Abstract
Concerns regarding the rise of drug-resistant tuberculosis (DR-TB) infections and the need for new drugs with shorter treatment time and fewer side effects have been voiced by the World Health Organization (WHO). The WHO revised its guideline to treat multidrug resistant tuberculosis (MDR-TB) with a 6-month course of BPaLM (bedaquiline, pretomanid, linezolid and moxifloxacin) in 2022. However, a thorough study and meta-analysis of available evidence is required due to the limited confidence of the evidence confirming the effectiveness of pretomanid-containing regiments. The aim of this systematic review and meta-analysis was to evaluate the effectiveness of pretomanid-containing regiments in treating DR-TB patients. Data from six search engines were searched using inclusion criteria based on the PICOS framework. The keywords of pretomanid and tuberculosis or their alternatives were used. Using RoB2 Cochrane risk-of-bias tool for randomized clinical trials, data were independently extracted and the quality of the data was evaluated. Odds ratio (OR) and heterogeneity tests were used and the findings were presented in ORs and forest plots. A total of four studies with 237 patients was included in the final analysis and 204 (86%) patients had favorable outcome (cured) and 33 (14%) was not cured. Pretomanid-containing regimen (OR: 46.73; 95%CI: 11.76–185.7) and BPaLM/BPaL (OR: 41.67; 95%CI: 8.86–196.73) regimens were associated with favorable outcome (cured). This meta-analysis indicates that the pretomanid-containing regimen and the BPaLM/BPaL regimen could increase the chance to have favorable outcome in DR-TB patients.
Keywords: Tuberculosis, drug-resistant tuberculosis, pretomanid, BPaLM, efficacy
Introduction
Drug-resistant tuberculosis (DR-TB) is a form of tuberculosis (TB) resistant to several drugs used to treat drug-sensitive tuberculosis (DS-TB). DR-TB comes in a variety of forms, including rifampicin resistant tuberculosis (RR-TB), multi-drug resistant tuberculosis (MDR-TB), and others. The World Health Organization (WHO) reported that there has been an increase in DR-TB cases worldwide, from 437,000 in 2020 to 450,000 in 2021 with an increase of 2.9% [1]. This a global concern because dealing with DR-TB cases is challenging, because it required extended course of therapy (9 to 20 months), patients frequently break their regimen, and side effects impact the adherence to treatment [2,3]. Treatment options for patients with extremely drugresistant strains of tuberculosis are restricted. The conventional course of therapy is costly, timeconsuming, and hazardous, and the percentage of patients who do not improve is still very high. Additionally, patients in nations with high TB incidence rates were more likely to get standard therapy and to receive it with significant delays, which increased the risk of treatment failure [4-6]. Therefore, the discovery of new drugs against DR-TB cases, especially those with a relatively shorter duration of therapy with minimum side effects.
The WHO modified its MDR-TB treatment recommendations in 2022 to address these issues [7]. In 2022, WHO published two new recommendations for MDR/RR-TB treatment regimens: (i) a 6-month treatment using BPaLM regiment (bedaquiline, pretomanid, linezolid (600 mg), and moxifloxacin) as opposed to a 9-month or longer (18-month) plan; and (ii) MDR/RR-TB patients that sensitive to fluoroquinolones (FQ), the 9-month all-oral regimen is used rather than longer (18-month) regimens [7]. In short, in 2022 WHO recommendation, pretomanid was added, and the short-term treatment period (9 to 11 month) was reduced to 6 months only [7,8]. Pretomanid-based regimens may be more effective than bedaquiline-based regimens in preventing acquired resistance since the former's early bactericidal activity (EBA) began earlier [9]. The WHO recommendation, however, is "very low certainty of evidence" for this 6-month program [7]. Pretomanid suitability for use in routine regimens for DR-TB patients is thus called into doubt. There have been several clinical studies but no meta-analysis to aggregate the data to boost confidence, particularly in the effectiveness, safety, and side effect profiles. Therefore, the aim of this systematic review and meta-analysis was to assess the efficacy of pretomanid regimens in treating DR-TB patients. The use of this meta-analysis is anticipated to benefit WHO decision to include pretomanid-containing regimens as treatment options for DR-TB patients in its recommendations.
Methods
Study setting and registry
The protocol of the study containing details of the literature search strategy has been registered and published in PROSPERO with number register CRD42023422982. This systematic review and meta-analysis was written accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement for reporting systematic reviews and meta-analyses [10].
Data sources and searching strategy
The available studies published in PubMed, Cochrane, Science Direct, Google Scholar, Epistemonikos and clinicaltrial.gov were searched. Inclusion criteria as followed: (1) clinical trial, (2) last 10-years, (3) published in Indonesia or English, (4) the intervention used pretomanid-containing regiment, and (5) treatment duration of six months (24 weeks). The combination of keywords (“pretomanid” OR “PA-824”) AND (“tuberculosis” OR “TB” OR “drug-resistant tuberculosis” OR “DR-TB”) were used during the searches.
The studies were selected based on PICOS framework: DR-TB/MDR-TB/XDR-TB/RR-TB/Pre XDR-TB (Patient), pretomanid-containing regiments (Intervention), no comparison restricted (Comparison), efficacy such as favorable and unfavorable (Outcome), and clinical trial (Study). Pretomanid containing regimen is a combination of pretomanid and antibiotics used for DR-TB therapy; the combination can be a combination with other antibiotics recommended by WHO such as bedaquiline, linezolid, moxifloxacin, and others. AFB sputum positive, not being cured during treatment, and confirmed relapse during follow up period were considered unfavorable outcomes. Favorable outcomes were defined as patients who were cured throughout the course of treatment observation.
Data extraction and quality assessment
The data were independently extracted by four investigators (AMS, RD, PMA, and AH) as per the PRISMA process, and any disagreement were resolved through discussion with the other authors (IY, SS, and DA). First author, year of publication, sample size, country, population, regimen used, time on treatment, number of favorable and unfavorable patients were extracted from each article.
The quality of the included studies was checked using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) consisting five domains [11] and was assessed by all authors. All included articles were assessed and disagreements were resolved by discussion.
Statistical analysis
The number of patients with favorable outcome (cured) and unfavorable outcome (uncured) are the key outcome that were statistically examined. The odds ratios (ORs) were calculated and weighed in accordance with the capability of each research. The RevMan 5.4 program was used for all statistical testing. The Chi-squared value and the statistical inconsistency index (I2) were used to evaluate the statistical heterogeneity test. Chi-squared with p<0.05 or the I2 value is greater than 50% was identified there is heterogeneity between studies. The results of the meta-analysis were presented as OR values and forest plots. An overall effect result was considered statistically significant if the p-value is less than 0.05.
The primary outcome of this study is favorable (cured) and unfavorable outcome (uncured) of pretomanid containing regiment in DR-TB patients. Secondary outcome of this study is sub-group analysis between all pretomanid containing regiment compared to WHO pretomanid regiment for DR-TB. Both outcomes used Random Effect Model to generalize to the general population.
Results
Systematic review and characteristics of the included studies
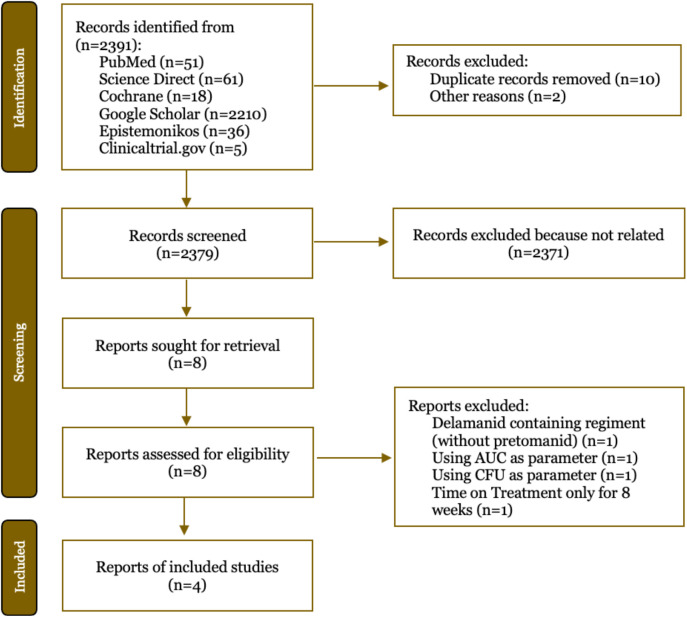
The searches yielded 2391 articles and 2379 of them were screened for the titles and abstracts screening, leaving four studies that satisfied the eligibility requirements (Figure 1). After carefully screening process eight studies were fully accessed. Additional four studies were excluded with the reasons: using delamanid without pretomanid, using colony forming unit (CFU) as parameter, using area under curve (AUC) as parameter and time on treatment only for eight weeks. Four studies met the eligibility requirements with a total of 237 patients received DR-TB treatment therapy with pretomanid-containing therapy [12-15]. The complete characteristics of the included articles are presented in Table 1. Different DR-TB types such as RR-TB, XDR-TB, and MDR-TB are reported in all clinical trials. Conradie et al. in 2020 [10] and 2022 [14] conducted two clinical studies using different samples (different NCT registry number). The study, which used populations from various countries, evaluated the treatment for a period of six months and some for 6.5 months. Those studies were still included because they are still within six months.
Figure 1. Search and selection of literature using the PRISMA flowchart. AUC: area under curve; CFU: colony forming unit.
Table 1. Baseline characteristics of included studies.
| Study, Year | NCT registry | Country | Population | Sample size | Regimen | Time on treatment weeks (month) |
|---|---|---|---|---|---|---|
| Nyang'wa | NCT02589782 | Belarus, | RR-TB | 145 | BPaLM | 24 (6) |
| et al., | South Africa, | |||||
| 2022 [12] | and | |||||
| Uzbekistan | ||||||
| Conradie | NCT03086486 | South Africa, | XDR-TB, Pre- | 45 | BPaL | 26 (6.5) |
| et al., | Georgia, | XDR-TB, or | ||||
| 2022 [14] | Moldova, | RR-TB | ||||
| Russia | ||||||
| Tweed et | NCT02342886 | South Africa, | RR-TB | 38 | PaMZ | 24 (6) |
| al., 2021 | Tanzania, | |||||
| [15] | Philippines, | |||||
| Kenya, | ||||||
| Malaysia, | ||||||
| Uganda, | ||||||
| Thailand, | ||||||
| and Ukraine | ||||||
| Conradie | NCT02333799 | Cape Town | XDR TB, MDR- | 109 | BPaL | 26 (6.5) |
| et al., | and Durban | TB (treatment | ||||
| 2020 [13] | intolerant/non- | |||||
| responsive) |
B: bedaquiline; Pa: pretomanid; L: linezolid; M: moxifloxacin; Z: pyrazinamide.
Effectiveness of pretomanid-containing regimen
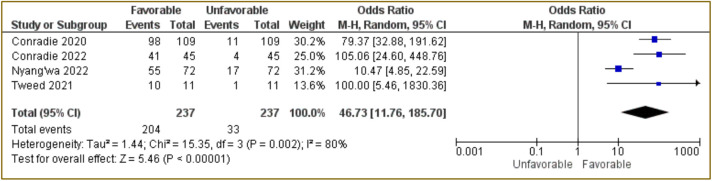
The meta-analysis was conducted and the results are presenting two forest plots: (a) all four included studies (Figure 2) and (b) studies that used BPaLM/BPaL only (Figure 3). The combined results of the four studies using Random Effect Model showed that pretomanid-containing regimen was associated with favorable outcome with OR: 46.73; 95%CI: 11.76–185.7, p<0.00001 (Figure 2). This indicates that the use of a pretomanid-containing regimen had 46.73 times the odds of favorable outcome compared to unfavorable outcome. In this analysis, the study conducted by Tweed et al., [15] has a large OR value (1830.36) with a long 95%CI value because small number of unfavorable patients were found. Nevertheless, this study is considered to be in accordance with the inclusion criteria that have been described, therefore this study was still included.
Figure 2. Forest plot of all included studies using pretomanid-containing regimen.
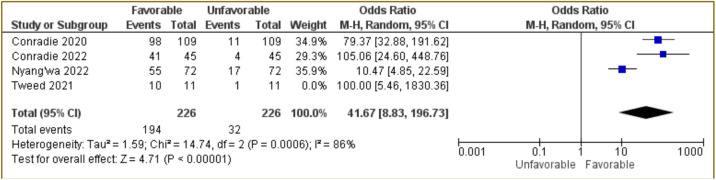
Figure 3. Forest plot of studies using BPaLM/BpaL regiment only.
The next meta-analysis test was carried out by excluding Tweed et al. [15]. The combined results of the three studies for BPaLM/BPaLM regimen only using Random Effect Model are presented in Figure 3. The results indicated that BPaLM/BPaLM regimen associated with favorable outcome with OR: 41.67; 95%CI: 8.83–196.73, p<0.00001 (Figure 3). This indicates BPaLM/BPaLM therapy had 41.67 times more favorable than unfavorable outcome.
Heterogeneity and risk of bias
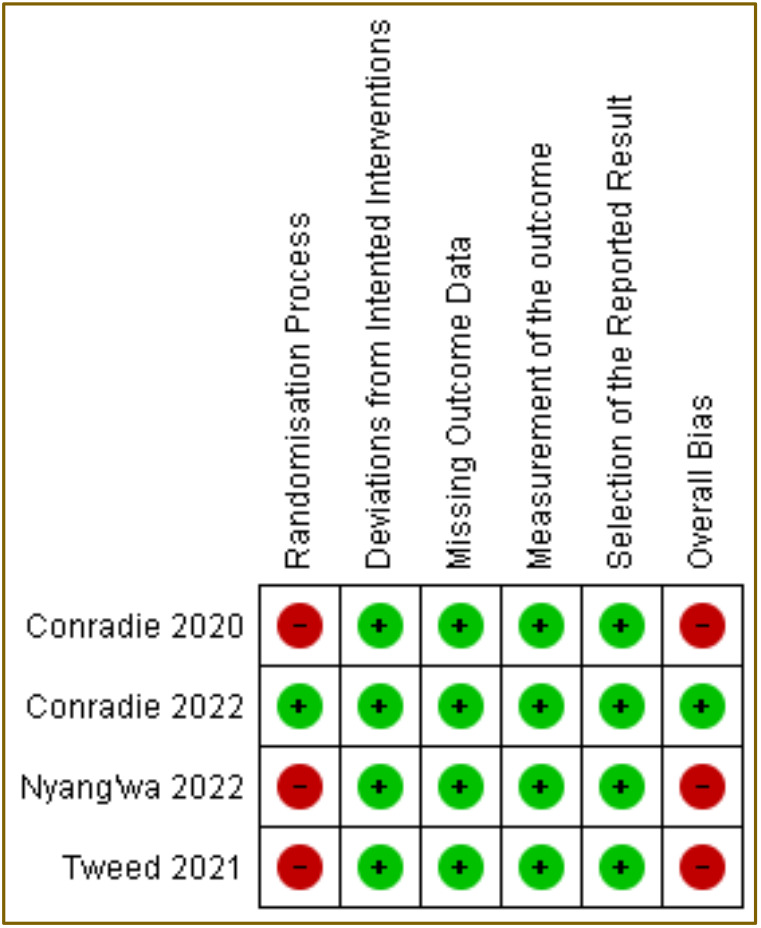
To assess the homogeneity or heterogeneity of the included studies, Chi-squared test and also I2 were used. The Chi-squared test and I2 for all four studies using pretomanid-containing regimen had a p=0.002 and 80%, respectively (Figure 2). The meta-analysis of BPaLM/BpaL regiment only had p=0.0006 of Chi-squared test with I2 of 86% (Figure 3). These data suggested that there were variations between studies for both analyses. The results of RoB2 also indicted a high risk of bias in the randomization process (domain 1) because some studies were open-label and some did not use randomization to specific groups (Figure 4).
Figure 4. Summary of risk-of-bias from included studies with RoB2 Cochrane appraisal tool for randomized control trials (RCTs). Green indicates low risk of bias and red indicates high risk of bias.
Discussion
Our data indicated that the pretomanid-containing regimen associated with favorable outcome 46.73 times (95%CI: 11.76–185.70) compared to non-pretomanid regimen and BPaLM/BPaL regiment for six months associated with 41.67 times chance to have outcome in DR-TB patients. The ORs were rather big since there were studies that had few patients with poor outcomes. We did not exclude those studies to avoid the possible bias that would be later produced if the studies were omitted. The 95%CI values also had a wide range, which might be taken as higher uncertainty or less accuracy in the data. That is proof of the data as it is, without any additions or subtraction. Comparable meta-analysis contrasting the results of the present meta-analysis is unavailable. Our results have a practical consequence since this meta-analysis has the potential to be additional power evidence of the use of pretomanid-containing regimen.
A previous systematic review evaluating the effectiveness and safety of the pretomanid-containing regimen in TB patients found that the regiment had 90% success rate [16]. This is consistent with this meta-analysis. This present meta-analysis is different from the previous systematic review [16] that included all TB cases, including those that were rifampicin-susceptible TB.
It is anticipated that pretomanid, a highly lipophilic substance with limited solubility, will diffuse across lipid membranes with ease [17]. When administered in the fed condition as opposed to the fasted state, the body parts are exposed to pretomanid at a higher level. When a high-fat, high-calorie meal was combined with a 200 mg dosage of pretomanid, the mean Cmax rose by 76% and mean AUC by 88% in comparison to the fasting state [4]. In humans, it has high bioavailability at dosages ranging from 50 to 1500 mg, and as dose increases, bioavailability rises proportionally [18]. When taken orally once daily, it was well tolerated and reached maximal plasma drug levels in 4–5 hours [19].
Pretomanid use in DR-TB patients was observed to have a wide range of adverse effects. However, Nyang'wa et al. [12] found that BPaLM had less adverse effects than the earlier DR-TB regimens. Anemia (2/72), neutropenia (3/72), pancreatitis (2/72), hepatic disorder (3/72), creatinine renal decrease (1/72) and acute kidney injury (1/72) were among the adverse effects of the BPaLM regimen [12]. Other adverse effects such peripheral neuropathy, optic neuritis, and myelosuppression were also recorded; however, they were all reported in very modest numbers [16]. Data suggested that moxifloxacin-containing regimens had more arthralgias and gastrointestinal issues, the pretomanid-containing regimens had more neurological and hepatic abnormalities [16,20]. Furthermore, a Phase 2b clinical trial using eight-week of MPaZ, found comparable incidence of adverse events in the intervention and control groups [9].
Despite being a relatively new treatment of choice for DR-TB, there have been several reports of resistance to pretomanid [21]. Animal and in-vitro models revealed pretomanid resistance; nevertheless, there is a dearth of information from clinical studies [22-24]. Pretomanid resistance is associated with mutations in ddn (Rv3547) and fgd1 (Rv0407) genes, which are linked to prodrug activation; or in fbiA (Rv3361), fbiB (Rv3261), fbiC (Rv1173), and fbiD (Rv2983) genes, which are linked to the coenzyme F420 biosynthesis pathway [21,25].
Some study limitations are need to be discussed. The lack of clinical studies that directly contrasting the pretomanid-containing regimens with earlier WHO-recommended DR-TB treatments are limited. Since, the duration of the pretomanid-containing regimen in included trials was just six months, the possibility of recurrence in DR-TB patients using this regimen was not discussed. We further advise that pretomanid-treated DR-TB patients to be monitored to detect the possibility of recurrence.
Conclusion
Our meta-analysis suggests that pretomanid-containing regimens or BPaL/BPaLM regimen associated with approximately 46- and 41-times chance to have favorable outcome, respectively for favorable outcome (cured) in DR-TB patients, compared to previous DR-TB regiments. The data of this meta-analysis strengthen the evidence supporting the use of pretomanid in patients with DR-TB.
Acknowledgments
The author would like to thank the continuous support from the Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, Universitas Riau, Indonesia and also the Department of Internal Medicine, Faculty of Medicine, Universitas Riau.
Ethics approval
Not required.
Competing interests
All the authors declare that there are no conflicts of interest.
Funding
This study received no external funding.
Underlying data
Derived data supporting the findings of this study are available in the article.
How to cite
Simanjuntak AM, Daenansya R, Aflandhanti PM, et al. Efficacy of pretomanid regiment for drugresistant tuberculosis: A systematic review and meta-analysis of clinical trials. Narra J 2023; 3 (3): e402 - http://doi.org/10.52225/narra.v3i3.402.
References
- 1.World Health Organization. Global tuberculosis report 2022. Geneva. 2022. [Google Scholar]
- 2.Kurz SG, Furin JJ, Bark CM. Drug-resistant tuberculosis: Challenges and progress. Infect Dis Clin North Am 2016;30(2):509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephanie F, Saragih M, Tambunan USF. Recent progress and challenges for drug-resistant tuberculosis treatment. Pharmaceutics 2021;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fekadu G, Tolossa T, Turi E, et al. Pretomanid development and its clinical roles in treating tuberculosis. J Glob Antimicrob Resist 2022;31:175–184. [DOI] [PubMed] [Google Scholar]
- 5.Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. New Engl J Med 2020;382(10):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grüber G. Introduction: Novel insights into TB research and drug discovery. Prog Biophys Mol Biol 2020;152:2–5. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: Treatment drug-resistant tuberculosis treatment 2022 update. Geneva. 2022. [PubMed] [Google Scholar]
- 8.Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J 2021;57(6):2003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diacon AH, Dawson R, von Groote-Bidlingmaier F, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 2012;380(9846):986–993. [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019:4898. [DOI] [PubMed] [Google Scholar]
- 12.Nyangwa BT, Berry C, Kazounis E, et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. New Engl J Med 2022;387(25):2331–2343. [DOI] [PubMed] [Google Scholar]
- 13.Conradie F, Diacon AH, Ngubane N, et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. New Engl J Med 2020;382(10):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med 2022;387(9):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tweed CD, Wills GH, Crook AM, et al. A partially randomised trial of pretomanid, moxifloxacin and pyrazinamide for pulmonary TB. Int J Tuberc Lung Dis 2021;25(4):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gils T, Lynen L, de Jong BC, et al. Pretomanid for tuberculosis: A systematic review. Clin Microbiol Infect 2022;28(1):31–42. [DOI] [PubMed] [Google Scholar]
- 17.Stancil SL, Mirzayev F, Abdel-Rahman SM. Profiling pretomanid as a therapeutic option for tb infection: Evidence to date. Drug Des Devel Ther 2021;15:2815–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter H, Ginsberg A, Egizi E, et al. Effect of a high-calorie, high-fat meal on the bioavailability and pharmacokinetics of PA-824 in healthy adult subjects. Antimicrob Agents Chemother 2013;57(11):5516–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manjunatha U, Boshoff HIM, Barry CE. The mechanism of action of PA-824. Commun Integr Biol 2009;2(3):215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tweed CD, Dawson R, Burger DA, et al. Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: A multicentre, open-label, partially randomised, phase 2b trial. Lancet Respir Med 2019;7(12):1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TVA, Nguyen QH, Nguyen TNT, et al. Pretomanid resistance: An update on emergence, mechanisms and relevance for clinical practice. Int J Antimicrob Agents 2023;62(4):106953. [DOI] [PubMed] [Google Scholar]
- 22.Rifat D, Li SY, Ioerger T, et al. Mutations in fbiD (Rv2983) as a novel determinant of resistance to pretomanid and delamanid in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2020;65(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen S, Jing W, Zhang T, et al. Comparison of in vitro activity of the nitroimidazoles delamanid and pretomanid against multidrug-resistant and extensively drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis 2019;38(7):1293–1296. [DOI] [PubMed] [Google Scholar]
- 24.Haver HL, Chua A, Ghode P, et al. Mutations in genes for the F 420 biosynthetic pathway and a nitroreductase enzyme are the primary resistance determinants in spontaneous in vitro-selected PA-824-resistant mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2015;59(9):5316–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi KP, Bair TB, Bae YM, et al. Use of transposon Tn 5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F 420 biosynthesis by Mycobacterium bovis BCG. J Bacteriol 2001;183(24):7058–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available in the article.