Abstract
Background:
To evaluate effect of maximal anterior cortical lens density, iris scatter and anterior chamber depth on laser flare photometry.
Methods:
Patients diagnosed with clinical uveitis were enrolled in the study. Clinical flare gradings were recorded upon the Standardization of Uveitis Nomenclature. Aqueous flare was measured with an automated device (Kowa FM-700). Back-scattering from anterior cortical lens and anterior iris surface was calculated from Scheimpflug images. A curvilinear regression model was used to calculate estimated values for each clinical grade. These values were used to split cases in Group I (laser flare photometry lower than estimated) and Group II (laser flare photometry higher than estimated). Mean anterior chamber depth, pupil aperture, maximal anterior cortical lens density and iris scatter values were compared between two groups. A stepwise multiple regression analysis was performed to determine the effect of clinical flare gradings and ocular parameters on aqueous flare measurements.
Results:
The study included 228 eyes of 114 cases. Scheimpflug images were obtained from 105 eyes. Estimated aqueous flare measurements (in photons/milliseconds) were 4.87, 8.50, 14.81, 25.83, 45.04 and 136.93 for 0, 0.5+, 1+, 1.5+, 2+ and 3+ clinical flare respectively. Group II had higher maximal anterior cortical lens density than Group I (96.6 ± 37.1 vs 77.9 ± 17.1 pixel unit, p = 0.001). The measured aqueous flare was significantly related to clinical flare, maximal anterior cortical lens density and pupil aperture (adjusted R2: 0.480, p < 0.001).
Conclusion:
The back-scattered light from anterior cortical lens could affect laser flare photometry measurements. This effect might be quantified by Scheimpflug imaging.
Keywords: Anterior chamber, aqueous flare, laser flare photometer, lens, uveitis
Introduction
Aqueous flare is an ocular finding that indicates an increased protein concentration in the aqueous humour following a breakdown in blood-aqueous barrier. Clinical assessment of anterior chamber cells and flare by slit-lamp examination constitutes the basis of the Standardization of Uveitis Nomenclature clinical grading system for ocular inflammation.1 In this classification, the grading of flare is based on visibility of iris and lens details and scored on a scale between 0 (no flare) and 4+ (intense flare). This subjective method of judgement of intraocular inflammation with slit-lamp biomicroscope, and the categorical grading, however, led to some confusion in certain cases wherein clinical experience would indicate that the examination findings would fall in between the suggested classification grades. Such challenge is in addition to the notable length of training required to become proficient at clinical grading method that is subject to intra- and interobserver variations.2 Therefore, there have been efforts to find new ways to measure aqueous flare in an objective and reliable manner, since 1950s.
Laser flare photometry, an instrument-based, in vivo method for quantitative measurement of aqueous flare, was introduced in 1988.3 Since its invention, laser flare photometer has been proven to be an objective, quantitative and highly reproduceable measurement method4 to evaluate a wide variety of ocular inflammatory disorders.5 The device measures the back-scattering of a monochromatic, in phase He-Ne laser beam that is directed into a measurement window in the anterior chamber by a photodetector-photomultiplier system.6 Due to its working principle, laser flare photometer measurement could be affected by any condition or anatomical landmark such as iris, lens, cornea that alters light back-scattering from the anterior segment of the eye.7 This effect of ‘background scattering’ of light on laser flare photometry measurements is already well known. For this reason, laser flare photometer device gives a warning of ‘background’ when back-scattered light affects the measurement. If there is a ‘background’ warning, that measurements are considered as unreliable. However, in clinical practice, there may be variations even after this elimination, because of the sensitivity level of this measure. Laser photometry measurement of aqueous flare is known to be influenced by various factors other than ocular inflammation such as ageing,8 pupillary dilation9 and various medications7 that may have an effect on either aqueous flare concentrations or the amount of light scattering. Although flare alterations due to possible blood ocular barrier disruptions were investigated thoroughly, only a few studies looked into the light scattering effect of cataract and other anterior segment features and laser photometer readings10,11 As far as is known, the effect of hyperreflectivity of anterior chamber structures on automated flare measurements that are already inside the critical background levels has not been reported quantitatively in the literature. This altered scattering effect may be best detected with Scheimpflug camera systems,12 which already emerged as a promising method for objective assessment of lens density that is constantly verified against the established Lens Opacity Classification System III.13–16
This paper aims to evaluate the effect of anterior segment structures including anterior cortical lens density, anterior chamber depth, iris surface and pupil aperture on the automated flare meter measurements due to light back-scattering that is detected quantitatively from the anterior segment Scheimpflug images.
Methods
Patient selection
The current study was approved by the Institutional Review Board of Gazi University in Ankara, Turkey. The study adhered to the tenets of the Declaration of Helsinki. In this retrospective cohort study, patients diagnosed with clinical uveitis were enrolled from a uveitis clinic at a tertiary referral centre between January 2018 and June 2020. All subjects underwent laser flare photometer and all available corneal topography measurements were collected, retrospectively. All patients underwent a full ophthalmologic examination including a clinical flare evaluation by a senior uveitis specialist. Full ophthalmologic examination included best-corrected visual acuity, slit-lamp biomicroscope, intraocular pressure with Goldmann applanation tonometry, and clinical grade of anterior chamber cells and flare with slit-lamp through a dilated pupil. Patients who had corneal pathologies, significant posterior synechiae and previous intraocular surgery, including pseudophakia patients, were excluded.
Clinical flare grading and measurement
The clinical flare grading () of the anterior chamber was evaluated according to a modified type of Standardization of Uveitis Nomenclature grading scale,17 in which additional 0.5+ and 1.5+ flare grades were introduced to grade cases that were considered between 0 to 1.0+ and 1.0+ to 2.0+ clinical grades respectively. Anterior chamber flare was recorded by trained operators using Kowa laser flare-cell photometer FM-700 (Kowa Co, Tokyo, Japan). Each laser flare photometer examination included a minimum of ten (10) flare measurements () and measured as photons per milliseconds. Measurements where the output had background and cell warnings from the device were excluded from the study. Both clinical flare grading and laser flaremetry measurements were performed during the same visit.
Image acquisition and processing
Scheimpflug imaging (Sirius Topographer, CSO, Italy) was performed after pupil dilation with an eyedrop of cyclopentolate 1% and phenylephrine 2.5%. The patient was seated in front of the Scheimpflug camera with the chin supported on a chin rest. To reduce operator-dependent variability, the automatic-release mode was used. Three measurements were taken of each eye, and the best horizontal section image was selected for further analysis. Anterior chamber depth values measured by the Scheimpflug system software were noted.
Images obtained by the Scheimpflug camera were imported into ImageJ software for processing. Pupil aperture was measured in pixels and converted to millimetres. Polygonal selection was performed to select anterior cortical lens and anterior iris surface on the immediate border of pupil aperture as separate regions of interest. The light artefacts in the acquired images were excluded from calculations. Maximum anterior cortical density and iris scatter inside regions of interests were measured for each patient in pixel units on a scale of 0 to 255 (Figure 1).
Figure 1.
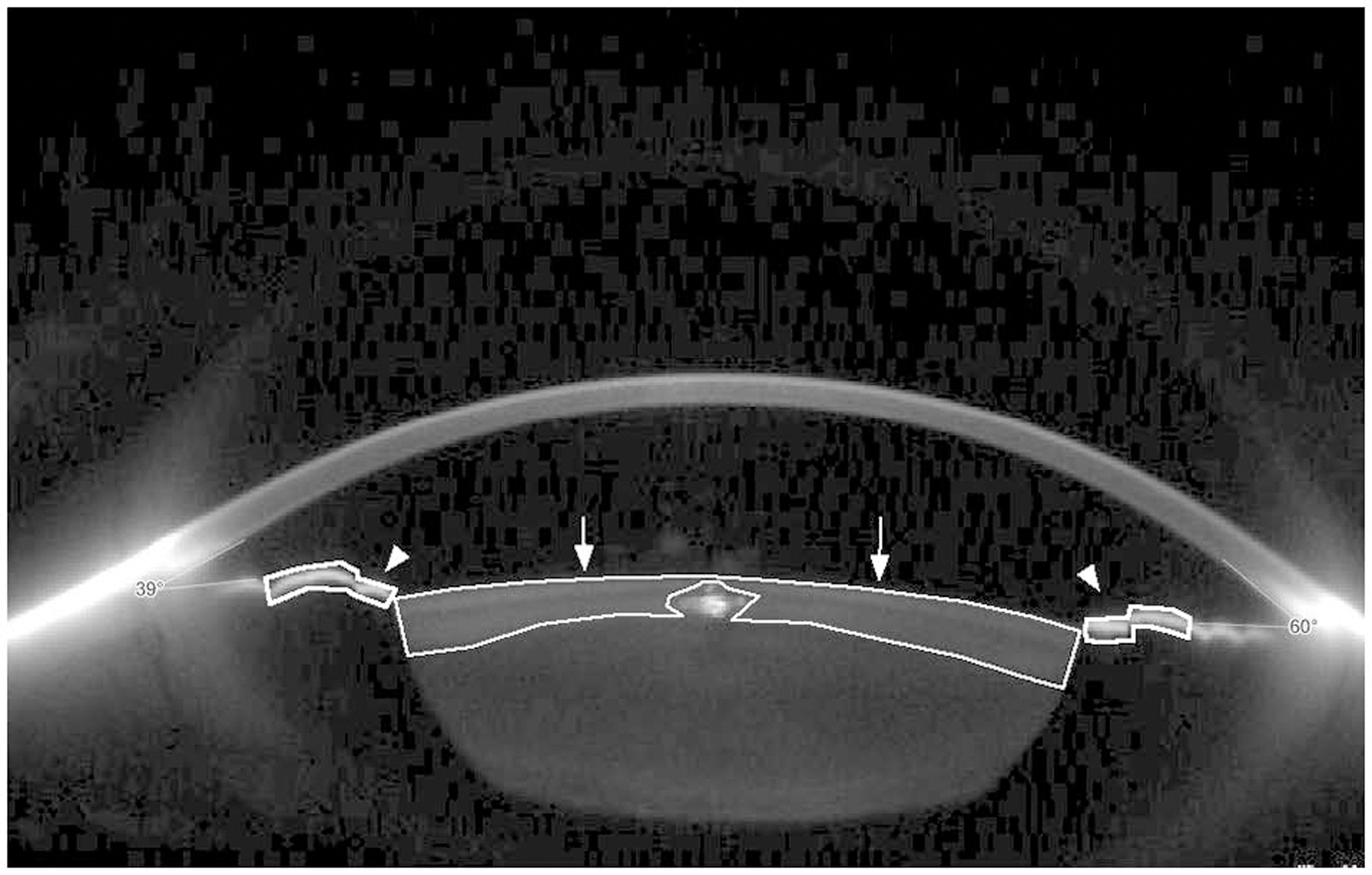
Scheimpflug image mounted with ImageJ software to measure maximum density of anterior cortical lens and anterior iris surface. Selected anterior cortical lens (arrows) and iris anterior surfaces (arrowheads). Note that the intra-lenticular light artefact is excluded from the measurement.
Statistical analysis
Statistical analysis was performed using SPSS software (Version 20.0, IBM, Armonk, NY, USA). Data distribution was determined with Kolmogorov-Smirnov test. Mean and median values of measured flare () (photons/milliseconds), anterior chamber depth (millimetre), pupil aperture (millimetre), maximum anterior cortical density (pixel unit) and maximum iris scatter (pixel unit) were calculated. Curvilinear regression analysis was performed to evaluate the best-matched curve that estimated the correlation between modified Standardization of Uveitis Nomenclature grading and laser flare photometer measurements of cases. Laser flare photometer values that were estimated by regression analysis for each modified Standardization of Uveitis Nomenclature grade were calculated. These values were used as cut-off values to split eyes in Group I (laser flare photometer measurement lower than estimated) and Group II (laser flare photometer measurement higher than estimated). Categorical variables were compared with chi-square test. The mean age, anterior chamber depth, pupil aperture, maximum cortical density and maximum iris scatter between two groups were compared with independent samples t-test. To create the prediction equation, a stepwise multiple regression analysis was performed. The natural logarithm of was set as a dependent variable, and , maximum cortical density, anterior chamber depth and pupil aperture were set as independent variables. A p value less than 0.05 was considered statistically significant.
Results
Two hundred and twenty-eight eyes (228) of 114 patients were enrolled in the study. The demographics and baseline clinical characteristics of patients are demonstrated in Table 1. Fifty eight percent (58%) of the patients were female and mean age was 35.1 ± 16.9 years. Of these subjects, 121 (53%) were clinically assessed as with no flare, 25 (11%) with 0.5+ flare, 54 (24%) with 1+ flare, 14 (6%) with 1.5+ flare, 13 (6%) with 2+ flare and 1 with 3+ flare.
Table 1.
Demographics and baseline clinical characteristics of subjects.
| Total (n = 228) (%) | Group I (n = 123) (%) | Group II (n = 105) (%) | p Value | |
|---|---|---|---|---|
| Female: male ratio | 11:8 | 10:8 | 12:8 | 0.598 |
| Age in years (mean ±SD) | 35.1 ± 16.9 | 34.5 ± 16.0 | 35.8 ± 17.8 | 0.582 |
| BCVA in logMAR units (mean ±SD) | 0.14 ± 0.30 | 0.11 ± 0.28 | 0.17 ± 0.31 | 0.127 |
| Uveitis classification | ||||
| Anterior | 111 (48.7) | 57 (46.3) | 54 (51.4) | 0.454 |
| Intermediate | 48 (21.1) | 26 (21.1) | 22 (21.0) | |
| Posterior | 23 (9.9) | 16 (13.0) | 7 (6.7) | |
| Panuveitis | 46 (19.8) | 24 (19.5) | 22 (21.0) |
n: number of eyes; BCVA: best corrected visual acuity; logMAR: logarithm of minimal angle resolution.
p < 0.05 is considered statistically significant.
The mean ± SD, median and range values of on each clinical grading were given in Table 2. Curvilinear regression analysis between and have shown that an exponential model was best matched to estimate the correlation between clinical flare score and laser flare photometer measurement (R2: 0.733, p < 0.001) with following equations (Figure 2):
Table 2.
Flare meter results (photons/milliseconds) of cases in each group of modified Standardization of Uveitis classification.
| MSUN | n (%) | LFP (ph/ms) |
Group I (n) | Group II (n) | |||
|---|---|---|---|---|---|---|---|
| Mean ±SD | Median | Range | Model | ||||
| 0 | 121 (53) | 5.69 ± 3.05 | 5.0 | 1.6–25.6 | 4.87 | 55 | 66 |
| 0.5+ | 25 (11) | 7.92 ± 3.09 | 7.4 | 4.0–20.0 | 8.50 | 17 | 8 |
| 1+ | 54 (24) | 13.56 ± 6.16 | 12.0 | 4.3–32.0 | 14.81 | 38 | 16 |
| 1.5+ | 14 (6) | 25.96 ± 15.34 | 19.6 | 12.3–67.8 | 25.83 | 9 | 5 |
| 2+ | 13 (6) | 77.82 ± 28.36 | 84.1 | 31.1–133.1 | 45.04 | 2 | 11 |
| 3+ | 1 | 118 | 118 | NA | 136.93 | 1 | 0 |
MSUN: Modified Standardization of Uveitis Nomenclature; n: number of eyes; LFP: Laser flare photometry; ph/ms: photons/milliseconds; SD: standard deviation.
Model values are calculated from curvilineal estimation formula of LFP for each clinical flare grading.
Figure 2.
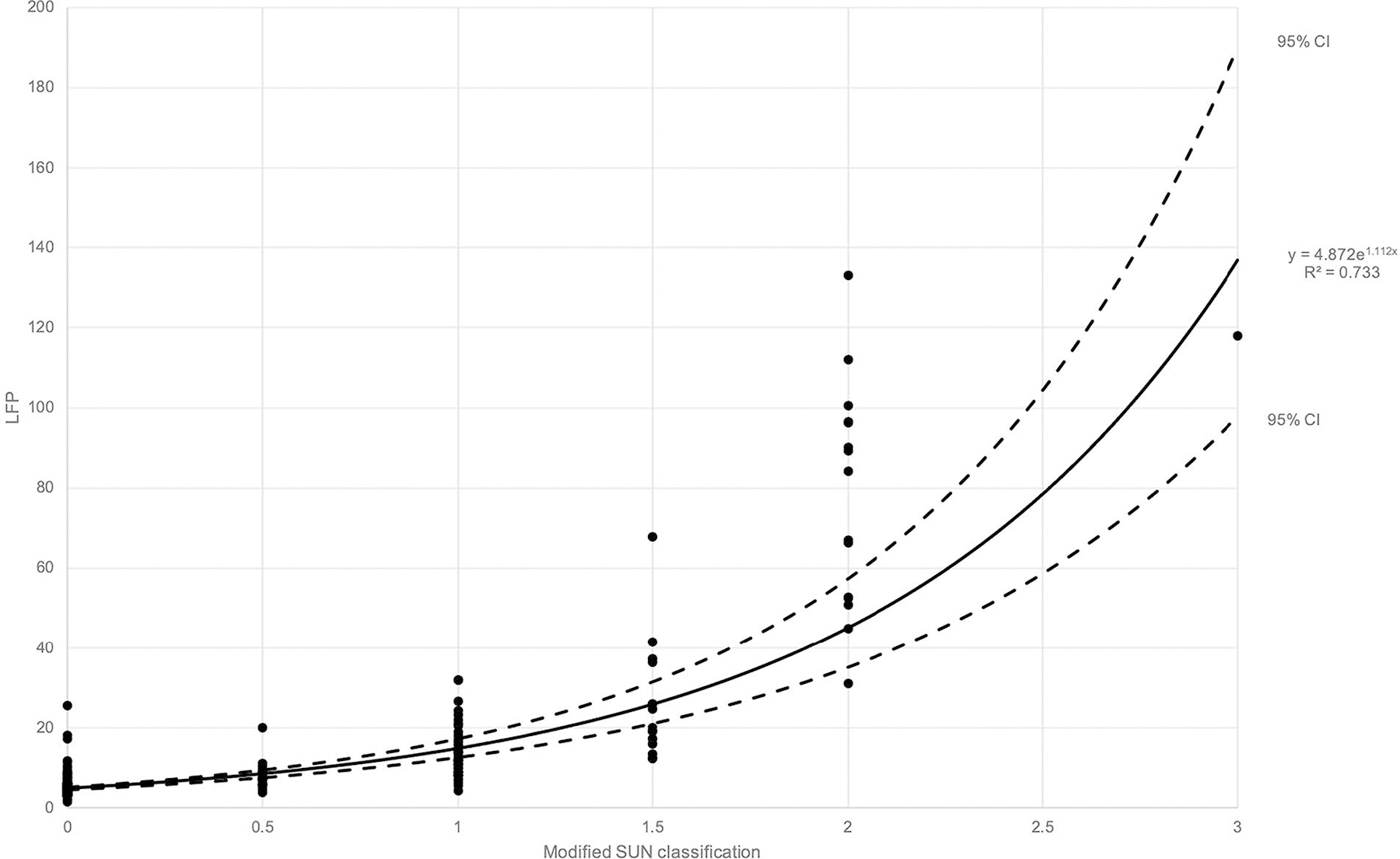
Scatter plot and nonlinear regression model of cases comparing modified Standardization of Uveitis Nomenclature classification and laser flare photometer measurements (n = 228). An exponential equation curve had the best match with R2 value equal to 0.733. 95% confidence interval curves were also added.
The model-based laser flare photometer values corresponding to clinical flare scores 0, 0.5+, 1+, 1.5+, 2+ and 3+ were calculated from the regression formula (added in Table 2) and considered as cut-off values to split cases that are under and over the regression curve in two groups: Groups I and II were consisted of eyes who had actual measurements that were lower and higher than estimated by the model, respectively.
Scheimpflug images that were previously acquired from 105 eyes in the study were used for further anterior segment structural analysis (Table 3). Of these eyes, 49 (46.7%) were in Group I and 56 (53.3%) in Group II. Anterior chamber depth, pupil aperture and maximum cortical lens density measurements in two groups are given in Table 4. The mean maximum anterior cortical lens density of Group II was significantly higher than Group I (p = 0.001). No significant difference was found in mean anterior chamber depth, pupil diameter and maximum iris scatter values between two groups (p = 0.098, 0.130 and 0.996, respectively).
Table 3.
Flare meter results (photons/milliseconds) of cases with Scheimpflug images (n = 105) in each group of modified Standardization of Uveitis classification.
| MSUN | n (%) | LFP (ph/ms) |
|---|---|---|
| Mean ±SD | ||
| 0 | 58 (55) | 5.84 ± 3.80 |
| 0.5+ | 12 (11) | 8.21 ± 4.10 |
| 1+ | 22 (21) | 14.53 ± 6.50 |
| 1.5+ | 6 (6) | 20.53 ± 10.32 |
| 2+ | 7 (7) | 87.21 ± 33.70 |
MSUN: Modified Standardization of Uveitis Nomenclature; n: number of eyes; LFP: laser flare photometry; ph/ms: photons/milliseconds; SD: standard deviation.
Table 4.
Comparison of anterior segment measurements (n = 105) between Groups I (n = 49) and II (n = 56).
| Parameters | Mean ±SD |
p Value | |
|---|---|---|---|
| Group I (n = 49) | Group II (n = 56) | ||
| ACD (millimetre) | 3.31 ± 0.51 | 3.15 ± 0.48 | 0.098 |
| Pupil aperture (millimetre) | 5.55 ± 2.19 | 4.91 ± 2.00 | 0.128 |
| Dmax (pixel unit) | 77.9 ± 17.06 | 96.6 ± 37.1 | 0.001 |
| Iris scatter (pixel unit) | 212.1 ± 35.7 | 212.1 ± 36.2 | 0.996 |
ACD: anterior chamber depth; Dmax: maximum anterior cortical lens density; SD: standard deviation. p < 0.05 is considered statistically significant.
The results of stepwise multiple regression analysis have shown and maximum cortical density as explanatory variables (adjusted R2 = 0.486, : b = 0.499, p < 0.001; maximum cortical density: b = 0.335, p = 0.001) to establish a new relation between laser flare photometer and clinical flare with the following equation:
Discussion
Herein, we present the first quantitative study that investigates the effect of back-scattered light from anterior segment structures on laser flare photometer measurements. Although laser flare photometry measurements are correlated with slit-lamp based clinical grading,7,18 significant overlap of laser flare photometer results within lower grades of Standardization of Uveitis Nomenclature classification is reported,5,17 which made the definition of precise cut-off laser flare photometer values between clinical grades challenging. Therefore, no consensus has been established to translate quantitative laser flare photometer measurements into clinical flare grading until present. In this study, an exponential relationship between clinical flare grading and automated flare measurements was found in accordance with the previous studies where the natural logarithm of laser flare photometer measurements was used to compare with clinical grading scores.4,5,19 It was assumed that laser flare photometer values that were calculated for each clinical grade from this mathematical model would be less biased by the possible effects that were subject to this study when compared to mean and median values, thus model-based laser flare photometer values were used to split cases in two groups.
Several techniques are applied during automated flare measurements to minimize the signal noise created by back-scattered light from anterior segment structures.6 These techniques include the use of a narrow beam for a vertical scanning of sampling window, 1 ms photon sampling time and mathematical elimination of background scattering that is detected outside sampling window.6 In the current study, it was possible to quantify the effect of back-scattered light from the anterior cortical part of the crystalline lens over laser flare photometer measurements while there was no evidence found to indicate an influence of anterior chamber depth or iris scatter. Given that none of the eyes in the study had cataract, the highest density was observed on the anterior cortical segment of the crystalline lens in the present Scheimpflug images. Another fact was that contrary to lens nucleus and posterior cortex which were partially visible, pupillary part of the anterior cortex of the lens was entirely visible for the most of cases, leading us to choose anterior cortical lens for backscatter analysis. Besides the known effect of the cataractous lens, based on findings of the present study, non-cataractous crystalline lens might also have a quantifiable effect over laser flare photometer measurement. It could be argued that although light scattering from anterior iris surface was reduced by the inherent scatter minimizing methods that were mentioned above, the back-scattered light from lens surface was not completely eliminated by these methods. In this measure, analysis of back-scattered light from acquired Scheimpflug images might be helpful to further adjust the measured laser flare photometer. The pupillary dilation might have an ambivalent effect over light scattering that while reducing the scatter from the iris surface and decreasing protein leakage into aqueous humour,20 it might enhance the scatter originated from the lens. The anterior chamber was also found to be narrower in Group II though the difference did not reach a statistically significant level. The intensity of back-scattered light at a given point is inversely correlated with its distance to the scatter source.21 In automated flare measurement, anterior chamber depth alone is not sufficient to predict the distance between lens-iris plane and sampling window, therefore the distance between posterior corneal surface and sampling window should also be known precisely to evaluate the possible effect of narrow anterior chamber over laser flare photometer measurement quantitatively.
The main limitations of the present study are its retrospective design and small sample size. Nearly half of the study population was graded with 0 flare based on modified Standardization of Uveitis Nomenclature classification. The results, however, give valuable information to interpret high variations of flare measurements in lower clinical grades. As this was a retrospective study, not all of participants had previous Scheimpflug imaging. Participants who do not have Scheimpflug images were still included in the study to calculate more accurately estimated LFP values for each clinical grade, thus more accurate grouping of cases. The stepwise multiple regression analysis that was performed in this study gives a mere insight regarding the effect of anterior cortical lens density over laser flare photometer findings in a limited number of patients. Future studies to aim for accurate analysis that encompasses an adjustment formula should include additional parameters such as corneal scatter, depth of sampling window inside the anterior chamber and pupillary area. These formulas should however be advised to adjust high laser flare photometer variations that cause overlapping flare counts between cases with low clinical grades (0 to 1+); the larger laser flare photometer variations which result in a similar overlapping between high clinical grades (1+ to 3+) appear more likely due to the logarithmic nature of the clinical grading system. On the other hand, bias should be taken into account due to the inaccuracies on subjective clinical grading of flare. However, being considered as the gold standard, the feasibility of novel approaches still needs to be assessed in comparison with the current clinical grading systems. Furthermore, it should be acknowledged that any effort to eliminate the undesired backscatter effects over laser flaremetry readings with additional data acquired from clinical examination and ancillary tests would be limited, for that hardware-related modifications to improve the optics of flare measurement (e.g. use of near infrared light beam, better dark-room isolation, etc.) are needed.
Conclusion
The back-scattered light from anterior cortical lens opacities may have significant effect laser flare photometer measurements which potentially be quantified by Scheimpflug imaging. Adjustment of measurements accordingly may enable further standardization of current automated flare meter technique.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Jabs DA, Nussenblatt RB and Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 2005; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempen JH, Ganesh SK, Sangwan VS, et al. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol 2008; 146: 813–818.e1. [DOI] [PubMed] [Google Scholar]
- 3.Sawa M, Tsurimaki Y, Tsuru T, et al. New quantitative method to determine protein concentration and cell number in aqueous in vivo. Jpn J Ophthalmol 1988; 32: 132–142. [PubMed] [Google Scholar]
- 4.Konstantopoulou K, Del’Omo R, Morley AM, et al. A comparative study between clinical grading of anterior chamber flare and flare reading using the Kowa laser flare meter. Int Ophthalmol 2015; 35: 629–633. [DOI] [PubMed] [Google Scholar]
- 5.Tugal-Tutkun I and Herbort CP. Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int Ophthalmol 2010; 30: 453–464. [DOI] [PubMed] [Google Scholar]
- 6.Sawa M Laser flare-cell photometer: principle and significance in clinical and basic ophthalmology. Jpn J Ophthalmol 2017; 61: 21–42. [DOI] [PubMed] [Google Scholar]
- 7.Ladas JG, Wheeler NC, Morhun PJ, et al. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol 2005; 50: 27–47. [DOI] [PubMed] [Google Scholar]
- 8.Shah SM, Spalton DJ and Smith SE. Measurement of aqueous cells and flare in normal eyes. Br J Ophthalmol 1991; 75: 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Harazi SM, Ruiz RS, Feldman RM, et al. Quantitative assessment of aqueous flare: the effect of age and pupillary dilation. Ophthalmic Surg Lasers 2002; 33: 379–382. [PubMed] [Google Scholar]
- 10.Küchle M, Hannappel E, Nguyen NX, et al. [Correlation between tyndallometry with the “laser flare cell meter” in vivo and biochemical protein determination in human aqueous humor]. Klin Monatsbl Augenheilkd 1993; 202: 14–18. [DOI] [PubMed] [Google Scholar]
- 11.Ursell PG, Spalton DJ and Tilling K. Relation between postoperative blood-aqueous barrier damage and LOCS III cataract gradings following routine phacoemulsification surgery. Br J Ophthalmol 1997; 81: 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegener A and Laser-Junga H. Photography of the anterior eye segment according to Scheimpflug’s principle: options and limitations - a review. Clin Exp Ophthalmol 2009; 37: 144–154. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez-Vicent A, Birkeldh U, Laurell CG, et al. Objective assessment of nuclear and cortical cataracts through scheimpflug images: agreement with the LOCS III Scale. PLoS One 2016; 11: e0149249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta M, Ram J, Jain A, et al. Correlation of nuclear density using the lens opacity classification system III versus Scheimpflug imaging with phacoemulsification parameters. J Cataract Refract Surg 2013; 39: 1818–1823. [DOI] [PubMed] [Google Scholar]
- 15.Grewal DS, Brar GS and Grewal SP. Correlation of nuclear cataract lens density using Scheimpflug images with lens opacities classification system III and visual function. Ophthalmology 2009; 116: 1436–1443. [DOI] [PubMed] [Google Scholar]
- 16.Chylack LT Jr, Wolfe JK, Singer DM, et al. ; The Longitudinal Study of Cataract Study Group. The lens opacities classification system III. Arch Ophthalmol 1993; 111: 831–836. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal R, Keane PA, Singh J, et al. Classification of semi-automated flare readings using the Kowa FM 700 laser cell flare meter in patients with uveitis. Acta Ophthalmol 2016; 94: e135–e141. [DOI] [PubMed] [Google Scholar]
- 18.el-Maghraby A, Marzouki A, Matheen TM, et al. Reproducibility and validity of laser flare/cell meter measurements as an objective method of assessing intraocular inflammation. Arch Ophthalmol 1992; 110: 960–962. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal R, Keane PA, Singh J, et al. Comparative analysis of anterior chamber flare grading between clinicians with different levels of experience and semi-automated laser flare photometry. Ocul Immunol Inflamm 2016; 24: 184–193. [DOI] [PubMed] [Google Scholar]
- 20.Oshika T and Kato S. Changes in aqueous flare and cells after mydriasis. Jpn J Ophthalmol 1989; 33: 271–278. [PubMed] [Google Scholar]
- 21.TJTP van den Berg. Intraocular light scatter, reflections, fluorescence and absorption: what we see in the slit lamp. Ophthalmic Physiol Opt 2018; 38: 6–25. [DOI] [PubMed] [Google Scholar]


