Abstract
Since the arrival of leadless pacemakers (LPs), they have become a cornerstone in remedial treatment of bradycardia and atrioventricular (AV) conduction disorders, as an alternative to transvenous pacemakers. Even though clinical trials and case reports show indisputable benefits of LP therapy, they also bring some doubts. Together with the positive results of the MARVEL trials, AV synchronization has become widely available in LPs, presenting a significant development in leadless technology. This review presents the Micra AV, describes major clinical trials, and introduces the basics of AV synchronicity obtained with the Micra AV and its unique programming options.
Keywords: leadless pacing, Micra AV, transcatheter pacing system, atrioventricular synchrony, atrioventricular block
Introduction
The well-established consensus for high-degree atrioventricular (AV) block treatment is an implantable pacemaker therapy. Even though primary VVI mode is enough to reduce mortality, current guidelines underline the clinical significance of AV synchronization obtained via atrial sensing for improved quality of life and avoidance of pacemaker syndrome [1].
Leadless pacemakers (LPs) were first mentioned in cardiac pacing and cardiac resynchronization therapy guidelines in 2021 [1]. The first LP device — NANOSTIM — implanted in 2012, offered VVIR mode as the most sophisticated pacing option [2], but it was withdrawn from the market due to battery issues and docking button detachments during implantation or retrieval.
The first steps in the development of an AV synchrony algorithm were taken with the MASS and MASS2 trials. Participants had software downloaded into a Micra VR (MVR), allowing accelerometer signal telemetry. After a series of manoeuvre tests, the signal was collected from Micra’s accelerometer vectors detailing four heart signals: A1, A2, A3, and A4 [3].
The subsequent trial exploring the field of AV synchrony in LPs was MARVEL, which proved the feasibility of ventricle pacing with AV synchronization in LPs [3]. The developed algorithm was downloaded into previously implanted Micra devices, which allowed for an average 87% AV synchrony, with 80% in high-degree AV block patients and 94.4% in patients with intrinsic AV conduction. After enhancing the algorithm, the MARVEL2 trial improved the median AV synchrony in high-degree AV block patients from 26.9% with VVI pacing to 94.3% in VDD mode, and the left ventricular outflow tract velocity–time integral (which stands for left ventricular stroke volume) by 8.8 ± 15.4% [4].
The rising importance of understanding indications for Micra AV (MAV) implantation, unique programming options, and precautions in specific clinical situations are necessary to achieve satisfactory results.
Build of the Micra AV-MC1AVR1
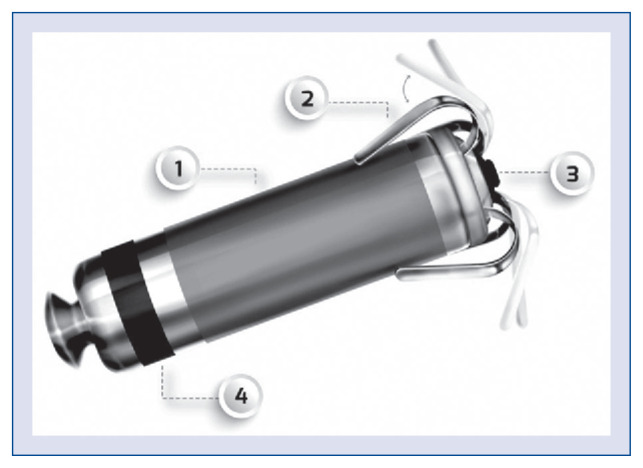
With a mass of 1.75 g, dimensions of 25.9 mm × 6.7 mm, and volume of 0.8 cc — around 1% of the hearts’ right ventricle (RV) [5] volume — the MAV duplicates Medtronic’s previous LP external construction: the MVR. The main body of the MAV is composed of a capsule (Fig. 1 — — 1), a dexamethasone-coated stimulation cathode (Fig. 1 — 3), and an anode (Fig. 1 — 4). There are 4 fixation nitinol tines (Fig. 1 — 2) between the cathode and anode (Fig. 1 — — 1) to attach the device to the heart tissue — myocardium. The MAV detects atrial contraction with a built-in accelerometer, providing AV synchronous pacing. The battery has (typical for pacemakers) 3 indicator states: recommended replacement time (RRT), which is shown 6 months before the end of service (EOS); elective replacement indicator, 3 months before EOS; and the state of EOS, which starts after 3 consecutive daily automatic measurements with ≤ 2.5 V.
Figure 1.
Build of the Micra AV; 1 — capsule; 2 — fixation tine; 3 — stimulation cathode; 4 — stimulation anode.
Micra AVs longevity is estimated between 8 and 13 years and depends on the pacing mode, ventricle pacing percentage, impedance, and threshold.
Implantation technique
Because the MAV’s external build is identical as the MVR, the implantation technique is the same. Vascular access is obtained by puncturing the femoral vein, preferably the right one, but it can also be achieved through the jugular vein [6]. With sheaths placed for vascular access, the introducer is inserted via a stiff guidewire to the right atrium. The delivery system is advanced into the introducer up to the mid-atrium. Subsequently, with the help of fluoroscopy guidance, the LP system is placed in the septal portion of the RV. Micra’s position should be verified using different fluoroscopy views and contrast flow to achieve satisfactory adherence to the wall of the myocardium. The target location is the mid-high interventricular septum. Too high a position of the LP can interfere with the tricuspid valve or pulmonary valve and has less chance of being hooked in the trabeculae, which can happen especially in the right ventricular outflow tract. Avoiding the RV apex is also vital due to its thin wall approaching around 1 mm musculature.
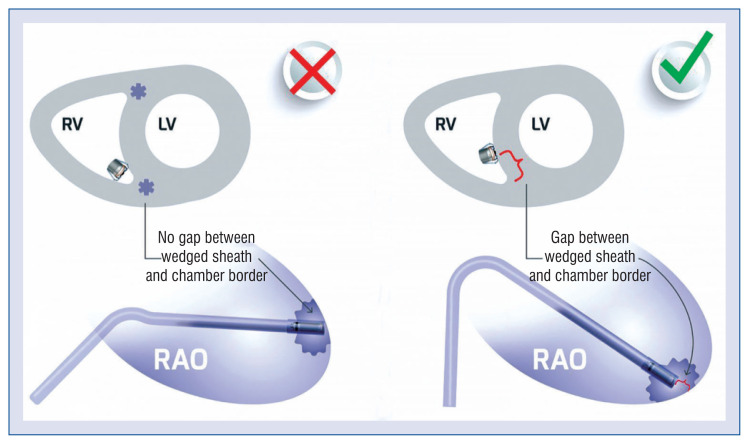
Different projections and radiological signs help with appropriate LP navigation. The right anterior oblique (RAO) view allows recognition of the tricuspid valve passing and avoiding the true apex. Moreover, in this view, with a contrast-push and the presence of a space between the wedged sheath and myocardium border, one can achieve the confidence of Micra’s septal contact, called the RAO space sign (Fig. 2) [7].
Figure 2.
Right anterior oblique (RAO) space sign [7]; LV — left ventricle; RV — right ventricle.
Left anterior oblique (LAO) and full lateral view give additional information and allows avoiding Micra implantation in the anterior wall, which is crucial to decrease the risk of free wall perforation. In the LAO view the catheter should be pointed towards the spine, and in the full lateral view the cup should point directly at the camera. During contrast injections, a flat flow pattern against the septum in LAO 30–40° indicates optimal contact, and the contrasted trabeculated surface only reassures a good position. If doubt persists, the use of intraprocedural transesophageal echocardiography can prevent the LP’s implantation to the heart’s free wall [8].
Recommended parameters for the device are R-wave ≥ 5 mV, impedance 400–1500 Ω, and threshold ≤ 1.00 V. A predictive model for the long-term electrical performance of an LP has been proposed by Kiani et al. [9]. Threshold less than or equal to 1 V after implantation seems to predict good future electrical properties of the positioned Micra. Higher values need to be re-checked after 3–5 min. With threshold of more than 2 V and impedance less than 800 Ω, repositioning of the Micra is strongly advised.
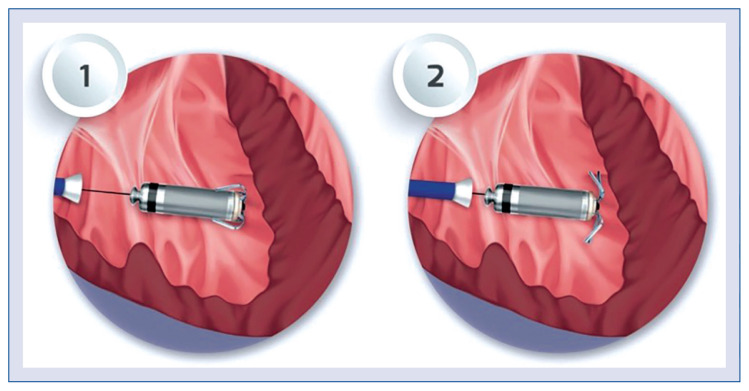
With stable parameters, one should assess the fixation of the tines with a pull-and-hold test and bending of the tines. Recognition of at least 2 out of 4 tines being straightened during a pull-and-hold test is necessary to confirm successful implantation (Fig. 3).
Figure 3.
Pull-and-hold test; 1 — before the pull-and-hold test, no fixation tines bend; 2 — after pulling, 4 out of 4 fixation tines are bending, confirming the correct implantation of the Micra.
Clinical indications for the Micra-AV
Leadless pacemakers should be considered for patients with frailty syndrome, chronic kidney disease, especially those on dialysis, less than 10 years of life span, hindered access for the transvenous pacemakers (TV-PM), i.e., stenotic vena cava superior or its branches, or history of cardiac device-related infective endocarditis (CDRIE) [1].
Moreover, the decision for LP implantation during severe viral infection, i.e., SARS-CoV-2, seems safe [10].
Due to the stroke volume improvement with AV synchrony, MAV is preferred in patients considered for the LP with an AV block [4].
One should be aware that sick sinus syndrome, especially with maintained retrograde ventriculoatrial conduction or persistent supraventricular arrhythmia (including bradycardia), should not be regarded as an indication for MAV [11].
Programming
With an introduction of AV synchronization in LPs due to a built-in accelerometer, programmable vectors were constituted, which characterize three space dimensions — X, Y, and Z, represented by numbers: 1, 2, and 3. They allow for a distinction between atrial and ventricle mechanical work with the possibility of being combined in different options for better signal quality to achieve optimal sensing results.
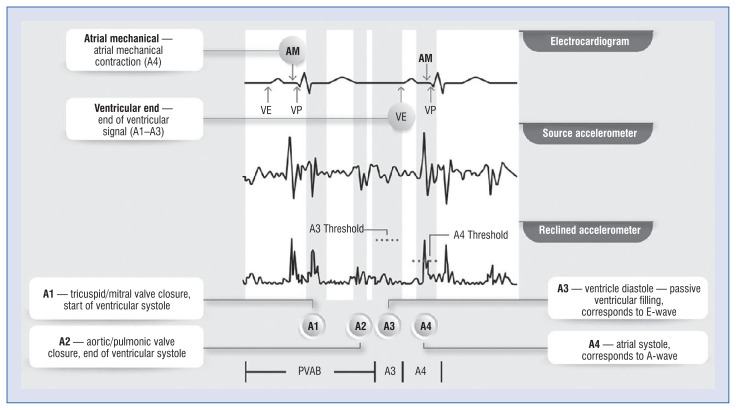
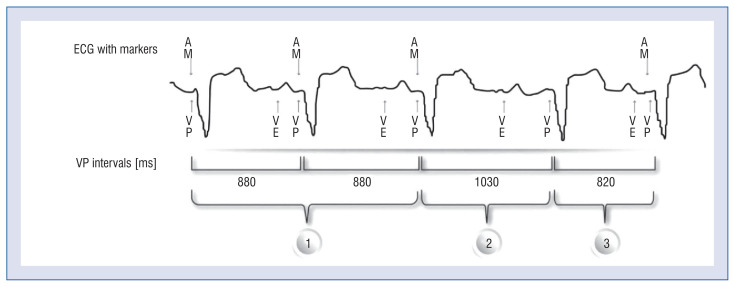
The heart work is split by an accelerometer into 4 waves (Central illustration). The A1 wave follows the QRS complex immediately. It consists of the mitral and tricuspid valve closure and represents the start of a ventricle contraction. Afterwards, the A2 wave occurs at the end of the ventricle contraction with an aortic and pulmonary valve closure. This signal is usually sharp and can be located near the end of the T-wave. The A1 and A2 signals should be located in a customizable postventricular atrial blanking (PVAB), nominally set to 550 ms. Moreover, there is also a post ventricular atrial refractory period (PVARP) extendable from 500 to 750 ms.
Central illustration.
Accelerometer-based waves split in Micra AV [31]; AM — atrial mechanical; PVAB — post-ventricular atrial blanking; VE — ventricular end; VP — ventricular pacing.
An A3 signal stands for the diastole of the ventricles. During that phase, the signal is usually rounder and corresponds to the passive filling of the ventricles. In Doppler ultrasonography, this part is known as an E wave. In Medtronic’s programmer, the end of the A3 wave is annotated with the letters “VE”, which is an abbreviation of “ventricular events”. It corresponds to the A1–A3 waves. The last one, the A4 signal, represents the atrial contraction, which comes around 100 ms after the P-wave and stands for the Doppler’s A wave. The A4 wave is aliased with the “AM” marker, as an abbreviation for “atrial mechanical”.
The A7 wave can appear with an accelerometer overlap of the A3 and the A4 wave and corresponds to the gallop sound during heart auscultation. It occurs when the passive and active filling of the ventricles happens simultaneously, with higher heart rates or lack of AV synchronization.
The VDD pacing mode is initiated by obtaining AV synchronization and can be achieved in two ways. After implantation and turning on the VDD mode, the atrial sensing setup process is held automatically for 30 min, including a collection of atrial activity detection. After the auto setup test, based on the obtained data, the device offers automatic values for A3 and A4 thresholds and the ventricular window end (including minimum and maximum values).
However, the manual atrial mechanical test allows the manual setting of the parameters mentioned above: A3 and A4 threshold and the ventricular window end, along with the gathered data.
Unfortunately, the mechanical signals obtained from the accelerometer can be interfered with by various factors, i.e., accelerated heart rate or suboptimally programmed A3 and A4 signals. Thus, surface electrodes should be connected between the programmer and patient during the manual atrial mechanical test to recognize atrial detection correctly.
The window end for the A3 wave should be fixed below 700 ms to deactivate automatic adjustment, which can lead to excessive prolongation of the A3 window and worsening p-wave detection [12, 13]. A fixed threshold for the A3 wave should be set around 1 m/s2 above the primary sensed A3 wave. It allows proper A7 signal sensing during higher sinus rates, the maintenance of AV synchrony and, at the same time, blanking of the A3 signal to avoid falsely recognizing the A4 waves [14].
In turn, the A4 threshold should be just below the A4 signal to recognize the AM, but it should not to be misled by signal noise.
Algorithms
Medtronic developed different algorithms that promote AV synchrony in VDD mode.
If intermittent A4 under sensing occurs, the rate smoothing algorithm (Fig. 4) maintains AV synchrony. With no sensed atrial contraction and when the rate smoothing interval times out, the device paces at the rate smoothing rate. Based on pacing history and an offset (which can be programmed via the smoothing delta), the MAV increases the probability of AV synchronous pacing and tracking the next atrial contraction.
Figure 4.
Rate smoothing algorithm: 1 — appropriate atrial mechanical (AM) sensing with synchronous ventricular pacing (VP); 2 — atrial undersense — VP occurs within the rate smoothing interval, instead of the lower rate; 3 — atrioventricular synchrony recovery [29]; ECG — electrocardiogram; VE — ventricular end.
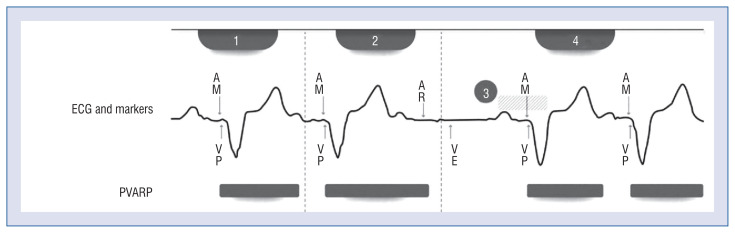
Tracking check is another pacing feature that ensures the sensed atrial origin of the A3 and the A4 wave and prevents falsely induced paced tachycardia (Fig. 5). During a ventricular rate above the programmable tracking check rate, the algorithm extends the PVARP. Tracking check estimates the location of the subsequent AM signal with the stored and tracked patient sinus rate. The appropriate sinus tracking is confirmed if the next AM is within that window, and the device returns to the standard PVARP value for approximately 1.5 min.
Figure 5.
Tracking check algorithm; 1 — atrial mechanical ventricular pacing (AM-VP) rhythm at or above the tracking check rate; 2 — after the median ventricular rate reaches tracking check rate, the tracking check extends the post-ventricular atrial refractory period (PVARP) until the following AM occurs within; 3 — the tracking check estimates the location of the next AM; 4 — AM occurs in the expected range — appropriate tracking is confirmed, and the PVARP is returned to its basic value. Copyright Medtronic. Used with permission [30]; ECG — electrocardiogram; AR — atrial refractory sensing.
Oversensing-induced tachycardia is confirmed if the next AM signal occurs outside the estimated range — tracking check maintains the extended PVARP for 40 s. The following falsely sensed AM signal should occur within the next refractory period, and ventricular pacing should be inhibited.
The tracking check algorithm can lead to an abrupt change in cycle length with arrhythmogenic short-long-short sequences. In rare situations, the algorithm can cause polymorphic ventricular tachycardia, especially in patients with long QT syndrome [15]. In this situation, tracking check should be turned off.
The MAV is also equipped with mode switching algorithms. The activity mode switch turns into a rate-responsive mode (VDIR) when the accelerometer detects high activity, and the ventricular rate is low. Such a situation can happen due to loss of atrial tracking during VDD mode. After high activity stops, the MAV switches back to VDD mode.
Another mode switching option introduced with the MAV is AV conduction mode switch — the so-called “VVI+”. It promotes intrinsic conduction and reduces battery usage and ventricular pacing by scanning for AV conduction by periodically switching to VVI mode with a lower rate of 40/min, regardless of the presence of an AV block. This option should be turned off in the case of complete AV block or clinically significant P-R interval prolongation with a heart rate above 40/min.
Clinical trials
While comparing LP safety to TV-PMs, the MVR and MAV should be considered as similar devices and combined into one group due to their similar external build.
One in 8 patients with TV-PM may experience peri- and post-procedural complications [16].
Thus, the vital clinical issue for Micra was safety assessment in the short- and long-term, which was proven in 2 studies: Micra VR Investigational Device Exemption (IDE) and Post-Approval Registry (PAR) — showing indisputable benefits. The IDE study showed 48% (hazard ratio [HR]: 0.52; 95% confidence interval [CI]: 0.35–0.77) fewer complications compared to TV-PMs, a high implant success rate (99.2%), and stable low pacing thresholds at 6 months in 98.3% of patients [17].
The PAR, which had 1817 participants with at least 12 months of follow-up for 465 of them, confirmed the general safety from the IDE study and proved a low rate of major complications throughout 12 months (2.7%; 95% CI: 2.0–3.6%) with no device-related infections [18]. The reduction in major complications was mainly driven by a 47% relative risk reduction in hospitalizations and 82% relative risk reduction in system revisions. Such outcomes can be associated with the lack of pocket and leads, which account for two-thirds of transvenous pacemaker complications [19].
Even though patients obtaining LP are usually burdened with more comorbidities, there is no difference in all-cause mortality at 2-year follow-up compared to the TV-PM comparator population. Moreover, after 2 years the benefits of LP in comparison to TV-PM were maintained — MVR was associated with a 38% lower rate of reinterventions and a 31% lower rate of chronic complications [20].
The feasibility of obtaining and maintaining high AV synchrony in patients with complete AV block and normal sinus function was proven with the AccelAv trial — a single-arm prospective study. After MAV implantation, at the first month, mean resting AV synchrony was 85.4% (95% CI: 81.1–88.9%; median 90.0%), and ambulatory AV synchrony, obtained via 24-hour Holter recording, was 74.5% (95% CI: 70.4–78.2%; median 75.0%). With A4 wave recognition optimization, the mean ambulatory AV synchrony increased to 82.6% (95% CI: 75.8–87.7%; median 85.3%). It was achieved mainly by fixing the A3 threshold approximately 1.0 m/s2 greater than the obtained A3 signal to help track the sinus rate between 80 and 110 bpm. The authors also proved with the EuroQol Five-Dimensions Three-Level questionnaire that quality of life improved after MAV implantation [13]. An even higher percentage of AV synchrony was achieved by optimizing atrial signal sensing by Briongos-Figuero et al. [12]. Deactivating the automatic A3 window end and manually shortening the A3 window end increased AV synchrony as determined by device counters significantly from 68.7 ± 14.7% to 87.3 ± 11.1% in the 6-month follow- up. More importantly, the 24-hour Holter monitoring in the follow-up demonstrated 87.3 ± 6.3% AV synchrony in patients undergoing daily activities. At the same time, most of the A4 thresholds were set automatically [12].
The rate of pericardial effusion following Micra implantation is similar to that observed with TV-PMs, i.e., 1.1%, with similar occurrence risk factors [21]. Increasing age, body mass index < 20, female sex, heart failure, prior myocardial infarction, chronic obstructive pulmonary disease, absence of prior cardiothoracic surgery, and hemodialysis raises the risk of post-procedure pericardial effusion. Several deployments of Micra are also associated with increased risk of pericardial effusion, especially in patients with elevated risk at baseline [15]. There has not been a reported case of CDRIE in LPs, even though LPs are often the first choice after the previous CDRIE. Its intracardiac fixation, small volume, and tendency for rapid encapsulation might be the reason for this [17].
Unfortunately, encapsulation may complicate extraction. Even though there is a report of successful retrieval of a 4-year-old MVR with commonly available tools [22], LPs lack a retrieval registry.
Between November 2020 and June 2021, 20 patients underwent transcatheter aortic valve implantation followed by MAV implantation. The safety and performance of the LP were proved with a 1-month follow-up. Atrial under sensing was listed as the main issue, which occurred in 2 patients, and the issue was resolved by reprogramming the MAVs [23].
Micra’s implantation safety, performance, and post-procedural complications were also evaluated in a retrospective study comparing patients with pre-procedure AV-node ablation (AVNA). The study proved the procedural and performance safety of concomitant AVNA and LP implantation with the precaution of a higher risk of major complications in patients undergoing AVNA [24].
The AVNA patients were older, more frequently female, and tended to have more co-morbid conditions than non-AVNA patients. With high implantation success (99.5%) and a mean pacing threshold at implant of 0.58 ± 0.35 V, stable values during follow-up — major complications within 30 days occurred more frequently in AVNA patients than non-AVNA patients (7.3% vs. 2.0%, p < 0.001). Intermittent loss of capture occurred in 3 AVNA patients (1.6%) within 30 days of implant, requiring system revision.
Another exciting field for LPs is cardioinhibitory vasovagal syncope (VVS) treatment. Micra’s battery performance has been evaluated in a retrospective study on patients suffering from frequent VVS episodes. Even though LPs provide a promising treatment option for patients with VVS with satisfactory battery performance (estimated during the study for 13.65 ± 2.97 years), the lack of a Micra retrieval registry and limited possibilities of LP reimplantation raises concerns in this strategy, especially in younger patients [25].
Currently, MAVs’ PAR is ongoing, with the end of the study expected in 2025. It is a prospective, multicenter, single-arm registry with the aim of assessing the safety and performance of the MAV on more than 750 patients. The primary endpoint is a rate of pacemaker syndrome requiring revision at 3 years and a secondary endpoint is to assess acute and chronic complications after implantation.
Because the MAV is implanted in the heart’s RV, atrial pacing is unavailable, unlike the TV-PM. However, Abbot’s Aveir DR Leadless Pacemaker — a dual-chamber leadless pacemaker — is currently the subject of a pivotal Aveir DR i2i study.
Another ongoing study — MODULAR ATP — assess the safety, performance, and effectiveness of the mCRM™ Modular CRM System (EMBLEM™ S-ICD System and EMPOWER™ Modular Pacing System), which would deliver ATP as well as high-voltage therapy.
MC1AVR1 with EOS battery state
Usually, the Micra is recommended for patients with a shorter lifespan due to the absence of long-term data on LP performance and limited data on retrievability and end-of-life strategy [1]. However, the number of patients who encounter an EOS state of LP will rise. Even though the Micra occupies around 1% of the RV volume [5], the area of possible implantation is much more limited, and the Micra is considered an unremovable device. If further pacing is needed after RRT, the implantation of another LP in the RV is undertaken. With available data, there have been cases showing the feasibility of the procedure with 3 LPs implanted simultaneously [26].
Micra AV in Poland
Currently, there is no national registry for the MAV or LPs, although experts recommend the introduction of such a registry [27]. In 2021 there were 47 MC1AVR1 implanted, mainly in 2 high-volume centers. A medical center willing to implant the MAV must write a document to the NFZ for reimbursement. Till the 10th implantation, each procedure needs to be performed with the supervision of a Medtronic technical expert.
Specific clinical situations [28]
Electric cardioversion
Electric cardioversion can be safely performed with several precautions. Firstly — use the lowest clinically efficient energy level, because an increased energy level raises the probability of damage to the device. Secondly, defibrillator paddles should be placed more than 15 cm from the implanted device. One should note that the procedure can temporarily or constantly raise the threshold level.
Radiotherapy
The cumulative exposition dose should not exceed 500 cGy. Asynchronous therapy should be considered during radiotherapy to reduce intervention detection.
MRI
Magnetic resonance imaging (MRI) scans can be safely performed under certain conditions. Scanning is allowed only after turning on an MRI SureScan option. This function cannot be allowed while RRT is on, which needs to be underlined. In this situation, VOO should be considered. Other requirements are stimulation amplitude equal to or lower than 4.5 V, no phrenic stimulation observed during MRI while the SureScan is on, MRI of the strength 1.5 T or 3.0 T, and maximal volume gradient ≤ 25 T/m (2500 Gs/cm).
It is worth noting that it is not recommended to perform MRI during the stabilization period, i.e., the first 6 weeks after implantation.
Cremation
There is no need to explant Micra post-mortem; cremation is possible due to no significant emission expected.
Conclusions
Leadless pacing has indisputable advantages. Among them is the reduced number of major complications in the peri- and postprocedural period. Even though nowadays LP technology is limited compared to TV-PM, technological advancements have allowed them to be overcome, and AV synchrony is the next step in LP evolution. One should also remember about the small implantation area, restricted catheter flexibility, programming issues with the AV synchrony, and economic issues. Moreover, to date, there is no registry of long-term outcomes and retrieval. Atrial pacing, conduction-system pacing, simple retrieval protocol, and high-voltage therapy are crucial issues that must be resolved in the future.
Footnotes
Conflict of interest: Bruno Hrymniak and Dariusz Jagielski received speaker honoraria fees from Medtronic. Przemysław Skoczyński, Bartosz Biel, and Waldemar Banasiak declare no conflict of interest.
References
- 1.Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42(35):3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 2.Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129(14):1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 3.Chinitz L, Ritter P, Khelae SK, et al. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: Results from the Micra atrioventricular feasibility studies. Heart Rhythm. 2018;15(9):1363–1371. doi: 10.1016/j.hrthm.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Steinwender C, Khelae SK, Garweg C, et al. Atrioventricular Synchronous Pacing Using a Leadless Ventricular Pacemaker: Results From the MARVEL 2 Study. JACC Clin Electrophysiol. 2020;6(1):94–106. doi: 10.1016/j.jacep.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Omdahl P, Eggen MD, Bonner MD, et al. Right ventricular anatomy can accommodate multiple Micra transcatheter pacemakers. Pacing Clin Electrophysiol. 2016;39(4):393–397. doi: 10.1111/pace.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleem-Talib S, van Driel VJ, Nikolic T, et al. The jugular approach for leadless pacing: A novel and safe alternative. Pacing Clin Electrophysiol. 2022;45(10):1248–1254. doi: 10.1111/pace.14587. [DOI] [PubMed] [Google Scholar]
- 7.Contractor T, Co ML, Cooper J. Is there a way to confirm true septal placement of leadless pacemakers? Proposal of the ”RAO space” sign. Pacing Clin Electrophysiol. 2021;44(1):176–177. doi: 10.1111/pace.14138. [DOI] [PubMed] [Google Scholar]
- 8.Kaczmarek K, Cygankiewicz I, Czarniak B, et al. Septal implantation of the Micra transcatheter pacing system guided by intraprocedural transesophageal echocardiography. Kardiol Pol. 2019;77(12):1190–1192. doi: 10.33963/KP.15043. [DOI] [PubMed] [Google Scholar]
- 9.Kiani S, Wallace K, Stromberg K, et al. A predictive model for the long-term electrical performance of a leadless transcatheter pacemaker. JACC Clin Electrophysiol. 2021;7(4):502–512. doi: 10.1016/j.jacep.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Hrymniak B, Biel B, Szwarc B, et al. Implantation of a leadless pacemaker in a patient with an atrioventricular block and COVID-19. Kardiol Pol. 2021;79(11):1294–1295. doi: 10.33963/KP.a2021.0111. [DOI] [PubMed] [Google Scholar]
- 11.Kempa M, Mitkowski P, Kowalski O, et al. Expert opinion of a Working Group on Leadless Pacing appointed by the National Consultant in Cardiology and the Board of the Heart Rhythm Section of the Polish Cardiac Society. Kardiol Pol. 2021;79(5):604–608. doi: 10.33963/KP.15982. [DOI] [PubMed] [Google Scholar]
- 12.Briongos-Figuero S, Estévez-Paniagua Á, Sánchez Hernández A, et al. Optimizing atrial sensing parameters in leadless pacemakers: Atrioventricular synchrony achievement in the real world. Heart Rhythm. 2022;19(12):2011–2018. doi: 10.1016/j.hrthm.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Chinitz LA, El-Chami MF, Sagi V, et al. Ambulatory atrioventricular synchronous pacing over time using a leadless ventricular pacemaker: Primary results from the AccelAV study. Heart Rhythm. 2023;20(1):46–54. doi: 10.1016/j.hrthm.2022.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Sagi V, Sheldon T, Cornell S. B-PO04-209 achieving optimal ambulatory performance with an AV synchronous leadless pacemaker during high sinus rates: a case report. Heart Rhythm. 2021;18(8):S364. doi: 10.1016/j.hrthm.2021.06.901. [DOI] [Google Scholar]
- 15.Burkman G, Saltsburg M, Buck M, et al. Leadless pacemaker--induced torsades de pointes. HeartRhythm Case Rep. 2021;7(2):79–82. doi: 10.1016/j.hrcr.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udo EO, Zuithoff NPA, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9(5):728–735. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds D, Duray GZ, Omar R, et al. Micra Transcatheter Pacing Study Group A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med. 2016;374(6):533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 18.Roberts PR, Clementy N, Al Samadi F, et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm. 2017;14(9):1375–1379. doi: 10.1016/j.hrthm.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Cantillon DJ, Exner DV, Badie N, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. 2017;3(11):1296–1305. doi: 10.1016/j.jacep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 20.El-Chami MF, Bockstedt L, Longacre C, et al. Leadless vs. transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur Heart J. 2022;43(12):1207–1215. doi: 10.1093/eurheartj/ehab767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccini JP, Cunnane R, Steffel J, et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: experience with the Micra transcatheter pacemaker. Europace. 2022;24(7):1119–1126. doi: 10.1093/europace/euab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minami K, Shtëmbari J, Petrů J, et al. Successful retrieval of a 4-year-old micra transcatheter pacemaker system in a patient with leadless biventricular pacing therapy. JACC Case Rep. 2020;2(14):2249–2252. doi: 10.1016/j.jaccas.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mechulan A, Prevot S, Peret A, et al. Micra AV leadless pacemaker implantation after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2022;45(11):1310–1315. doi: 10.1111/pace.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Chami MF, Shinn T, Bansal S, et al. Leadless pacemaker implant with concomitant atrioventricular node ablation: Experience with the Micra transcatheter pacemaker. J Cardiovasc Electrophysiol. 2021;32(3):832–841. doi: 10.1111/jce.14881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turagam MK, Gopinathannair R, Park PH, et al. Safety and efficacy of leadless pacemaker for cardioinhibitory vasovagal syncope. Heart Rhythm. 2020;17(9):1575–1581. doi: 10.1016/j.hrthm.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Omdahl P, Eggen MD, Bonner MD, et al. Right ventricular anatomy can accommodate multiple Micra transcatheter pacemakers. Pacing Clin Electrophysiol. 2016;39(4):393–397. doi: 10.1111/pace.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempa M, Mitkowski P, Kowalski O, et al. Expert opinion of a Working Group on Leadless Pacing appointed by the National Consultant in Cardiology and the Board of the Heart Rhythm Section of the Polish Cardiac Society. Kardiol Pol. 2021;79(5):604–608. doi: 10.33963/KP.15982. [DOI] [PubMed] [Google Scholar]
- 28.MicraTMAV MC1AVR1 MR. Conditional Dual Chamber Transcatheter Pacing System with SureScanTM Technology (VDD) Device Manual [Google Scholar]
- 29.Rate Smoothing for Micra AV. https://www.medtronicacademy.com/features/rate-smoothing-micra-av .
- 30.Tracking Check for Micra AV. https://www.medtronicacademy.com/features/tracking-check-micra-av .
- 31.El-Chami MF, Bhatia NK, Merchant FM. Atrio-ventricular synchronous pacing with a single chamber leadless pacemaker: Programming and trouble shooting for common clinical scenarios. J Cardiovasc Electrophysiol. 2021;32(2):533–539. doi: 10.1111/jce.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]