Abstract
The urgency of implementing the One Health approach to overcome zoonotic diseases cannot be overstated. By recognizing the interconnectedness of human health, animal health, and the environment, we can effectively prevent and respond to emerging infectious disease threats. This review article provides information on the importance of generating research on zoonotic diseases, especially in Indonesia, where research is still relatively scarce. The Indonesian government has taken steps to implement the One Health by establishing the One Health Coordinating Unit and the National Zoonosis Committee; however, implementation has not been optimal. The urgency and challenges are focused on critical implementation aspects in the community. The urgency of implementing One Health includes that Indonesia has experienced several outbreaks of zoonotic diseases; high environmental degradation; and the antimicrobial resistance issue in Indonesia has increased. The challenges faced in implementing One Health are overcoming fragmentation due to incohesive communication between important sectors, securing funding and resource investment, aligning policies to eliminate regulation barriers, capacity building to increase awareness and professionals, and addressing critical socioeconomic factors. By prioritizing implementing the One Health approach and addressing existing challenges, Indonesia can build a more resilient and integrated system to protect the well-being of all species, protect ecosystems, and prevent the devastating effects of zoonotic diseases on global health. In this review, we present the urgency of One Health implementation and its challenges comprehensively.
Keywords: One Health, zoonotic diseases, communicable disease, Indonesia, tropical disease
Introduction
Zoonotic diseases are infections caused by pathogens that can be transmitted from animals to humans [1-3]. This disease has long been part of human history, as have rabies, influenza, and Japanese encephalitis originating in animals. However, the emergence of new zoonotic diseases in recent years, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Ebola, Zika, dengue, schistosomiasis, and mpox, has highlighted the urgent need for a comprehensive approach to disease prevention and control [4-8]. One critical aspect of this linkage is the emergence and spread of zoonotic diseases, which significantly threaten human health. Understanding health principles and the complexity of zoonotic diseases is critical to maintaining global health and preventing future pandemics [9-11].
The presence and development of zoonotic diseases in Indonesia have had an alarming impact on public health and pose a significant national health burden over time [1]. This is evident from massive prevention and control efforts, accompanied by the need for considerable resources and funding every year. In addition, the high interaction between humans and animals facilitates the transmission of zoonotic diseases, which have been studied through the sylvatic cycle (pathogenic invasion into non-human primates) [7]. Zoonotic diseases that are a priority for handling in Indonesia today include avian influenza, including bird flu (H5N1) and swine flu (H1N1), which have been infecting poultry since 2003 and are highly pathogenic to poultry, causing losses to the poultry sector in Indonesia [12]. In addition, SARS-CoV-2 and MERS-CoV infections, which have resulted in a worldwide pandemic with reported mortality reaching 161,918 in Indonesia, have had devastating impacts and paralysis in various sectors [8,13]. Furthermore, zoonotic tuberculosis disease is included as a priority program that generally affects cattle (Mycobacterium bovis) and zoonosis in humans and domestic animals [14,15]. These three zoonotic diseases tend to affect the respiratory system [16].
Another priority zoonotic disease is rabies, which is endemic in 26 of 34 provinces in Indonesia and causes 99% mortality in animals and humans [17]. In recent months, rabies cases have experienced a spike in Indonesia, such as Bali, Kalimantan, and Sulawesi, resulting in death within just <48 hours after being bitten [18,19]. In addition to infectious diseases caused by viruses, bacterial zoonoses have also had a negative impact on farmers, one of which is anthrax, which in recent months has experienced a resurgence in various provinces of Indonesia [1]. The last zoonotic disease that is a priority is leptospirosis, which is transmitted through rodents. High climate change, poor sanitation, and flooding in several regions of Indonesia have great potential for causing this disease [20,21]. These six priority diseases are included in the Regulation of the Coordinating Minister for Human Development and Culture of the Republic of Indonesia Number 7 of 2022, concerning Guidelines for the Prevention and Control of New Zoonoses and Infectious Diseases [22].
In addition to these six priority diseases, other examples of zoonotic diseases have become a concern in all related sectors, namely vector-borne diseases, including dengue hemorrhagic fever, chikungunya, yellow fever, Japanese encephalitis, malaria, and Zika, which are reported to occur and fluctuate throughout the year [7,23-27]. Zoonotic diseases that pose a health burden in Indonesia include salmonellosis, brucellosis, trichinosis, trematodoses, toxoplasmosis, Ebola, psittacosis, echinococcosis, and bovine spongiform encephalopathy, which can have a negative impact nationally if not addressed [28-30].
The One Health approach recognizes that human, animal, and environmental health are interconnected [31]. It recognizes that the health of each sector is affected by the other and calls for collaborative efforts among human health professionals, veterinarians, ecologists, environmental scientists, and policymakers to address complex health challenges effectively. By integrating knowledge and expertise from different disciplines, One Health seeks to prevent and control zoonotic diseases at their source, mitigating their impact on public health. This review seeks to explain the urgency of implementation and challenges of implementing the concept of One Health in efforts to prevent and control zoonotic diseases in Indonesia.
Urgency of applying the One Health concept to zoonotic diseases in Indonesia
Zoonotic diseases are infectious diseases that can be transmitted between animals and humans through direct contact with infected animals, ingestion of contaminated food or water, or exposure to vectors such as fleas, mosquitoes, snails, and others [32,33]. Zoonotic diseases that until now have not been appropriately handled in Indonesia include dengue virus infections, malaria, rabies, chikungunya, leptospirosis, bubonic plague, brucellosis, and others [34-36]. The Indonesian government has taken steps to implement the One Health approach. The country has established the One Health Coordinating Unit and the National Zoonosis Committee, demonstrating the government’s commitment to comprehensively address zoonotic diseases [37,38]. However, field implementation has not been optimal. This can be seen from the results of the Joint External Evaluation (JEE) assessment, which is used as a standard assessment of a country in the implementation of One Health concept, including detection, prevention, and response. The result is that Indonesia’s capacity reaches only 63% of the ideal capacity value of 100% [39]. This indicates that detection, prevention and response to outbreaks or extraordinary events have not been going well.
For example, the high number of rabies cases in various endemic provinces, one of which is Bali, has not even received services. If there is a case report of handling in the field that does not directly carry out surveillance of zoonotic diseases but awaits confirmation from an agency that has the authority to do so, in addition to the prevention side, the community has not evenly obtained information related to control and prevention. As a result, if there is a bite in humans, early prevention is not carried out, which results in handling delays that cause mortality and morbidity. The last thing is the lack of coordination between agencies related to the procurement of anti-rabies vaccines (VAR) or pre-exposure prophylaxis (PrEP) vaccines for animals, resulting in high fatalities caused by rabies virus infection and pets with a high potential for transmitting or contracting the rabies virus from infected animals, as a result of which cases of Rabies Infectious Animal Bites (GHPR) increase throughout the year [40-42].
The contrast of policies with the results of implementation in the field highlights the importance of the Indonesian government in implementing policies that lead to efficient program improvements. Limited monitoring and reporting systems, low funding, healthcare infrastructure, and trained resources are the main areas of improvement. The lack of coordination between agencies, including control of wildlife trafficking practices and consumption of wildlife meat that contributes to transmitting zoonotic diseases, is of particular concern and must be corrected starting at the household level. Weak policies and terms of reference have resulted in failures in the prevention and control of zoonotic diseases in Indonesia [39,43-45].
This is evident from the assessment of the Zoonotic Diseases Action Package (ZDAP), which includes surveillance systems, veterinary or animal health workforce, and mechanisms for responding to zoonoses and potential zoonoses obtained by Indonesia, only 53% of the ideal capacity value of 100% [39]. This can also be seen from the various studies we analyzed using bibliometric analysis. We found that the research focus related to One Health, especially in Indonesia, is minimal (32 articles) from 2016 to 2022 and has shifted, primarily focusing on the latest issues, so that the topic of One Health is only associated with and not employing One Health as the research foundation [46,47].
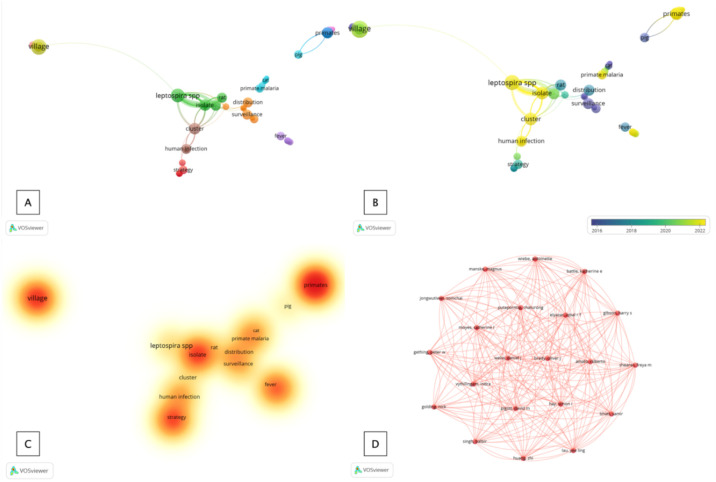
Related terms that have changed annually and the focus on topics that still need further development and research are presented in Figure 1. The concept of One Health is not directly a central topic [48]. However, it is only associated with it, so future research is essential to understand disease dynamics through a One Health approach [49,50]. Our findings consisted of 11 clusters containing “One Health” in 32 research articles obtained in PubMed and Scopus (Figure 1A). This indicates that the One Health topic still needs to be studied more deeply. Co-occurrences that are immensely discussed are “leptospirosis,” “malaria primates,” “primates,” “human infections,” “anthrax,” “rat,” and “village” (Figures 1A and Figure 1B). These zoonotic diseases are emerging, but a comprehensive approach has yet to be widely taken. Furthermore, density visualization analysis showed that current research focuses on diseases originating in rural areas, especially “primates,” by identifying isolates and prevention and control strategies (Figure 1C). Finally, based on the distribution and network of the authors, one network was obtained, which indicates that only this group of authors contributed to the development of the concept of One Health in zoonotic diseases.
Figure 1. Mapping and visualization of the term “co-occurrence” (One Health) in its application to zoonotic diseases. Description: (A) clusters and terms used as keywords; (B) overlay visualizations related to developments and changes in research topics from 2016–2022; (C) density visualizations related to the depth of the One Health concept in each research topic; and (D) author networks on the topic of one-health and zoonotic diseases.
The concept of One Health is very relevant to zoonotic diseases in Indonesia due to the country’s unique ecological and socioeconomic characteristics, with a large population and rich biodiversity, making it vulnerable to zoonotic disease outbreaks. By adopting the One Health approach, Indonesia can collaborate with interdisciplinary approaches to enhance its preparedness, surveillance, and response to zoonotic diseases [51,52]. Thus, improving the health and well-being of people and animals, ensuring a safer and healthier future for the country.
There are several reasons why the government, society, and related sectors must implement the concept of One Health throughout Indonesia. First, Indonesia has a high burden of zoonotic diseases. Along the way, Indonesia has experienced several outbreaks of zoonotic diseases, such as bird flu, rabies, and especially the coronavirus disease 2019 (COVID-19) pandemic. These infectious diseases originate in animals and can spread rapidly to humans. By addressing the health of animals and their ecosystems, we can effectively detect, prevent, and respond to these emerging threats [27,53].
Second, there has been high environmental degradation, mediated by rapid urbanization, deforestation, and wildlife trafficking, contributing to habitat loss, increased human-wildlife interaction, and an overflow of zoonotic diseases. Consumption of meat contaminated with viruses or pathogens can result in new sources of outbreaks that endanger human health. Zoonotic disease outbreaks have severe economic consequences impacting various sectors, including agriculture, tourism, and high public health spending. One Health approach can help mitigate these economic losses by preventing and controlling zoonotic diseases at their source and considering efficiency and effectiveness in their implementation [54,55].
Third, the antimicrobial resistance issue in Indonesia has increased [53,56-58], so efforts are needed to reduce it. Zoonotic diseases contribute significantly to the emergence and spread of antimicrobial resistance [53]. Overusing and abusing antimicrobials in human medicine and animal husbandry contribute to the development of drug-resistant pathogens. The One Health approach promotes responsible antimicrobial use, surveillance, and coordinated action to combat antimicrobial resistance effectively. One Health recognizes these interconnected drivers and emphasizes the importance of a holistic and collaborative approach to disease surveillance, prevention, and response [59,60]. By strengthening surveillance systems at the human-animal-environment interface, potential outbreaks can be detected and contained before escalating into a full-blown pandemic.
Challenges of implementing the One Health concept in Indonesia
Despite the high urgency of implementing One Health in Indonesia, several challenges contribute to the slowdown in implementing this approach. First, the approach and communication could be more cohesive since the various sectors involved are fragmented, including human health, veterinary medicine, and environmental conservation. These sectors have traditionally operated independently, resulting in a need for coordination and information sharing [44,60,61]. This has been conveyed and negotiated in meetings and discussions on the global implementation of the concept of One Health in each country. As outlined in the policy brief, Indonesia’s current condition has not been able to realize the integration of one datum (interconnectivity) between one ministry and another and ineffective collaboration between institutions, so that the absorption of information related to cases of emerging and emerging diseases, mitigation, and control efforts that tend to be different, and the urgency and priorities of each sector are different direct obstacles to the implementation of One Health in Indonesia [61]. Moreover, effective communication and collaboration among these sectors are critical for successful implementation. Second, the country has limited funding and resources; implementing the One Health approach requires adequate financial investment and resources. However, its initiation still needs improvements, hampering its implementation and sustainability [62,63]. This has been conveyed in the T20 policy brief related to implementing and financing One Health, in which it is stated that the hampering of implementing One Health is caused by inadequate financing capabilities, with a score of 66 out of 100 [61]. In addition, a previous study explained that the estimated financing for pandemic primary prevention is about $20 billion, less than one-tenth of the global pandemic financing [64]. These data indicate a lack of interest in countries investing in infectious disease prevention programs (emerging and re-emerging) with a one-health approach, in addition to the fact that the funding allocated annually is inadequate [61]. In addition to the prevention side, adequate health resources also require sufficient funding, so governments, international organizations, and stakeholders should prioritize funding and resource allocation for research, surveillance, capacity building, and infrastructure development.
Third, policy and regulatory barriers exist; current policies and regulations often must align with the One Health approach. Harmonizing regulations and developing cross-sector policies encouraging collaboration and data sharing are necessary. Overcoming bureaucratic hurdles and encouraging interdisciplinary cooperation can be challenging but critical for effective implementation.
Fourth, capacity building and education need improvements. Increasing the capacity of professional resources in human and animal health is critical to successful implementation. This requires training programs that foster interdisciplinary collaboration and knowledge exchange. In addition, public awareness and education campaigns are needed to promote understanding and support for One Health.
Fifth, socioeconomic factors that are hard to change. Many communities depend on livestock for their livelihoods, and their economic concerns may conflict with disease control measures such as culling or restrictions on movement. Balancing economic considerations with public health priorities is a complex challenge that requires careful navigation [2,48].
Key to the successful implementation of the One Health concept in Indonesia
Based on the urgency and challenges identified, several recommendations have become critical aspects of the One Health concept in relation to zoonotic diseases, especially in Indonesia, including the need for multidisciplinary collaboration. The One Health approach brings together experts from various fields to work with synergy. These include human and veterinary medicine professionals, epidemiology, ecology, environmental science, and others [2,37].
Furthermore, disease surveillance and early warning systems are needed, which is possible by implementing One Health to establish a comprehensive disease surveillance system to monitor animal and human populations [10,36]. By detecting and reporting disease outbreaks early, interventions can be implemented immediately to prevent further spread. Regarding research and data sharing, the One Health approach encourages zoonotic disease research, including their origin, transmission dynamics, and prevention strategies. Sharing data and findings across disciplines and institutions enhances our understanding of the disease and informs evidence-based interventions [50].
Other vital considerations to successfully implement the One Health concept include preventive and control measures. The One Health concept emphasizes preventive actions to reduce the risk of zoonotic diseases [65]. These include promoting animal vaccination programs, improving hygiene practices, improving biosecurity measures in livestock production, and controlling disease vectors. In addition, considering the environment is vital because the health of ecosystems and the environment is significant for preventing zoonotic diseases. One Health recognizes the impact of environmental factors, such as deforestation, climate change, and biodiversity loss, on disease emergence. Protecting natural habitats and promoting sustainable practices can help reduce these risks [38,47].
Finally, public awareness and education contribute to applying this concept by advocating for public awareness and education campaigns to promote a better understanding of zoonotic diseases and their prevention [33,46]. By increasing knowledge and changing behavior, individuals can adopt practices that reduce the transmission of zoonotic diseases.
Conclusion
One Health offers a comprehensive framework to address the complex challenges posed by zoonotic diseases. This approach fosters collaboration, information sharing, and coordinated action across disciplines by recognizing the interconnectedness of human, animal, and environmental health. Implementing the One Health principle is imperative as we continue to address the impact of the COVID-19 pandemic and prepare for future health threats. Through a holistic and integrated approach, we can protect the well-being of all species, protect ecosystems, and prevent the devastating effects of zoonotic diseases on global health. In the future, it is expected that many studies will generate the concept of One Health to eradicate zoonotic diseases and improve the quality of the concept so that it can be used sustainably.
Acknowledgments
The author would like to thank the Prodia Education and Research Institute (PERI) for funding the writer to study at the Faculty of Medicine, Universitas Airlangga.
Ethics approval
Ethics approval was waived for this study because no patient data were reported.
Competing interests
The author states that there is no conflict of interest in this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Underlying data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
How to cite
Adnyana IMDM, Utomo B, Eljatin DS, Sudaryati NLG. One Health approach and zoonotic diseases in Indonesia: Urgency of implementation and challenges. Narra J 2023; 3 (3): e257 - http://doi.org/10.52225/narra.v3i3.257.
References
- 1.Negara KSP, Sari Yulia, Wijayanti Lilik, et al. One health strategy in prevention and control of parasitic zoonosis globally and Indonesia - from theory to practice: A mini-review. Bali Med J 2022;11(3):1537–1542. [Google Scholar]
- 2.Erkyihun GA, Alemayehu MB. One health approach for the control of zoonotic diseases. Zoonoses 2022;2(1):0037. [Google Scholar]
- 3.Agustiawan, Adnyana IMDM, Ashriady, et al. Epidemiologi penyakit menular. 1st ed. Asir Annisa Ishmat, editor. Bandung: CV Media Sains Indonesia; 2022;236. [Google Scholar]
- 4.Mulakoli F, Gachara G, Ndombi E, Khamadi S.. Dengue virus surveillance and blood safety: A One Health perspective. Dengue fever in a one health Perspective. IntechOpen 2022. [Google Scholar]
- 5.Kurniawan W, Suwandono A, Widjanarko B, et al. The effectiveness of the One health SMART approach on dengue vector control in Majalengka, Indonesia. J Health Res 2020;35(1):63–75. [Google Scholar]
- 6.Martinez S, Sullivan A, Hagan E, et al. Living safely with bats: Lessons in developing and sharing a global One Health educational resource. Glob Health Sci Pract 2022;10(6):e2200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adnyana IMDM. Monkeypox and genital skin diseases : New challenges from a dermatological perspective. J Pak Assoc Dermatol 2023;33(3):811–812. [Google Scholar]
- 8.Aisyah DN, Mayadewi CA, Budiharsana M, et al. Building on health security capacities in Indonesia: Lessons learned from the COVID-19 pandemic responses and challenges. Zoonoses Public Health 2022;69(6):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adnyana IMDM. Prinsip epidemiologi. Epidemiologi Penyakit Menular. 1st ed. Bandung: CV Media Sains Indonesia; 2022;19–40. [Google Scholar]
- 10.Adisasmito WB, Almuhairi S, Barton Behravesh C, et al. One health action for health security and equity. The Lancet 2023;401(10376):530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird BH, Mazet JAK. Detection of emerging zoonotic pathogens: An integrated One Health saproach. Annu Rev Anim Biosci 2018;6(1):121–139. [DOI] [PubMed] [Google Scholar]
- 12.Rehman S, Effendi MH, Witaningruma AM, et al. Avian influenza (H5N1) virus, epidemiology and its effects on backyard poultry in Indonesia: a review. F1000Res 2023;11:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiyono L, Rocha ICN, Cedeño TDD, et al. Dengue and COVID-19 infections in the ASEAN region: a concurrent outbreak of viral diseases. Epidemiol Health 2021;43:e2021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olea-Popelka F, Muwonge A, Perera A, et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis a call for action. Lancet Infect Dis 2017;17(1):e21–e25. [DOI] [PubMed] [Google Scholar]
- 15.Müller B, Dürr S, Alonso S, et al. Zoonotic Mycobacterium bovis - induced tuberculosis in humans. Emerg Infect Dis 2013;19(6):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kock R, Michel AL, Yeboah-Manu D, et al. Zoonotic tuberculosis - The changing landscape. Int J Infect Dis 2021;113 Suppl 1:S68–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aptriana CD, Sudarnika E, Basri C.. Nationally and locally-initiated One Health approach in controlling rabies in West Kalimantan, Indonesia. Vet World 2022;15(12):2953–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehman S, Rantam FA, Rehman A, et al. Knowledge, attitudes, and practices toward rabies in three provinces of Indonesia. Vet World 2021;14(9):2518–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suseno PP, Rysava K, Brum E, et al. Lessons for rabies control and elimination programmes: A decade of One Health experience from Bali, Indonesia. Rev Sci Tech 2019;38(1):213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widiasih DA, Lindahl JF, Artama WT, et al. Leptospirosis in ruminants in Yogyakarta, Indonesia: A serological survey with mixed methods to identify risk factors. Trop Med Infect Dis 2021;6(2):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djati RRAP, Kusnoputranto H, Utomo SW, et al. Leptospirosis control based on eco-social factors: Modeling combination in Demak, Central Java, Indonesia. Biodiversitas 2020;21(12):5818–5828. [Google Scholar]
- 22.Indonesian Coordinating Minister for Human Development and Cultural Affairs. Regulation of the coordinating Minister for Human Development and Cultural Affairs of the Republic of Indonesia Number 7 of 2022 on The Guideline Prevention and Control of Zoonosis and Emerging Infectious Diseases in Indonesia, 2022. Available from: https://cdn.who.int/media/docs/default-source/searo/indonesia/permenko-no-7-tahun-2022-small-file.pdf?sfvrsn=8d8e8c94_1&download=true. Accessed: 3 October 2023.
- 23.Harapan H, Michie A, Mudatsir M, et al. Epidemiology of dengue hemorrhagic fever in Indonesia: analysis of five decades data from the National Disease Surveillance. BMC Res Notes 2019;12(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harapan H, Michie A, Mudatsir M, et al. Chikungunya virus infection in Indonesia: A systematic review and evolutionary analysis. BMC Infect Dis 2019;19(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoso MS, Haryanto S, Rulian F, et al. Continuous circulation of chikungunya virus during COVID-19 pandemic in Jambi, Sumatra, Indonesia. Trop Med Infect Dis 2022;7(6):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diptyanusa A, Herini ES, Indarjulianto S, et al. Estimation of Japanese encephalitis virus infection prevalence in mosquitoes and bats through nationwide sentinel surveillance in Indonesia. PLoS One 2022;17(10):e0275647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lempang MEP, Dewayanti FK, Syahrani L, et al. Primate malaria: An emerging challenge of zoonotic malaria in Indonesia. One Health 2022;14:100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman MDT, Sobur MDA, Islam MDS, et al. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020;8(9):1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nugroho D, Husein W, Pacheco D, et al. The evaluation of One health initiative on zoonoses prevention and control program in Indonesia. Proceedings of the Conference of the International Society for Economics and Social Sciences of Animal Health - South East Asia 2019 (ISESSAH-SEA 2019) 2019;125927948. [Google Scholar]
- 30.Tazerji SS, Nardini R, Safdar M, et al. An overview of anthropogenic actions as drivers for emerging and re-emerging zoonotic diseases. Pathogens 2022;11(11):1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. One health zoonotic disease prioritization (OHZDP). Available from: https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/index.html#:∼:text=The. Accessed: 13 June 2023.
- 32.Saba VPM, Gumpangseth N, Songhong T, et al. Emerging and re-emerging zoonotic viral diseases in Southeast Asia: One health challenge. Front Public Health 2023;11:1141483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varela K, Goryoka G, Suwandono A, et al. One health zoonotic disease prioritization and systems mapping: An integration of two One health tools. Zoonoses Public Health 2023;70(2):146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waltner-Toews D. Zoonoses, One health and complexity: Wicked problems and constructive conflict. Philos Trans R Soc Lond B Biol Sci 2017;372(1725):20160171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morcatty TQ, Pereyra PER, Ardiansyah A, et al. Risk of viral infectious diseases from live bats, primates, rodents and carnivores for sale in Indonesian wildlife markets. Viruses 2022;14(12):2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elnaiem A, Mohamed-Ahmed O, Zumla A, et al. Global and regional governance of one health and implications for global health security. Lancet 2023;401(10377):688–704. [DOI] [PubMed] [Google Scholar]
- 37.Tomori O, Oluwayelu DO. Domestic animals as potential reservoirs of zoonotic viral diseases. Annu Rev Anim Biosci 2023;11(1):33–55. [DOI] [PubMed] [Google Scholar]
- 38.Webster JP, Gower CM, Knowles SCL, et al. One health - An ecological and evolutionary framework for tackling neglected zoonotic diseases. Evol Appl 2016;9(2):313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemenkes RI. Koordinasi zoonotic diseases action package (ZDAP). Available from: http://p2p.kemkes.go.id/koordinasi-zoonotic-diseases-action-package-zdap/. Accessed: 3 October 2023.
- 40.Ekowati RV, Sudarnika E, Purnawarman T.. Spatial analysis of Rabies cases in dogs in Bali Province, Indonesia. Adv Anim Vet Sci. 2019;8(1):32–40. [Google Scholar]
- 41.Dewi APM, Riono P, Farid MN. Effects of Rabies elimination program on Rabies cases in Bali, 2008-2015. KnE Life Sci 2018;4(1):62. [Google Scholar]
- 42.Purnama SG, Utami NWA, Subrata M, et al. Assessment of Rabies control attitudes during the COVID-19 pandemic through partial least square-structural equation modeling. Kesmas 2023;18(2):145–151. [Google Scholar]
- 43.Destoumieux GD, Mavingui P, Boetsch G, et al. The One health concept: 10 years old and a long road ahead. Front Vet Sci 2018;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebreyes WA, Dupouy-Camet J, Newport MJ, et al. The global One Health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl Trop Dis 2014;8(11):e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO. Engaging multisectoral stakeholders to strengthen One Health. Training of trainers (ToT) on zoonoses prevention and control with a one health approach 2022. Available from: https://www.who.int/indonesia/news/detail/14-07-2022-engaging-multisectoral-stakeholders-to-strengthen-one-health. Accessed: 3 October 2023.
- 46.Aptriana CD, Sudarnika E, Basri C.. Nationally and locally-initiated One Health approach in controlling rabies in West Kalimantan, Indonesia. Vet World 2022;15(12):2953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basham C, Billings E, El Rifay AS, et al. Designing and validating a One Health research translation framework through literature-based case studies in Egypt. One Health 2022;15:100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adisasmito WB, Almuhairi S, Behravesh CB, et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog 2022;18(6):e1010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ihekweazu C, Michael CA, Nguku PM, et al. Prioritization of zoonotic diseases of public health significance in Nigeria using the One-health approach. One Health 2021;13:100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khairani S, Sagasiousman R.. Implementation of One health approach for malaria zoonotic control in Indonesia: Past, present, and future. BIO Web Conf 2022;49:04003. [Google Scholar]
- 51.Dasgupta R, Tomley F, Alders R, et al. Adopting an intersectoral One Health approach in India: Time for One Health committees. Indian J Med Res 2021;153(3):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Socha W, Kwasnik M, Larska M, et al. Vector-borne viral diseases as a current threat for human and animal health-One Health perspective. J Clin Med 2022;11(11):3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biswas R, Debnath C, Bandyopadhyay S, et al. One Health approaches adapted in a low resource setting to address antimicrobial resistance. Sci One Health 2022;1:100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhama K, Chandran D, Chakraborty S, et al. Zoonotic concerns of Marburg virus: Current knowledge and counteracting strategies including One Health approach to limit animal-human interface: An update. Int Surg J 2022;106:106941. [DOI] [PubMed] [Google Scholar]
- 55.Kibenge FSB. Continuous surveillance and viral discovery in animals and humans are a core component of a one-health approach to address recent viral reverse zoonoses. J Am Vet Med Assoc 2023;261(6):1–9. [DOI] [PubMed] [Google Scholar]
- 56.Siahaan S, Herman MJ, Fitri N.. Antimicrobial resistance situation in Indonesia: A challenge of multisector and global coordination. J Trop Med 2022;2022:2783300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tauran PM, Djaharuddin I, Bahrun U, et al. Excess mortality attributable to antimicrobial-resistant bacterial bloodstream infection at a tertiary-care hospital in Indonesia. PLOS Glob Public Health 2022;2(7):e0000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Limato R, Lazarus G, Dernison P, et al. Optimizing antibiotic use in Indonesia: A systematic review and evidence synthesis to inform opportunities for intervention. Lancet Reg Health Southeast Asia 2022;2:100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh BT, Fujiwara PI, Ronello A, et al. The role of sub-national leaders implementing the One health approach. One Health Cases 2023;2023:0005. [Google Scholar]
- 60.Ferrinho P, Fronteira I.. Developing One health systems: A central role for the One Health workforce. Int J Environ Res Public Health 2023;20(6):4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suwandono A, Campbell A, Stevenson A, et al. Implementing and financing One Health. Task force 6: Global health security and COVID-19. 2022. Available from: https://www.t20indonesia.org/wp-content/uploads/2022/10/TF6_Implementing-and-Financing-One-Health.pdf. Accessed: 5 October 2023. [Google Scholar]
- 62.Asante A, Cheng Q, Susilo D, et al. The benefits and burden of health financing in Indonesia: analyses of nationally representative cross-sectional data. Lancet Glob Health 2023;11(5):e770–780. [DOI] [PubMed] [Google Scholar]
- 63.Vesterinen HM, Dutcher TV, Errecaborde KM, et al. Strengthening multi-sectoral collaboration on critical health issues: One health systems mapping and analysis resource toolkit (OH-SMART) for operationalizing One Health. PLoS One 2019;14(7):e0219197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernstein AS, Ando AW, Loch-Temzelides T, et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci Adv 2022;8(5):eabl4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leandro A, de S, Lopes RD, Martins CA, et al. The adoption of the One Health approach to improve surveillance of venomous animal injury, vector-borne and zoonotic diseases in Foz do Iguaçu, Brazil. PLoS Negl Trop Dis 2021;15(2):e0009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.