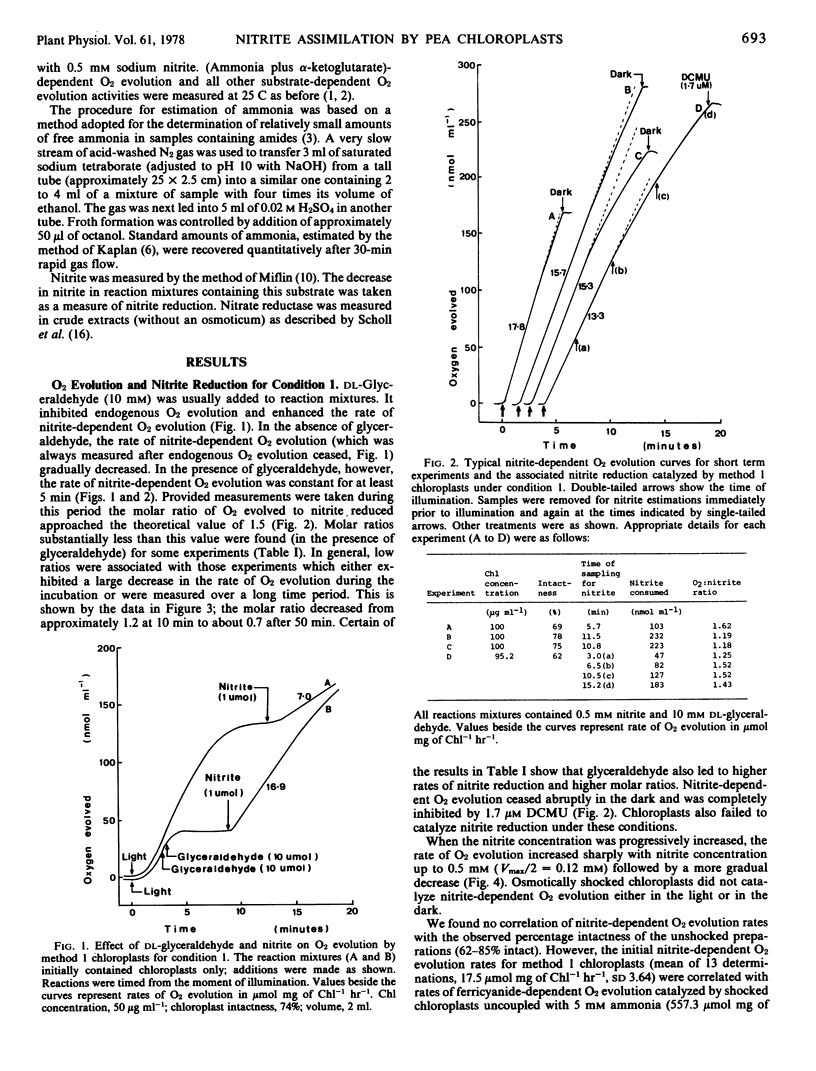
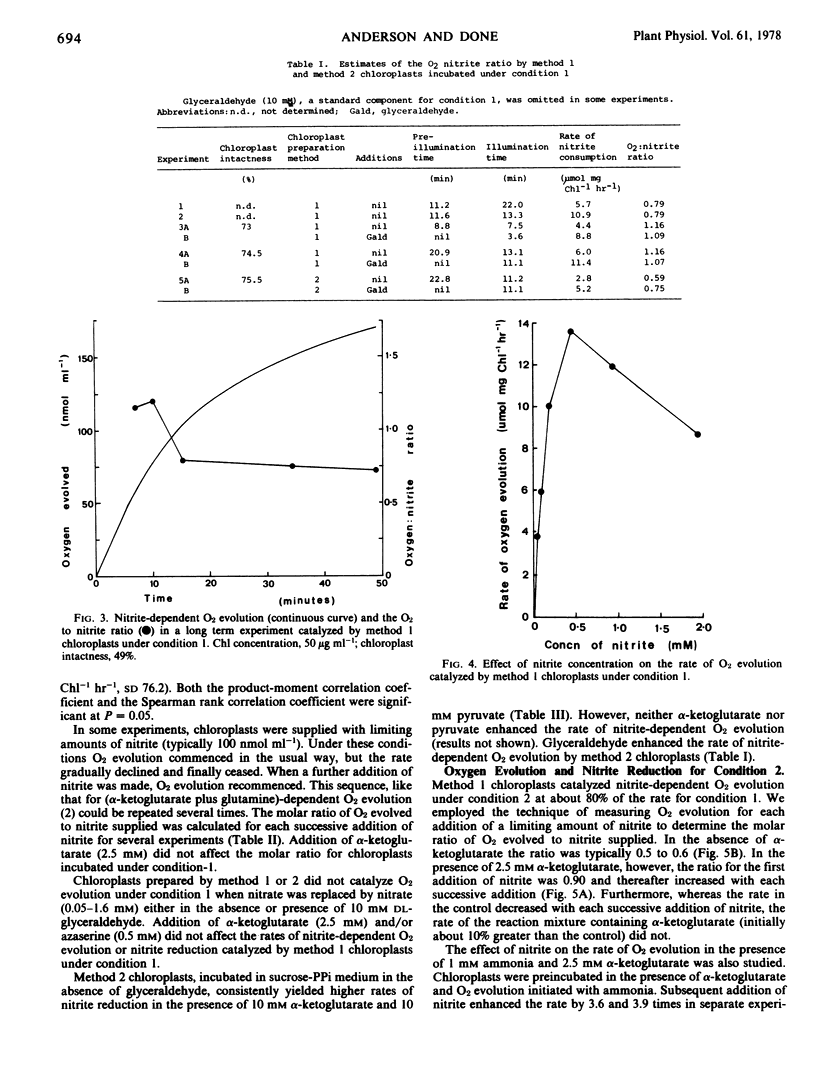
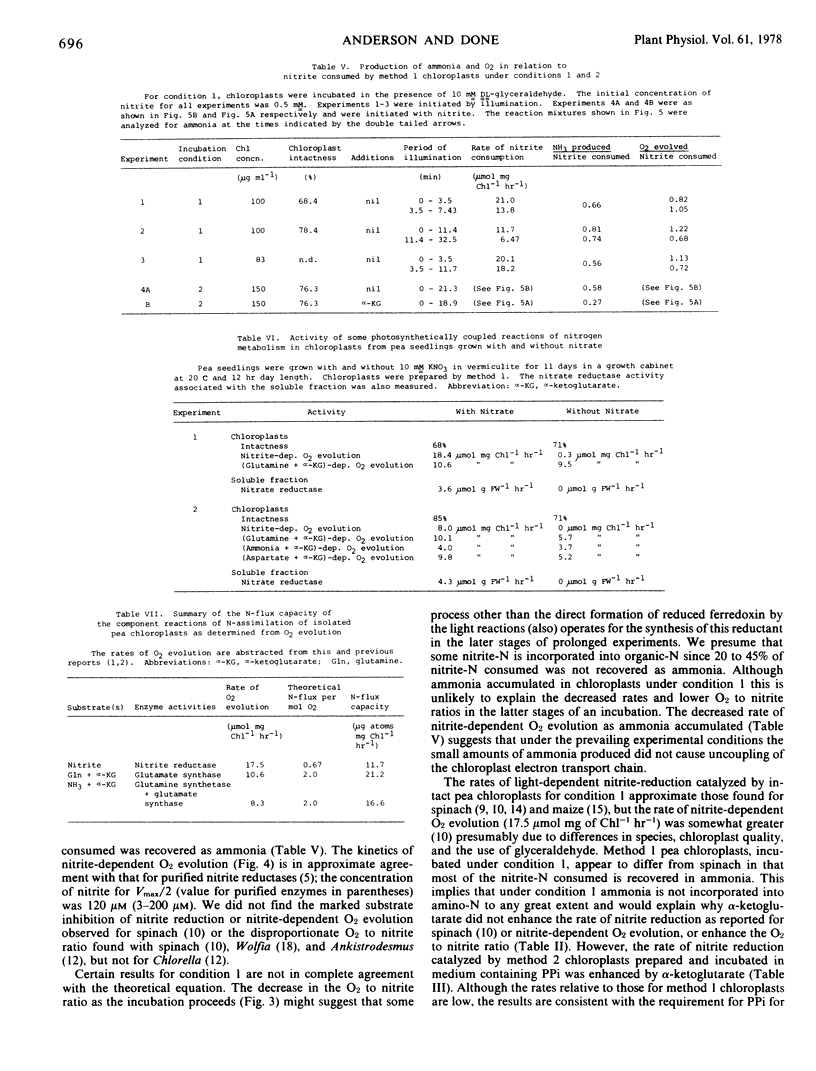
Chloroplasts were prepared from peas (Pisum sativum) in glucose-phosphate medium. In the presence of dl-glyceraldehyde, they catalyzed nitrite-dependent O2 evolution (mean of 13 preparations, 17.5 μmole per mg chlorophyll per hour, sd 3.64). The optimum concentration of nitrite was 0.5 mm; 0.12 mm nitrite supported Vmax/2. The reaction was accompanied by the consumption of nitrite; 55 to 80% of the nitrite-N consumed was recovered as ammonia. In short experiments (less than 10 minutes) the O2 to nitrite ratio approached 1.5, but thereafter decreased. There was no nitrite-dependent O2 evolution with chloroplasts from plants grown without added nitrate but such chloroplasts could assimilate ammonia at about the usual rate. The results are consistent with the reduction of nitrite to ammonia involving nitrate-induced nitrite reductase and a reductant generated by the chloroplast electron transport chain.
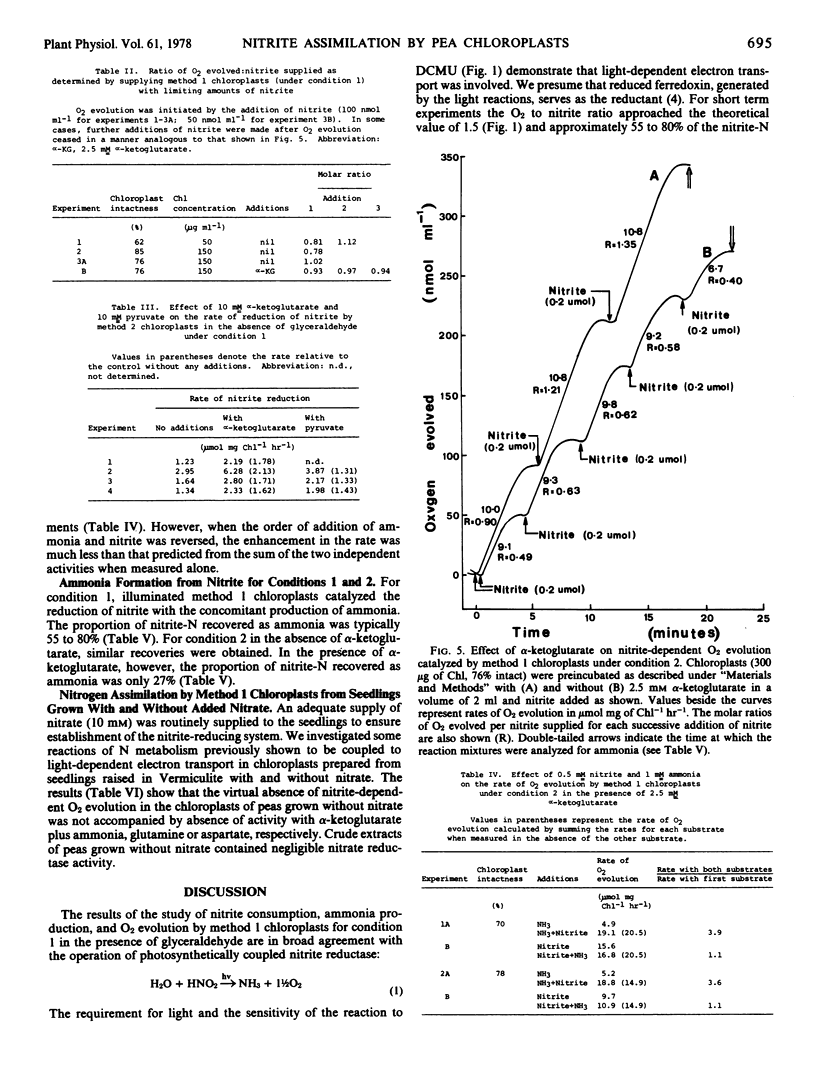
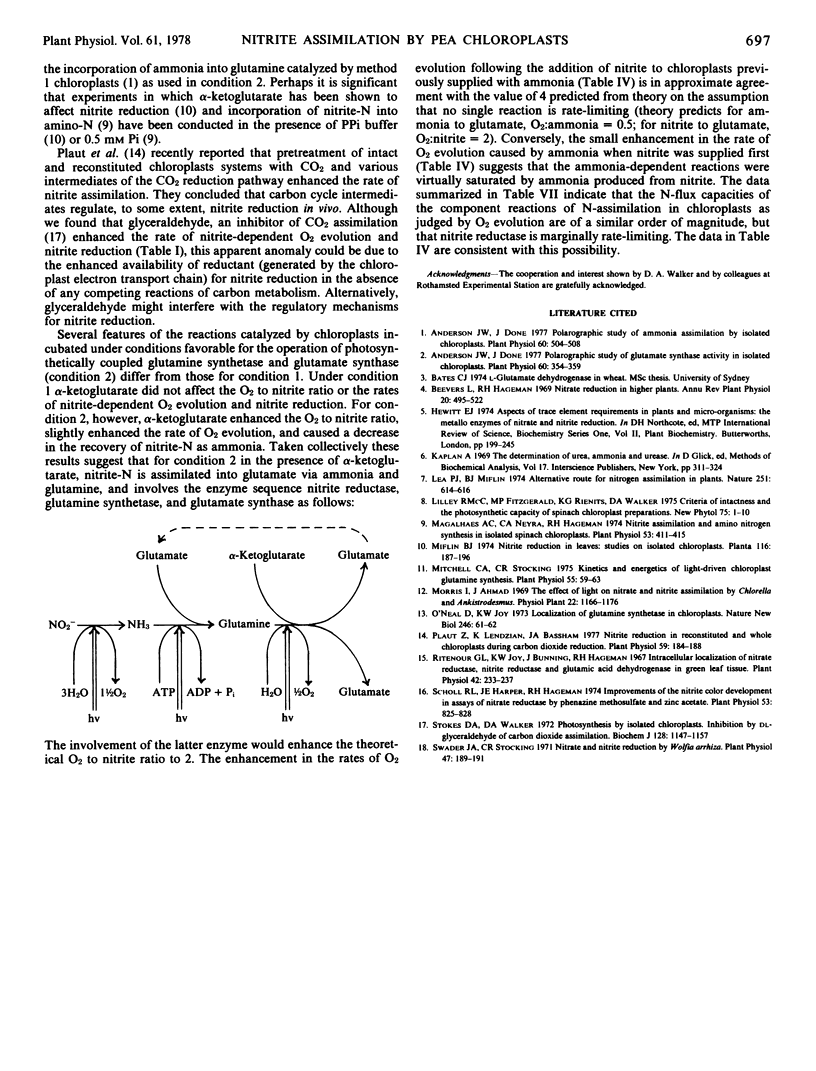
In the presence of ADP, pyrophosphate, and MgCl2 the O2 to nitrite ratio was typically 0.5 to 0.6 and the recovery of nitrite-N as ammonia about 60%. Under these conditions, α-ketoglutarate increased the O2 to nitrite ratio (0.9-1.35) and the recovery of nitrite-N as ammonia decreased to 27%. These data and the results of nitrite plus ammonia addition experiments (with and without α-ketoglutarate) are attributed to incorporation of nitrite-N into glutamate via the chloroplast enzymes nitrite reductase, glutamine synthetase, and glutamate synthetase.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. A polarographic study of glutamate synthase activity in isolated chloroplasts. Plant Physiol. 1977 Sep;60(3):354–359. doi: 10.1104/pp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. The determination of urea, ammonia, and urease. Methods Biochem Anal. 1969;17:311–324. doi: 10.1002/9780470110355.ch7. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Localisation of glutamine synthetase in chloroplasts. Nat New Biol. 1973 Nov 14;246(150):61–62. doi: 10.1038/newbio246061a0. [DOI] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swader J. A., Stocking C. R. Nitrate and Nitrite Reduction by Wolffia arrhiza. Plant Physiol. 1971 Feb;47(2):189–191. doi: 10.1104/pp.47.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]



