Abstract
Background
The prognostic impact of contrast-associated acute kidney injury (CA-AKI) in patients undergoing chronic total occlusion (CTO) percutaneous coronary intervention (PCI) remains underestimated.
Methods
We examined 2707 consecutive procedures performed in a referral CTO center between 2015 and 2019. CA-AKI was defined as an increase in serum creatinine ≥ 0.3 mg/dL or ≥ 50% within 48 h post-PCI. Primary endpoints were in-hospital major adverse cardiac and cerebrovascular events (MACCE, composite of all-cause death, myocardial infarction, target vessel revascularization, stroke) and at 1 year of follow-up.
Results
The overall incidence of CA-AKI was 11.5%. Technical success was comparable (87.2% vs. 90.5%, p = 0.056) whereas procedural success was lower in the CA-AKI group (84.3% vs. 89.7%, p = 0.004). Overall in-hospital MACCE was 1.3%, and it was similar in patients with and without CA-AKI (1.6% vs. 1.3%, p = 0.655); however, the rate of pericardial tamponade requiring pericardiocentesis was significantly higher in patients with CA-AKI (2.2% vs. 0.5%, p = 0.001). In multivariate analysis, CA-AKI was not independently associated with higher risk for in-hospital MACCE (adjusted odds ratio 1.34, 95% confidence intervals [CI] 0.45–3.19, p = 0.563). At a median follow-up time of 14 months (interquartile range [IQR], 11 to 35 months), 1-year MACCE was significantly higher in patients with vs. without CA-AKI (20.8% vs. 12.8%, p < 0.001), and CA-AKI increased the risk for 1-year MACCE (adjusted hazard ratio 1.46, 95% CI 1.07–1.95, p = 0.017) following CTO PCI.
Conclusions
Contrast-associated acute kidney injury in patients undergoing CTO PCI occurs in approximately one out of 10 patients. Our study highlights that patients developing CA-AKI are at increased risk for long-term MACCE.
Keywords: percutaneous coronary intervention, contrast-associated acute kidney injury, outcomes
Introduction
Percutaneous coronary intervention (PCI) for chronic total occlusion (CTO) remains the most technically challenging lesion subset in contemporary interventional practice, which requires a larger amount of contrast media compared to PCI for non-occlusive coronary artery disease [1]. Furthermore, pre-existing chronic kidney disease in patients undergoing CTO PCI has been associated with an increased risk for major adverse cardiac and cerebrovascular events (MACCE) both in-hospital and in the long term, studied in various patient cohorts [2–9]. Novel strategies and techniques have been described that overcome the growing need for appropriate management to prevent renal function impairment, such as applying meticulous pre-procedural hydration protocols, device-based contrast reduction, or use of intracoronary imaging [10]. An expert consensus document [11] rephrased the definition of PCI-related renal function impairment by replacing the previously used contrast-induced nephropathy (CIN, defined as an increase of serum creatinine (seCr) ≥ 0.5 mg/dL and/or an increase of the baseline seCr ≥ 25% [12]) to contrast-associated acute kidney injury (CA-AKI, defined as an increase of baseline seCr ≥ 0.3 mg/dL or an increase of baseline seCr ≥ 50% [13]) with the ultimate goal of identifying patients in danger appropriately and to standardize further research efforts. Currently, however, the incidence of CA-AKI is rarely available in studies providing systematical renal function assessment following CTO PCI [14, 15]. Because of all the above, discrepancy between previously designed studies and current practices may exist, possibly leading to an underestimation of the impact of CA-AKI on the outcomes of CTO revascularization. Accordingly, we sought to examine a large single-center CTO PCI registry to examine the prognostic impact of CA-AKI on in-hospital outcomes and at 1 year following the index PCI.
Methods
Study population and patient selection
We analyzed retrospectively the clinical, angiographic, and procedural characteristics of overall consecutive 2707 procedures with 2801 target vessels, performed in a referral CTO PCI center at the Division of Cardiology and Angiology II, University Heart Center Freiburg, Bad Krozingen between 2015 and 2019. Patients undergoing CTO PCI for acute coronary syndrome or stable procedures without baseline renal function assessment were excluded. Informed consent for PCI was obtained from each patient, and the study was approved by the Ethics Committee of the Albert-Ludwigs – Universität Freiburg, Germany (ID: EK 21-1100) and is in accordance with the ethical guidelines of the Declaration of Helsinki as revised in 1983.
Endpoints and definitions
The primary endpoint was procedural success as a composite of technical success without in-hospital major adverse cardiac and cerebrovascular events (MACCE) and pericardial tamponade requiring either pericardiocentesis or surgery. Technical success was defined as successful revascularization of chronic occlusive coronary lesions with achievement of < 30% residual diameter stenosis within the treated segment and restoration or maintenance of thrombolysis in myocardial infarction (TIMI) grade 3 antegrade flow. In-hospital MACCE included any of the following adverse events prior to hospital discharge: mortality, myocardial infarction (MI), recurrent symptoms requiring urgent target vessel revascularization (TVR), target lesion revascularization (TLR) with PCI or surgery, and stroke. Myocardial infarction was defined using by the 4th Universal Definition (type 4a) described by Thygesen [16].
One-year MACCE is defined as the composite of adverse events after hospital discharge such as mortality, MI, urgent TVR or TLR with PCI or surgery, and stroke. Data collection on short- and long-term follow-up of patients who underwent PCI were obtained during office visits, via telephone contacts with the patient or family members, and careful assessment of medical records, as necessary.
Secondary endpoints were components of the primary endpoint, coronary perforation managed conservatively, and Bleeding Academic Research Consortium (BARC) class 3 to 5 during in-hospital stay.
All PCI procedures were performed in patients with stable angina. Chronic total occlusion was defined as a coronary lesion with TIMI grade 0 flow of at least 3 months duration as described by the 2019 Consensus Document of the EuroCTO Club [17]. The J-CTO score was calculated as described by Morino et al. [18]. A CTO procedure was defined as “retrograde” if an attempt was made to cross the lesion through a collateral vessel or bypass graft supplying the target vessel distal to the lesion; if not, the procedure was classified as “antegrade”. Contrast injection was done exclusively via manual injection on both ipsilateral and contralateral sides. Pre-procedural hydration was obtained in patients with pre-existing renal insufficiency and were discharged 48 hours after the procedure as the earliest. Aside from the pre-procedural hydration protocol, no other pharmacological regimens were used. Metformin intake was suspended 2 days prior to the index PCI in diabetic patients on oral antidiabetics. For all CTO PCIs non-ionic low osmolar contrast agent Iomeron 350 (Bracco Imaging, Milan, Italy) was used.
Renal function assessment
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and the serum creatinine measurement obtained prior to and temporally closest to the index procedure [19]. Chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m2, and eGFR level was considered normal > 60 mL/min/1.73 m2. Classification of CKD stages was based upon the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) [20] guidelines (normal or high [I] ≥ 90 mL/min/1.73 m2; mildly decreased [II] 60–89 mL/min/1.73 m2; mildly to moderately decreased [IIIa] 45–59 mL/min/1.73 m2; moderately to severely decreased [IIIb] 30–44 mL/min/1.73 m2; severely decreased [IV] 15–29 mL/min/1.73 m2; kidney failure [V] < 15 mL/min/1.73 m2).
Contrast-associated acute kidney injury (CA-AKI) was defined as proposed by Kidney Disease: Improving Global Outcomes (KDIGO) [11, 13], and was classified as an increase of baseline seCr ≥ 0.3 mg/dL or an increase of baseline seCr ≥ 50% (Stage I); an increase of baseline seCr by 100–200% (Stage II), and an increase of > 200% or a follow-up seCr of ≥ 4 mg/dL with an acute increase of ≥ 0.5 mg/dL (Stage III).
All patients had at least one creatinine measurement performed within 24 hours prior to the index procedure, and underwent post-procedural renal function assessment 8, 16, 24, and 48 hours post-PCI (data availability was 92.8%, 76.9%, 98.3%, and 72.3% of all patients, respectively).
Statistical analysis
Categorical variables were expressed as percentages and were compared using Pearson’s χ2 test or Fisher’s exact test. Continuous variables were presented as mean ± standard deviation or median (interquartile range [IQR]) unless otherwise specified and were compared using the t-test and one-way analysis of variance (ANOVA) for normally distributed variables; the Wilcoxon rank-sum test and the Kruskal-Wallis test were applied for non-parametric continuous variables, as appropriate.
Multivariable logistic regression was used to identify the predictors for CA-AKI in CTO PCI after adjusting for confounding variables selected on the grounds of a) univariable association in the present study (p < 0.05) or b) previously established links with CA-AKI [14]; such variables included age, diabetes mellitus, reduced left ventricular ejection fraction (< 40%), CKD, baseline serum level of hemoglobin, periprocedural major perforation, and fluoroscopy time. Stepwise backward elimination was used forming the final model. We also used multivariable logistic regression to determine the association between CA-AKI, primary, and secondary endpoints. Crude and adjusted odd ratios (OR) were calculated after selection of the confounding variables based on an univariable association with the given endpoints at p < 0.05. MACCE-free survivals during 1 year of follow-up were calculated using the Kaplan–Meier method and compared between groups using the log-rank test. An adjusted Cox proportional hazard regression model was used to identify predictors of long-term MACCE and to calculate hazard ratios (HR). Confounding variables for the Cox proportional regression model were selected based on an univariable association with 1-year MACCE at p < 0.05. All statistical analyses were performed with JMP 13.0 (SAS Institute, Cary, North Carolina). A 2-sided p value of 0.05 was considered statistically significant.
Results
Clinical, angiographic, and technical characteristics
The overall incidence of CA-AKI was 11.5%. The baseline clinical features of the study cohort are shown in Table 1, categorized according to procedures with (n = 312) and without (n = 2395) CA-AKI. Patients developing kidney injury had significantly higher prevalence of diabetes mellitus, hypertension, congestive heart failure, and prior previous coronary artery bypass graft surgery (CABG) compared to those without CA-AKI. Baseline serum hemoglobin levels and eGFRs were significantly lower in patients with CA-AKI. Pre-existing CKD was more commonly observed in patients with post-PCI renal dysfunction (41.7% vs. 20.3%, p < 0.001).
Table 1.
Clinical characteristics of patients undergoing percutaneous coronary interventions for chronic total occlusion with and without contrast-associated acute kidney injury (CA-AKI).
| Variable | Overall (n = 2707) | No CA-AKI (n = 2395) | CA-AKI (n = 312) | P |
|---|---|---|---|---|
| Age [years]* | 65.6 ± 10.4 | 65.0 ± 10.3 | 70.1 ± 9.9 | < 0.001 |
| Men | 83.6% | 83.9% | 81.7% | 0.344 |
| BMI [kg/m2]* | 28.5 ± 4.6 | 28.5 ± 4.6 | 28.4 ± 4.8 | 0.720 |
| Diabetes mellitus | 29.1% | 28.0% | 37.6% | < 0.001 |
| Hypercholesterinemia | 90.7% | 90.8% | 89.6% | 0.492 |
| Hypertension | 86.2% | 85.1% | 94.1% | < 0.001 |
| Smoking (current) | 19.7% | 20.2% | 16.0% | 0.079 |
| LVEF‡: | < 0.001 | |||
| Normal | 65.4% | 66.9% | 54.0% | |
| Moderately reduced | 20.0% | 19.5% | 23.5% | |
| Reduced | 10.0% | 9.6% | 12.8% | |
| Low | 4.6% | 4.0% | 9.7% | |
| Family history of CAD | 44.6% | 44.9% | 41.8% | 0.344 |
| Heart failure | 4.3% | 3.8% | 8.2% | 0.001 |
| NYHA classification: | < 0.001 | |||
| I | 18.6% | 19.5% | 12.6% | |
| II | 50.2% | 50.8% | 45.4% | |
| III | 29.0% | 27.8% | 37.8% | |
| IV | 2.1% | 1.9% | 4.2% | |
| Prior MI | 38.5% | 37.9% | 43.6% | 0.066 |
| Prior CABG | 18.7% | 17.7% | 26.6% | < 0.001 |
| Baseline hemoglobin [g/dL]* | 14.2 ±1.5 | 14.2 ± 1.5 | 13.6 ± 1.7 | < 0.001 |
| Baseline creatinine [mg/dL]† | 1.00 (0.89, 1.17) | 1.00 (0.88, 1.14) | 1.10 (0.93, 1.37) | < 0.001 |
| Baseline eGFR [mL/min/1.73 m2]* | 74.5 ± 18.9 | 75.7 ± 18.1 | 65.5 ± 21.6 | < 0.001 |
| Chronic kidney disease | 22.7% | 20.3% | 41.7% | < 0.001 |
Mean ± standard deviation;
Median (interquartile range);
Left ventricular function groups are indicated as follows: normal (52–100%) moderately reduced (41–51%), reduced (30–40%), low (0–29) in males, and normal (54–100%), moderately reduced (41–53%), reduced (30–40%), low (0–29%) in females;
BMI — body mass index; CABG — coronary artery bypass graft; CAD — coronary artery disease; eGFR — estimated glomerular filtration rate; LVEF — left ventricular ejection fraction; MI — myocardial infarction; NYHA — New York Heart Association
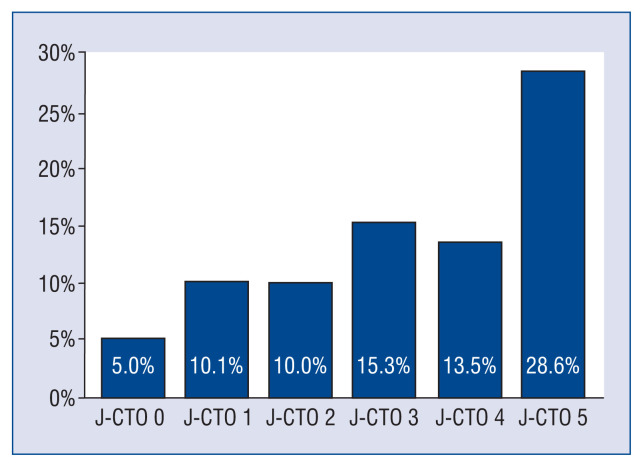
The angiographic and technical parameters of the study interventions are given in Table 2. The most common CTO PCI target lesions were the right coronary artery (49.1%) followed by the left anterior descending artery (24.9%) and circumflex artery (24.5%). Lesions were more complex in the CA-AKI group represented by higher J-CTO scores (2.9 ± 1.2 vs. 2.5 ± 1.2, p < 0.001) and the incidence of CA-AKI increased with lesion complexity (Fig. 1). Radial access was less frequently applied in patients post-PCI CA-AKI (23.2% vs. 28.9%, p = 0.034), whereas rotational atherectomy (11.3% vs. 5.3%, p < 0.001) and the retrograde approach (34.5% vs. 28.6%, p = 0.029) were more commonly used in these patients. The overall stent length was significantly longer in the CA-AKI cohort (64 [IQR 38–94 mm] vs. 56 [IQR 36–86 mm], p = 0.012).
Table 2.
Angiographic and technical characteristics of patients undergoing percutaneous coronary interventions (PCI) for chronic total occlusion with and without contrast-associated acute kidney injury (CA-AKI).
| Variable | Overall (n = 2801) | No CA-AKI (n = 2482) | CA-AKI (n = 319) | P |
|---|---|---|---|---|
| J-CTO Score* | 2.5 ± 1.2 | 2.5 ± 1.2 | 2.9 ± 1.2 | < 0.001 |
| Moderate/severe calcification | 67.0% | 65.8% | 76.0% | < 0.001 |
| Target vessel: | 0.875 | |||
| LM | 1.0% | 1.0% | 0.9% | |
| LAD | 25.2% | 24.9% | 27.3% | |
| CX | 24.2% | 24.5% | 22.3% | |
| RCA | 49.1% | 49.1% | 48.9% | |
| Graft | 0.6% | 0.6% | 0.6% | |
| Radial access used | 28.2% | 28.9% | 23.2% | 0.034 |
| Rotational atherectomy | 6.0% | 5.3% | 11.3% | < 0.001 |
| Crossing strategy used: | 0.029 | |||
| Antegrade-only | 70.8% | 71.4% | 65.5% | |
| Retrograde | 29.2% | 28.6% | 34.5% | |
| Number of DES used (n)† | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.209 |
| Overall stent length [mm]† | 56 (36, 86) | 56 (36, 86) | 64 (38, 94) | 0.012 |
| Multivessel PCI | 27.0% | 26.4% | 31.4% | 0.0601 |
| Technical success | 90.2% | 90.5% | 87.2% | 0.056 |
Mean ± standard deviation;
Median (interquartile range);
CTO — chronic total occlusion; CX — circumflex artery; DES — drug eluting stent; J-CTO — Japanese Chronic Total Occlusion; LAD — left anterior descending artery; LM — left main artery; RCA — right coronary artery
Figure 1.
The incidence of contrast-associated acute kidney injury increased with lesion complexity classified by J-CTO score (p = 0.009); CTO — chronic total occlusion; J — Japanese.
In-hospital outcomes
The overall technical and procedural success rates were 90.2% and 89.1%, respectively. The procedural characteristics are presented in Table 3. Median contrast volume, dose area product, and procedure and fluoroscopy time were 270 (200–470) mL, 10028 (5723–17624) cGy × cm2, and 86 (54–133) and 37.0 (22.0–65.0) minutes, respectively, and significantly differed across the groups (Table 3). The contrast-volume/eGFR ratio was significantly higher in patients with CA-AKI compared to patients with preserved post-PCI renal function (4.96 [IQR 3.03–7.83] vs. 3.63 [IQR 2.51–5.29], p < 0.001).
Table 3.
Procedural outcomes of patients undergoing percutaneous coronary interventions for chronic total occlusion with and without contrast-associated acute kidney injury (CA-AKI).
| Variable | Overall (n = 2707) | No CA-AKI (n = 2395) | CA-AKI (n = 312) | P |
|---|---|---|---|---|
| Procedural success | 89.1% | 89.7% | 84.3% | 0.004 |
| Length of hospital stay [days]* | 2 (2, 2) | 2 (2, 2) | 2 (2, 3) | < 0.001 |
| Procedural time [min]* | 86 (54, 133) | 84 (53, 129) | 100 (65, 170) | < 0.001 |
| Fluoroscopy time [min]* | 37.0 (22.0, 65.0) | 36.0 (21.0, 63.0) | 47.5 (28.3, 82.0) | < 0.001 |
| Contrast volume [mL]* | 270 (200, 400) | 270 (200, 400) | 300 (200, 418) | < 0.001 |
| Dose area product [cGy × cm2]* | 10028 (5723, 17264) | 9770 (5660, 16639) | 12993 (6509, 22366) | < 0.001 |
| CV/eGFR [min]* | 3.72 (2.55, 5.48) | 3.63 (2.51, 5.29) | 4.96 (3.03, 7.83) | < 0.001 |
| CV/eGFR ratio > 3.7 | 50.5% | 48.7% | 65.1% | < 0.001 |
| Major complications‡ | 2.1% | 1.8% | 3.9% | 0.019 |
| In-hospital MACCE: | 1.3% | 1.3% | 1.6% | 0.655 |
| Death | 0.3% | 0.3% | 0.6% | 0.233 |
| MI | 0.2% | 0.3% | 0.0% | — |
| Stroke | 0.2% | 0.1% | 1.0% | 0.013 |
| TVR | 1.0% | 1.1% | 0.6% | 0.465 |
| TLR | 1.0% | 1.0% | 0.6% | 0.761 |
| Pericardial tamponade§ | 0.7% | 0.5% | 2.2% | 0.001 |
| Vascular access complication | 2.1% | 1.8% | 4.4% | 0.031 |
| Bleeding# | 2.5% | 2.0% | 6.9% | < 0.001 |
| Perforation | 2.4% | 2.3% | 3.2% | 0.324 |
Median (interquartile range);
Composite of in-hospital major adverse cardiac and cerebrovascular events (MACCE) and pericardial tamponade requiring either pericardiocentesis or surgical evacuation;
Pericardial tamponade requiring pericardiocentesis or surgical evacuation;
Bleeding Academic Research Consortium (BARC) class 3 to 5;
CV — contrast volume; eGFR — estimated glomerular filtration rate; MI — myocardial infarction; TLR — target lesion revascularization; TVR — target vessel revascularization
On multivariable logistic regression the fluoroscopy time, level of serum hemoglobin at admission, age, left ventricular ejection fraction < 40%, hypertension, and pre-existing CKD were independent predictors for CA-AKI (Table 4).
Table 4.
Multivariate logistic regression to predict confounders for contrast-associated acute kidney injury in patients undergoing percutaneous coronary interventions for chronic total occlusion.
| Variable | Risk ratio | Lower 95% CI | Upper 95% CI | P |
|---|---|---|---|---|
| Fluoroscopy time [min]* | 1.03 | 1.01 | 1.05 | < 0.0001 |
| Hemoglobin at admission [g/dL]† | 0.85 | 0.78 | 0.93 | < 0.001 |
| Age [year]* | 1.18 | 1.11 | 1.26 | < 0.001 |
| LVEF < 40% | 1.63 | 1.15 | 2.30 | 0.007 |
| Diabetes mellitus | 1.28 | 0.97 | 1.69 | 0.087 |
| Hypertension | 2.21 | 1.29 | 4.09 | 0.003 |
| Contrast volume [mL]‡ | 1.02 | 0.99 | 1.05 | 0.233 |
| Rotational atherectomy | 1.33 | 0.82 | 2.07 | 0.234 |
| Chronic kidney disease | 1.39 | 1.01 | 1.89 | 0.041 |
| Pericardial tamponade | 2.72 | 0.96 | 7.17 | 0.060 |
Per 10-unit increment;
Per 1 unit increment;
Per 100-unit increment;
CI — confidence interval; LVEF — left ventricular ejection fraction
The overall in-hospital MACCE was 1.3% (Table 3), and was comparable between patients with and without post-procedural kidney injury (1.6% vs. 1.3%, p = 0.655). Pericardial tamponade (2.2% vs. 0.5%, p = 0.001), vascular access complications (4.4% vs. 1.8%, p = 0.031), and bleeding (6.9% vs. 2.0%, p < 0.001), were more commonly observed in patients with CA-AKI vs. without CA-AKI.
As shown in Table 5, CA-AKI was independently associated with an increased risk for in-hospital BARC class 3–5 bleeding (OR 3.47 [95% confidence interval [CI] 1.88–6.18], p < 0.001), whereas technical success (OR 1.36 [95% CI 0.90–2.02], p = 0.144), coronary perforation (OR 1.40 [95% CI 0.64–3.46], p = 0.422), and in-hospital major complications (OR 1.18 [95% CI 0.45–2.73], p = 0.721) were not significantly linked with CA-AKI on a multivariate level.
Table 5.
Multivariate analysis between secondary endpoints (technical failure, major complications, perforation, bleeding, one-year MACCE) and contrast-associated acute kidney injury.
| Variable | Non-adjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Technical failure | 1.38 | 0.95–1.97 | 0.079 | 1.36 | 0.90–2.02 | 0.144 |
| Perforation | 1.41 | 0.67–2.67 | 0.326 | 1.40 | 0.64–3.46 | 0.422 |
| In-hospital MACCE† | 1.24 | 0.42–2.95 | 0.664 | 1.34 | 0.45–3.19 | 0.563 |
| Major complications* | 2.14 | 1.07–3.96 | 0.033 | 1.18 | 0.45–2.73 | 0.721 |
| Bleeding | 3.64 | 2.08–6.16 | < 0.001 | 3.47 | 1.88–6.18 | < 0.001 |
| One-year MACCE†§ | 1.65 | 1.26–2.14 | < 0.001 | 1.46 | 1.07–1.95 | 0.017 |
Composite of in-hospital major adverse cardiac and cerebrovascular event (MACCE) and pericardial tamponade requiring either pericardiocentesis or surgical evacuation;
Composite of all-cause death, myocardial infarction, stroke, target vessel revascularization, and lesion revascularization;
Hazard ratio;
CI — confidence interval
Procedural outcomes at 1 year of follow-up
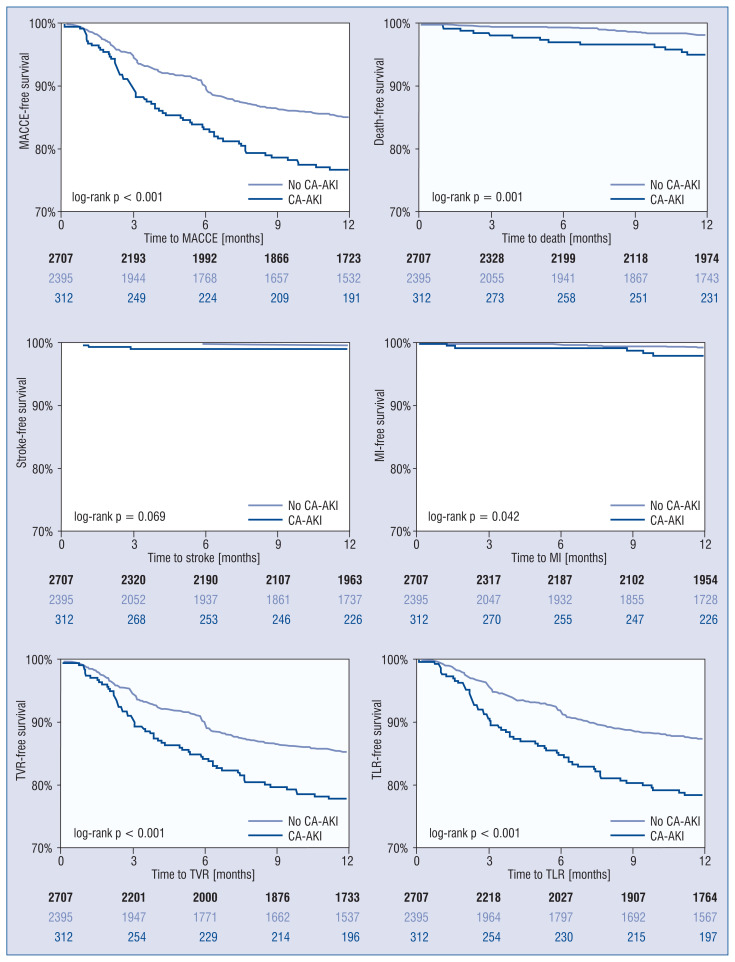
The median follow-up time was 14 months (IQR 11–35 months). The cumulative incidence of MACCE at 1 year was 13.7%, and it significantly differed in patients with vs. without post-procedural CA-AKI (20.8% vs. 12.8%, p < 0.001), mostly driven by TVR (19.6% vs. 10.9%, p < 0.001), all-cause mortality (4.5% vs. 1.7%, p = 0.001), and MI (1.9% vs. 0.9%, p = 0.038). The adjusted Cox regression model confirmed an independent association between CA-AKI and the risk of 1-year major adverse events (HR 1.46 [95% CI 1.07–1.95], p = 0.017).
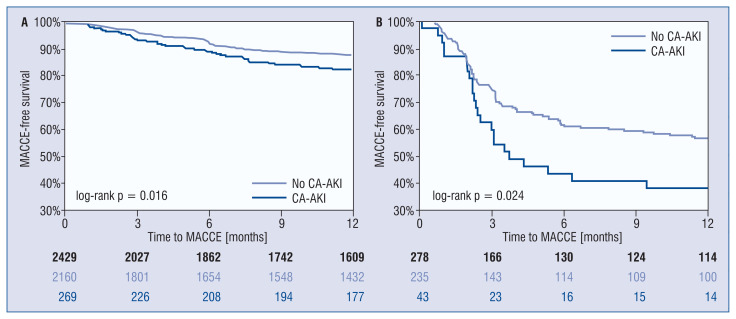
Patients with CA-AKI showed worse MACCE-free (log-rank p < 0.001) survival on Kaplan–Meier analysis (Fig. 2), mostly driven by higher rate of target vessel failure (log-rank p < 0.001), mortality (log-rank p = 0.001), and MI (log-rank p = 0.042). MACCE-free survivals significantly differed both in successful vs. failed CTO PCI, with less favorable outcomes in patients developing CA-AKI post-PCI (Fig. 3).
Figure 2.
Kaplan–Meier curves of 1-year major adverse cardiac and cerebrovascular events (MACCE) in patients undergoing chronic total occlusion percutaneous coronary interventions (PCI) with and without contrast-associated acute kidney injury (CA-AKI); MI — myocardial infarction; TLR — target lesion revascularization; TVR — target vessel revascularization
Figure 3.
Kaplan-Meier curves of one-year major adverse cardiac and cerebrovascular events (MACCE) in patients undergoing successful (A) versus failed (B) percutaneous coronary interventions (PCI) for chronic total occlusion (CTO) with and without contrast-associated acute kidney injury (CA-AKI).
Discussion
To the best of our knowledge, our study is the largest to date evaluating the impact of CA-AKI following CTO PCI. The major findings of our analysis are the following: (a) CA-AKI occurs frequently in patients undergoing CTO PCI (11.5%); (b) the incidence of in-hospital MACCE is similar in patients with and without post-PCI AKI; (c) at 1 year of follow-up, patients with CA-AKI are more likely to have major adverse events, (d) due to higher risk of mortality, and TVR.
Contrast-associated acute kidney injury has been identified as a major predictor for in-hospital mortality, MI, and bleeding in the all-comer PCI population regardless the severity of CA-AKI [21]. Nevertheless, the risk of acquiring sustained renal dysfunction seems dependent on the severity of CA-AKI [22]. These latter associations have raised awareness that CA-AKI may have an impact on adverse events following invasive angiography and angioplasty [11], presumably as a marker rather than a mediator. Adequately designed randomized controls trials, however, have yet to prove that prevention of CA-AKI could positively impact long-term survival, especially in complex patient subgroups such as with CTO PCI requiring larger amounts of contrast media or exposing patients to higher risk of hemodynamic instability. Our study implies that symptomatic pericardial tamponade, bleeding, and vascular access site complications were more frequent in patients developing CA-AKI. The latter observational finding is a potential explanation of how renal hypoxia (direct [cardiogenic shock, low cardiac output syndrome] or indirect [bleeding]) triggers CA-AKI [23]; however, our study was not designed to assess the underlying pathophysiology of CA-AKI. Nevertheless, clinically significant BARC 3–5 bleeding showed the strongest impact on triggering CA-AKI, in combination with a significant association between CA-AKI and decreasing pre-procedural serum level of hemoglobin (Table 4).
The novel standardized definition of AKI provides a stable ground for comparative studies estimating the true impact of CTO-PCI-related renal dysfunction [11]. Werner et al. [14] examined 1924 consecutive CTO PCIs in a single-center study, designed prospectively to have at least 48 hours post-PCI renal function assessment for the majority of the patients. The incidence of CA-AKI was 5.6%, and predictors for CA-AKI were identified, such as baseline hemoglobin, ejection fraction < 40%, age, fluoroscopy time, major coronary perforations, diabetes, and CKD. Patients with post-PCI AKI had higher in-hospital mortality, major coronary perforations, and pericardiocentesis. Our study represents a 2-fold higher incidence of CA-AKI compared to the latter study, which can be explained with the higher pre-existing CKD rates (22.7% vs. 17.7%). Despite the higher incidence, predictors for CA-AKI in our study fully matched Werner’s findings – extended fluoroscopy time, CKD, LVEF < 40%, and baseline hemoglobin level were similarly recognized in our cohort (Table 4). Additionally, our analysis shows that pericardial tamponade was significantly higher in the CA-AKI group (p = 0.001); however, on a multivariate level major coronary perforations requiring intervention were not significantly associated CA-AKI development (OR 2.78, p = 0.055). The potential role of significant pericardial effusion on post-PCI AKI, however, is inevitable because low cardiac output syndrome may cause prolonged medullar hypoxia, which leads to acute renal function impairment. In-hospital MACCE, major complications (composite of in-hospital MACCE and pericardial tamponade), and significant coronary perforations were not independently linked with newly developed AKI. The latter phenomenon is potentially explained by the immediate and appropriate treatment of coronary extravasation, which may decrease the probability of hemodynamic instability and the occurrence of any subsequent adverse event.
Azzalini et al. [15] reported 9.1% of CA-AKI from a more heterogenous but smaller cohort of 1092 patients undergoing CTO PCI cases, collected retrospectively from 5 dedicated international CTO centers. Although the primary endpoint of the study aimed to target the outcomes of CTO PCI in patients with baseline CKD (overall 19.6%), the impact of CA-AKI on long-term outcomes was also reported. There were significant differences reported at 1 year of follow-up (median follow-up time of 466 days [IQR 318–1124]) in terms of all-cause (9.3% vs. 3.7%, p = 0.001) and cardiac (8.1% vs. 1.7%, p < 0.001) mortality, but not with target-vessel MI and target lesion failure. Furthermore, on Kaplan–Meier curve analysis, only a higher trend was shown for CA-AKI related all-cause death at 1 year following the index PCI. Our study, however, showed slightly different outcomes because patients with CA-AKI suffered a higher rate of (a) all-cause death, (b) target vessel and lesion revascularization, and (c) MI on Kaplan–Meier analysis (Fig. 2) leading to inferiority in MACCE-free survival compared to patients with preserved renal function. These differences may be explained by the smaller patient cohort (n = 1092 vs. n = 2707), the patient heterogeneity (multi- vs. single-center design), lesion complexity (J-CTO score 1.7 ± 1.2 [Azzalini et al.] vs. 2.5 ± 1.2 [our cohort]), and other technical aspects of CTO PCI, such as the more frequent use of the retrograde approach (23% [Azzalini et al.] vs. 29% [our cohort]).
Novel advancements in CTO PCI, and the rapid evolution of wires, microcatheters, and other devices, have also reformed the preventive actions surrounding the PCI itself preserving the integrity of renal function post-PCI, with the ultimate goal of improving the long-term benefits of complex coronary interventions [10]. Strategies aiming to minimize the risk of CA-AKI are grouped as (a) pre-procedural preventive strategies (b) and procedural techniques. Preventive strategies have been thoroughly studied, but only appropriate hydration has been widely accepted, which can be adjusted to left ventricular end diastolic pressure [24], or central venous pressure [25]. In another aspect, various techniques have been described to reduce contrast media during complex PCIs including zero- or very low-contrast (< 10 mL) procedures [26, 27], extensive use of intravascular imaging, coronary road-mapping (by software or side branch wiring/metallic roadmap), or using a dedicated contrast-sparing system such as the DyeVert Plus [28].
Limitations of the study
Our study has limitations. First, it has a retrospective, observational design without core laboratory assessment of the study angiograms or independent clinical event adjudication. Second, procedural complications, such as perforation, are self-reported; however, the occurrence of MACCE has undergone a quality check performed by a dedicated independent committee in our institution. Third, study procedures were performed in a dedicated, high-volume CTO referral center. Fourth, there are no available data on the post-PCI dialysis rate. Fifth, contrast dye exposure peaks 3 to 5 days post-PCI; hence, the incidence of CA-AKI might be underestimated. Sixth, the follow-up of CTO PCIs ranged between 11 and 35 months because the majority of patients already had 3 years of follow-up, but some proportion of the study cohort only completed 1 year of follow-up after the index procedure.
Conclusions
Contrast-associated acute kidney injury in patients undergoing CTO PCI for stable coronary artery disease occurs in approximately one out of 10 patients, albeit its significance may still be underestimated in current practice. Our study implicates that patients developing CA-AKI have increased risk for long-term major adverse events, highlighting the importance of preserving renal function post-PCI, which could further improve patient-oriented outcomes.
Footnotes
Conflict of interest: None declared
Funding: Peter Tajti MD, PhD received support from the ÚNKP-21-4-II New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund, Hungary.
References
- 1.Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry) JACC Cardiovasc Interv. 2015;8(2):245–253. doi: 10.1016/j.jcin.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Lemos PA, Arampatzis CA, Hoye A, et al. Impact of baseline renal function on mortality after percutaneous coronary intervention with sirolimus-eluting stents or bare metal stents. Am J Cardiol. 2005;95(2):167–172. doi: 10.1016/j.amjcard.2004.08.089. [DOI] [PubMed] [Google Scholar]
- 3.Saltzman AJ, Stone GW, Claessen BE, et al. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4(9):1011–1019. doi: 10.1016/j.jcin.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Best PJM, Lennon R, Ting HH, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39(7):1113–1119. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 5.Latif F, Kleiman NS, Cohen DJ, et al. In-hospital and 1-year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug-eluting stents: a report from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) registry. JACC Cardiovasc Interv. 2009;2(1):37–45. doi: 10.1016/j.jcin.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Baber U, Giustino G, Sartori S, et al. Effect of chronic kidney disease in women undergoing percutaneous coronary intervention with drug-eluting stents: a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv. 2016;9(1):28–38. doi: 10.1016/j.jcin.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Gupta T, Paul N, Kolte D, et al. Association of chronic renal insufficiency with in-hospital outcomes after percutaneous coronary intervention. J Am Heart Assoc. 2015;4(6):e002069. doi: 10.1161/JAHA.115.002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stähli BE, Gebhard C, Gick M, et al. Outcomes after percutaneous coronary intervention for chronic total occlusion according to baseline renal function. Clin Res Cardiol. 2018;107(3):259–267. doi: 10.1007/s00392-017-1179-x. [DOI] [PubMed] [Google Scholar]
- 9.Tajti P, Karatasakis A, Danek BA, et al. In-Hospital outcomes of chronic total occlusion percutaneous coronary intervention in patients with chronic kidney disease. J Invasive Cardiol. 2018;30(11):E113–E121. [PubMed] [Google Scholar]
- 10.Almendarez M, Gurm HS, Mariani J, et al. Procedural strategies to reduce the Incidence of contrast-induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(19):1877–1888. doi: 10.1016/j.jcin.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 11.Mehran R, Dangas GD, Weisbord SD. Contrast-Associated acute kidney injury. N Engl J Med. 2019;380(22):2146–2155. doi: 10.1056/NEJMra1805256. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:S11–S15. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 13.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 14.Werner GS, Lorenz S, Yaginuma K, et al. A prospective study on the incidence of contrast-associated acute kidney injury after recanalization of chronic total coronary occlusions with contemporary interventional techniques. Int J Cardiol. 2021;337:38–43. doi: 10.1016/j.ijcard.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Azzalini L, Ojeda S, Demir OM, et al. Recanalization of chronic total occlusions in patients with vs without chronic kidney disease: the impact of contrast-induced acute kidney injury. Can J Cardiol. 2018;34(10):1275–1282. doi: 10.1016/j.cjca.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 17.Galassi AR, Werner GS, Boukhris M, et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention. 2019;15(2):198–208. doi: 10.4244/EIJ-D-18-00826. [DOI] [PubMed] [Google Scholar]
- 18.Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213–221. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 21.Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7(1):1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 23.McCullough PA, Choi JP, Feghali GA, et al. Contrast-Induced acute kidney injury. J Am Coll Cardiol. 2016;68(13):1465–1473. doi: 10.1016/j.jacc.2016.05.099. [DOI] [PubMed] [Google Scholar]
- 24.Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814–1823. doi: 10.1016/S0140-6736(14)60689-9. [DOI] [PubMed] [Google Scholar]
- 25.Qian G, Fu Z, Guo J, et al. Prevention of contrast-induced nephropathy by central venous pressure-guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc Interv. 2016;9(1):89–96. doi: 10.1016/j.jcin.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Ali ZA, Karimi Galougahi K, Nazif T, et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J. 2016;37(40):3090–3095. doi: 10.1093/eurheartj/ehw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzalini L, Laricchia A, Regazzoli D, et al. Ultra-low contrast percutaneous coronary intervention to minimize the risk for contrast-induced acute kidney injury in patients with severe chronic kidney disease. J Invasive Cardiol. 2019;31(6):176–182. [PubMed] [Google Scholar]
- 28.Tajti P, Xenogiannis I, Hall A, et al. Use of the DyeVert system in chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. 2019;31(9):253–259. [PubMed] [Google Scholar]