Abstract
Silent information regulator 3 is an essential component of the Saccharomyces cerevisiae silencing complex that functions at telomeres and the silent mating-type loci, HMR and HML. We show that expression of the N- and C-terminal-encoding halves of SIR3 in trans partially complements the mating defect of the sir3 null allele, suggesting that the two domains have distinct functions. We present here a functional characterization of these domains. The N-terminal domain (Sir3N) increases both the frequency and extent of telomere-proximal silencing when expressed ectopically in SIR+ yeast strains, although we are unable to detect interaction between this domain and any known components of the silencing machinery. In contrast to its effect at telomeres, Sir3N overexpression derepresses transcription of reporter genes inserted in the ribosomal DNA (rDNA) array. Immunolocalization of Sir3N-GFP and Sir2p suggests that Sir3N directly antagonizes nucleolar Sir2p, releasing an rDNA-bound population of Sir2p so that it can enhance repression at telomeres. Overexpression of the C-terminal domain of either Sir3p or Sir4p has a dominant-negative effect on telomeric silencing. In strains overexpressing the C-terminal domain of Sir4p, elevated expression of either full-length Sir3p or Sir3N restores repression and the punctate pattern of Sir3p and Rap1p immunostaining. The similarity of Sir3N and Sir3p overexpression phenotypes suggests that Sir3N acts as an allosteric effector of Sir3p, either enhancing its interactions with other silencing components or liberating the full-length protein from nonfunctional complexes.
Chromatin structure plays an important role in the regulation of gene expression in the eukaryotic cell. Cytological studies have long suggested that different domains of the genome are packaged into two structurally different types of chromatin, heterochromatin and euchromatin (48). In contrast to euchromatin, heterochromatin is rich in repetitive sequences and remains constitutively condensed throughout the cell cycle. In higher eukaryotic cells, these regions are generally poor in coding sequences and are replicated late in S phase, while most transcriptionally active genes are located in early replicating, euchromatic regions of the genome. Important for the genetic characterization of heterochromatin was the observation that it influences the transcription of genes transposed nearby. The resulting variegated repression of the euchromatic gene is known as position effect variegation (reviewed in reference 20).
Despite the absence of a cytologically visible heterochromatin, the yeast Saccharomyces cerevisiae has distinct chromosomal regions which, like heterochromatin, confer a heritable state of transcriptional repression on otherwise functional promoters. Repression at the silent mating-type loci, HML and HMR (hereafter collectively called HM loci), is stable, while repression of RNA PolII genes integrated near the telomeric TG1–3 repeat is variegated (called telomeric position effect [TPE]), much like the stochastic patterns of repression observed near centromeric heterochromatin in flies. In yeast, both these domains are less accessible to nucleolytic and methylase modification, and they contain a histone, H4, which is underacetylated on lysines 5 and 16 (reviewed in reference 32). In telomeric regions, the repressed chromatin state spreads along the chromosome, limited by the dosage of essential components, again reminiscent of the spread of centric heterochromatin in flies (16, 33). More recently, variegated expression has also been noted for reporter genes inserted into the tandemly repeated ribosomal DNA (rDNA) locus of yeast (4, 40).
A number of proteins are required for both telomeric and mating-type locus repression. These include repressor activator protein 1 (Rap1p), the silent information regulators Sir2 to -4, and the N termini of histones H3 and H4 (1, 17, 21, 23, 34, 44, 45). Of these, only Rap1p binds telomeric DNA directly, while Sir3p and Sir4p are both able to form homo- and heteromultimeric complexes (27, 29) that interact with the N termini of histones H3 and H4 (15). Combined immunofluorescence and in situ-hybridization experiments have shown that telomeres are clustered and that Rap1p, Sir3p, and Sir4p colocalize with telomeric foci in wild-type cells (9). Immunoprecipitation and cross-linking data confirm that Sir3p, Sir4p, histones, and Rap1p can be coimmunoprecipitated with subtelomeric DNA in wild-type cell extracts (16, 43). Sir2p is also part of this complex and can be cross-linked to telomeric chromatin through its interaction with Sir4p (43).
In addition to its telomeric localization, Sir2p was shown to be constitutively bound to the rDNA in a manner independent of Sir3p and Sir4p (10). This is consistent with the observation that the variegated repression of a PolII gene inserted in the rDNA repeats, as well as repression of recombination between rDNA repeats, requires SIR2 but not SIR3 or SIR4 (11, 40). The presence of Sir2p in the nucleolus suggests a direct effect on rDNA chromatin, perhaps through modulation of nucleosomal organization within the RNA PolI or PolIII promoter regions (4, 8, 40). In aging yeast cells, or in strains carrying mutant forms of Sir4p, Sir3p also relocalizes from telomeres to the nucleolar compartment (10, 22). Although the function of Sir3p in the nucleolus is unclear, Sir proteins do affect nuclear events other than HM and telomeric silencing: mitotic recombination increases fourfold in sir3-deficient strains, mitotic chromosome loss increases fivefold in sir4-deficient strains (31), and deletion of either SIR2, SIR3, or SIR4 increases sensitivity of a Δrad52 strain to ionizing radiation (47).
Sir3p plays a unique and central role in chromatin-mediated repression. Although Sir3p and Sir4p are present in approximately equimolar amounts (7), only Sir3p is limiting for the propagation of telomeric silencing (33). In SIR+ cells, TPE represses genes up to 4 kb from the telomere (core heterochromatin), while in cells overexpressing SIR3, telomeric repression extends roughly 20 kb from the telomeric TG1–3 repeat, coinciding with the spread of Sir3p along the repressed chromatin (16, 33, 43). The propagation of Sir3p is presumably mediated by interaction of its C-terminal domain with histone tails (15).
The N-terminal 214 amino acids (aa) of Sir3p have over 50% identity with the N terminus of Orc1p, the largest subunit of the origin recognition complex, and gene fusion experiments indicate that the N terminus of Orc1p can functionally substitute for that of Sir3p (2). Moreover, several point mutations in the N-terminal domain of Sir3p suppress silencing-deficient mutants in Rap1p and the N termini of histones H3 and H4, although these domains do not interact directly (15, 18, 24). Here we report that the ectopic expression of an N-terminal region (aa 1 to 503, hereafter called Sir3N) enhances TPE, while that of the C-terminal domain (aa 568 to 978 [Sir3C]) derepresses silencing. Expression of this Sir3N fragment in the presence of full-length Sir3p also extends telomere-proximal repression. In a strain overexpressing the C terminus of Sir4p, elevated expression of Sir3N, like that of full-length Sir3p, is able to restore both silencing and the punctate staining patterns of Sir3p and Rap1p.
In contrast to its effect at telomeres, overexpression of Sir3N derepresses a URA3 reporter gene inserted within the rDNA repeat. Localization of a Sir3N-GFP fusion protein indicates that it accumulates in the nucleolus in a Sir2p-dependent manner. Intriguingly, Sir3N overexpression leads to enhanced Sir2p staining at telomeres, coincident with the improvement in telomeric silencing, although we detect no direct interactions between Sir3N and Sir2p, nor between Sir3N and Sir4C. The hypothesis most consistent with the available data is that Sir3N counteracts the Sir4C-induced derepression and extends TPE by acting as an allosteric effector of full-length Sir3p.
MATERIALS AND METHODS
Plasmid construction.
Standard molecular biology techniques were used, following published protocols (37). pADH-SIR4 was described previously (7). pADH-SIR4C, used in this study, was constructed by replacing the LEU2 gene of pADH-SIR4C (7) with a HIS3 gene. pADH-SIR3N was constructed by subcloning a 1.5-kb BamHI-HindIII fragment of SIR3 into the HindIII site of vector pAAH5 after filling in overhangs. The 5′ BamHI site of SIR3 was engineered as described previously (31). pADH-SIR3 was described earlier as p2μ-ASir3 (26). pADH-SIR3C was constructed by subcloning a HindIII-HindIII 2.7-kb fragment into the HindIII site of vector pAAH5. pMG17 was constructed by subcloning a 3-kb BglII-BamHI fragment of SIR3 into the BamHI site of vector p423ADH (30). The pSIR3N-GFP1 and pSIR3N-GFP2 plasmids were constructed by cloning a 1.5-kb BamHI-HindIII fragment of SIR3 as before and a HindIII-EcoRI fragment containing GFP (pGFP; Clontech) into p414ADH and p424ADH, respectively (30). For two-hybrid assays DNA binding domains were fused both before and after the Sir3N open reading frame. N-terminal fusions lost the phenotypes associated with SIR3N expression, suggesting that they fold improperly. Therefore, for all SIR3 constructs used in this paper the N terminus was kept in its native state.
Yeast media and strains.
All yeast strains are described in Table 1. UCC18, YHR434, YHR440, and YHR441 are isogenic, and UCC18 was described previously (1). UCC518, UCC520, and UCC522 are isogenic (33), as are UCC3107, UCC3203, and UCC3207 (10, 42). Standard media were used for the growth of S. cerevisiae (12); all cultures were grown at 30°C. Yeast transformation was performed by the lithium acetate procedure (38), and other manipulations were as described previously (35).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| AJL275-2AVR | MATα ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 VR::URA3-Tel | 23 |
| AJL275-2AVIIL | MATα ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 VIIL::ADE2-Tel | 23 |
| EG37 | MATa leu2-3,112 ura3-52 trp1-289 his3 gal2 HML::E>I | 26 |
| GA509 | MATa ade2 can1::hisG his3 leu2 trp1 ura3 hhf1::HIS3 HHF2-gly17 VR::ADE2-Tel | 33a |
| GA510 | MATa ade2 can1::hisG his3 leu2 trp1 ura3 hhf1::HIS3 HHF2-gly16 VR::ADE2-Tel | 33a |
| GA513 | MATa ade2 can1::hisG his3 leu2 trp1 ura3 hhf1::HIS3 HHF2-gln16 VR::ADE2-Tel | 33a |
| JS231 | MATα ade2 trp1-63 leu2-1 his3Δ200 ura3-167 RDN::mURA3-HIS3 | 40 |
| UCC18 | MATa ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 adh4::URA3-Tel | 1 |
| UCC518, -520, -522 | MATa ade2-101 his3-Δ200 leu2Δ1 lys2-801 trp1-Δ1 ura3-52 ppr1::HIS3 VR::URA3 | 33 |
| UCC3107 | MATa ade2::hisG can1::hisG his3-11 leu2 trp1 ura3-52 VR::ADE2-Tel | 10, 42 |
| GA822 | MATa ade2::hisG can1::hisG his3-11 leu2 trp1 ura3-52 sir3::TRP1 VR::ADE2-Tel | |
| UCC3203 | MATa ade2::hisG can1::hisG his3-11 leu2 trp1 ura3-52 sir2::HIS3 VR::ADE2-Tel | 10, 42 |
| UCC3207 | MATa ade2::hisG can1::hisG his3-11 leu2 trp1 ura3-52 sir4::HIS3 VR::ADE2-Tel | 10, 42 |
| PT2 | MATα hom3 | |
| YHR434 | MATa ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 sir2::HIS3 adh4::URA3-Tel | 33a |
| YHR440 | MATa ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 sir3::HIS3 adh4::URA3-Tel | 33a |
| YHR441 | MATa ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 sir4::HIS3 adh4::URA3-Tel | 33a |
Repression assays.
The expression of the telomere-proximal URA3 was monitored by determining the fraction of cells capable of growth on 5 fluoro-orotic acid (5-FOA)-containing medium, which allows the growth of ura3− cells but not URA3+ cells (3, 12). The cells were grown for 3 to 5 days on selective medium at 30°C. Isolated colonies were resuspended in water, and 10-fold serial dilutions were spotted onto synthetic selective medium and onto the same medium containing 0.1% 5-FOA (12). 5-FOA resistance was determined as the average ratio of colonies formed on 5-FOA medium to colonies formed on selective medium. The number of colonies for each spot was determined after 3 to 4 days of growth at 30°C. Several independent transformants were tested, and the mean was calculated either directly (usually with eight independent colonies; performed in triplicate) or as described for standard fluctuation tests (discarding high and low extremes and taking the mean). We observed no significant differences in the values thus obtained.
Individual colonies carrying the ade2-1 mutation and the wild-type ADE2 gene adjacent to the left telomere of chromosome VII or the right arm of chromosome V (12, 23, 33) were streaked onto medium containing 10 mg of adenine/liter. Within single yeast colonies, the appearance of red and white sectors indicates metastable repression of the telomeric ADE2. Colonies were grown for 3 to 5 days at 30°C and then stored for 1 to 2 weeks at 4°C for pigment accumulation.
Repression of a URA3 reporter with a mutated promoter inserted at the rDNA [RDN1::(mURA3-HIS3)] was measured as follows: colonies from the JS231 strain transformed with either the vector or the plasmid overexpressing Sir3N, Sir3C, or full-length Sir3p were grown for 3 to 5 days at 30°C were resuspended in water. Tenfold serial dilutions were spotted onto selective media lacking leucine (to ensure maintenance of the plasmid) and lacking both leucine and uracil to measure repression of the rDNA URA3 gene. Derepression of URA3 results in bigger colonies after 3 days at 30°C.
Quantitative mating.
Quantitative mating was performed essentially as described previously (41). Strains UCC3107, the isogenic sir3::TRP1 (GA822), and the α tester strain PT2 were grown to a density of 5.0 × 106 to 1.5 × 107 cells per ml in selective medium lacking leucine and histidine to ensure maintenance of plasmids. The cells (2.0 × 106) were mixed with 107 cells of the tester strain, collected on a 0.8-μm-pore-size, 25-mm-diameter nitrocellulose filter disk (type AA; Millipore), and allowed to mate for 6 h at 30°C. The cells were resuspended in water and sonicated for 5 to 10 s to disperse clumps. Tenfold serial dilutions were plated on minimal medium to measure the titer of a/α diploids and on minimal medium complemented with adenine, tryptophan, and uracil, which allows the growth of both a/α diploids and the a haploid. Mating efficiency is expressed as the titer of a/α cells divided by the titer of a/α cells plus a cells.
Immunofluorescence and antibodies.
Immunofluorescence assays were performed as described previously (9), using affinity-purified rabbit antibodies. The anti-Sir3p antibody was raised against the C-terminal 537 aa of Sir3p. The affinity-purified rabbit anti-GFP antibody was a kind gift of K. Sawin (Imperial Cancer Research Fund, London, England) and was used at a 1:800 dilution. Secondary antibodies coupled to the fluorochrome 5-([4,6-dichlorotriazin-2-yl] (amino)-fluorescein (DTAF), and CY3 and GFP fluorescence, were visualized on a Zeiss Axiovert 100 microscope (Zeiss laser scanning microscope 410) with a 63× Plan-Apochromat objective (1.4 oil) as previously described (9). Under standard imaging conditions no signal from one fluorochrome could be detected on the other filter set. Standardized conditions for the image capture and subtraction of a background value (about 15% of the maximum signal) were carried out uniformly on all images.
RESULTS
Sir3N restores TPE in strains overexpressing Sir4C.
Overexpression of full-length Sir4p or its C-terminal domain (aa 743 to 1358 [Sir4C]) relieves silencing at HM and telomeric loci (7, 27). This may result either from disruption of the Sir2p-Sir3p-Sir4p complex or from the titration of an unknown, yet essential, silencing component. Coincident with the loss of silencing, the punctate staining patterns of Rap1p and Sir3p are disrupted upon Sir4C overexpression and Sir proteins are found diffused throughout the nucleus (7). We reasoned that if overexpression of Sir4C competes for the assembly of the multicomponent complex required for silencing, then overexpression of the limiting component might restore TPE.
To identify this limiting component, we screened for multicopy suppressors of Sir4C overexpression in a ade2-1 strain carrying a telomeric copy of ADE2 on the left arm of chromosome VII. In this strain, with or without the control pADH vector, silencing of ADE2 produces red sectors within white colonies (Fig. 1) (12). Cells overexpressing the Sir4C terminus, on the other hand, form white nonsectoring colonies, due to ADE2 expression (i.e., loss of TPE [Fig. 1]).
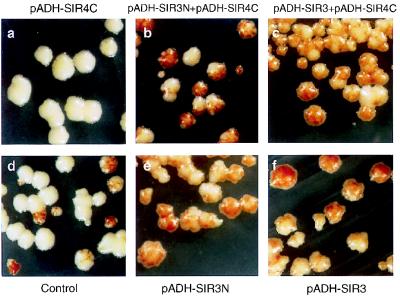
FIG. 1.
Overexpression of Sir3N restores sectoring in Sir4C-overexpressing cells. The strain AJL275-2AVIIL, which carries ADE2 adjacent to the VIIL telomere, was transformed with pADH-SIR4C (also called pFP340) and pAAH5 (a); pADH-SIR4C and pADH-SIR3N (b); pADH-SIR4C and pADH-SIR3 (p2μ-ASIR3 [26]) (c); control vectors (pAAH5 and p2HG) (d); pADH-SIR3N and p2HG (e); and pADH-SIR3 (p2μ-ASIR3 [26]) and p2HG (f). In all cases two plasmids were present and isolated colonies were streaked onto medium lacking histidine and leucine to ensure maintenance of the plasmids. Adenine concentrations are limiting. The colonies were allowed to grow for 5 days at 30°C. Following incubation, the plates were stored at 4°C to enhance the pigmentation of the cells.
A YEp13-based high-copy-number genomic library was introduced into the Sir4C-overexpressing strain, and transformants were screened for red sectoring, indicative of ADE2 repression. Screening of 5.0 × 103 transformants identified 30 sectored transformants, each carrying a different plasmid. Only one plasmid, however, reproducibly restored the sectored phenotype following plasmid isolation and retransformation. Southern blot analysis and sequencing revealed that this clone contained the first 1.8 kb of SIR3, encoding the N-terminal 503 aa of the protein (Sir3N [Fig. 2]), expressed under the control of its own promoter. No other SIR genes or known silencing factors were recovered in the screen. To see whether an increased dosage of this domain improves its ability to restore sectoring, we subcloned this fragment from another SIR3 vector so that it could be expressed under the control of the ADH promoter. As shown in Fig. 1, the resulting plasmid, pADH-SIR3N, is able to restore sectoring to levels higher than wild type in a Sir4C-overexpressing strain. Immunofluorescence with anti-Sir4C antibodies indicates no destabilization or down-regulation of the Sir4C fragment (see below).
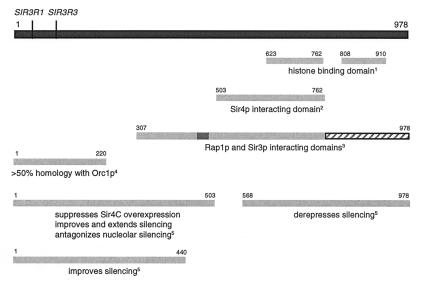
FIG. 2.
Functional domains of Sir3p. A schematic representation of full-length Sir3p and the functional domains revealed by genetic, two-hybrid, and biochemical studies is shown. Notes: 1, reference 15; 2, two-hybrid data indicate that the Sir4p binding domain is 3′ of aa 494 (6), and unpublished pull-down data indicate that there is only one site of interaction, not two as previously suggested (12a, 43), 3, reference 29 (as indicated by the shaded box, the domain necessary and sufficient for Rap1p interaction has been narrowed down to aa 455 to 481 of Sir3p, and the Sir3p homodimerization domain has been defined from aa 762 to the end of the protein [38a]); 4, reference 2; 5, this study. The two mutations isolated as suppressors of histone H4 mutants are labeled SIR3R1 and SIR3R3 (18). See the text for more details.
The simplest explanation for the ability of Sir3N to suppress the phenotype of Sir4C overexpression is that the two domains interact directly. However, extensive two-hybrid analysis with aa 1 to 503 of Sir3p, either as bait or as prey, failed to demonstrate an interaction with Sir4C (data not shown). We know that the Sir3N fusion used as bait in these assays is folded correctly, since it produces the same phenotypes as nonfused Sir3N (see below), and the Sir4C construct has been shown to interact with itself, Rap1p, and Sir3p (data not shown). Similarly, two-hybrid data of Moretti et al. (29) show that Sir4C binds a domain of Sir3p from aa 307 to the end of the protein. More recently the site of Sir4p binding was mapped by glutathione S-transferase interaction and two-hybrid assays to a central core of Sir3p (aa 481 to 734 [38a] and aa 503 to 763 [12a]) (Fig. 2). Finally, attempts to coimmunoprecipitate Sir3N with Sir4C overexpressed in yeast were negative (data not shown). Thus, in vitro binding assays, two-hybrid data, and coimmunoprecipitation results all suggest that Sir3N suppresses the Sir4C overexpression phenotype through a mechanism other than direct protein-protein interaction.
Overexpression of the Sir3N- and C-terminal domains have opposite effects on telomeric silencing.
One mechanism by which the Sir3N fragment could suppress the loss of silencing due to Sir4C overexpression is by stabilizing repressed chromatin structure through interactions with other silencing factors. Consistent with this, it has been reported that point mutations in the N terminus of SIR3 (SIR3R1 and SIR3R3) are able to suppress the loss of HML silencing that results from mutation of the N terminus of histone H4 (18). One of these SIR3 point mutations also suppresses the loss of telomeric and HML silencing conferred by mutations in the Rap1p C-terminal domain (24). This is surprising, since the binding sites for the histone N termini and for Rap1p, as well as the Sir3p homodimerization domain, map to regions C-terminal of aa 503 by two-hybrid and in vitro binding assays (15, 29, 38a). Using two-hybrid and coimmunoprecipitation methods, we could confirm the published interactions between Sir3C and Rap1p, Sir4C, and histones, while Sir3N showed no binding to any of these factors or to Sir2p (data not shown).
In view of this paradox, we decided to characterize the inherent functions of the N- and C-terminal domains of Sir3p by overexpressing these domains in a ura3 strain carrying a URA3-marked telomere. In this strain, transcriptional silencing of the telomere-proximal reporter can be measured quantitatively by the ability of cells to grow on 5-FOA (12) (see Materials and Methods). We confirmed that overexpression of full-length Sir3p increases the efficiency of telomeric silencing by 2 orders of magnitude (Table 2), as shown by Renauld et al. (33). Intriguingly, overexpression of Sir3N improves telomeric silencing with almost the same efficiency as full-length Sir3p (Table 2). This is not a bypass of the regular silencing mechanism, as the Sir3N-enhanced repression requires Sir2p, Sir4p, the histone N termini, and, importantly, full-length Sir3p (Table 3). Because the Sir3N domain shows a high degree of homology with the N-terminal domain of Orc1p (2), which was shown to bind Sir1p (46), we tested whether the Sir3N enhancement of telomere-proximal silencing requires SIR1. However, the Sir3N effect is identical in SIR1+ and sir1− strains, ruling out the possibility that Sir3N stabilizes telomeric silencing by recruiting Sir1p to telomeres (Table 3).
TABLE 2.
Effects of overexpression of the N- and C-terminal domains of Sir3p on telomeric position effect and requirements for enhanced repression: fraction of 5-FOAR colonies upon introduction of overexpression plasmidsa
| Strain | Plasmid | 5-FOARb | Fold variation |
|---|---|---|---|
| AJL275-2AVR | pADH-SIR3N | 3.0 × 10−2 (1.0 × 10−2–4.0 × 10−1) | 40 (repression) |
| pADH-SIR3C | 3.0 × 10−5 (1.7 × 10−6–7.0 × 10−5) | 30 (derepression) | |
| pADH-SIR3 | 7.0 × 10−2 (8.0 × 10−2–8.0 × 10−1) | 90 (repression) | |
| pADH | 8.0 × 10−4 (4.0 × 10−4–2.5 × 10−3) | 1 |
The AJL275-2AVR strain, which carries URA3 adjacent to the VR telomere, was transformed with the following 2μ-based plasmids: pADH-SIR3N, pADH-SIR3C, pADH-SIR3 (p2μ-ASIR3 [26]), and pADH (pAAH5).
Transcriptional repression of URA3 was determined by measuring resistance to 5-FOA from a minimum of four independent transformants. The range of values is given in parentheses. The effects of Sir3C and Sir3N overexpression were confirmed with both URA3 and ADE2 reporters at both TelVR and -VIIL in a range of strains, including UCC3107 and GA493 (6) as well as the AJL275 background.
TABLE 3.
Effects of overexpression of the N- and C-terminal domains of Sir3p on telomeric position effect and requirements for enhanced repression: fraction of FOAR colonies or ADE2 repression upon overexpression of Sir3N or Sir3pa
| Fraction 5-FOARb
|
ADE2 repressionc
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | Genotype | pADH-SIR3N | pADH-SIR3 | pADH | Strain | Genotype | pADH-SIR3N | pADH-SIR3 | pADH |
| UCC3107 | SIR+ | +++ | +++ | + | |||||
| YHR436 | SIR+ | 0.25 (0.16–0.33) | 0.34 (0.36–0.3) | 0.084 (0.015–0.12) | |||||
| UCC3242 | sir1 | +++ | +++ | + | |||||
| YHR434 | sir2 | <4.0 × 10−5 | <4.0 × 10−6 | <6.0 × 10−6 | |||||
| GA506 | HHF2 | +++ | +++ | + | |||||
| YHR440 | sir3 | <1.0 × 10−5 | 0.30 (0.42–0.21) | <1.0 × 10−5 | |||||
| GA509 | HHF2-gly17 | − | − | − | |||||
| YHR441 | sir4 | <2.0 × 10−5 | <4.0 × 10−6 | <2.0 × 10−5 | |||||
| GA510 | HHF2-gly16 | − | − | − | |||||
| GA513 | HHF2-gln16 | − | − | − | |||||
Shown are the frequency of resistance to 5-FOA in strains carrying URA3 adjacent to the VIIL telomere and repression of the ADE2 gene adjacent to the VR telomere. The strains were transformed with the same 2μ-based plasmids as for Table 2. The relevant genotypes of the strains are indicated.
The mean of the frequency of 5-FOA resistance from three independent transformants is presented; the range of values is given in parentheses.
+++, strong red color (strong repression); +, red-white color; −, white color (complete derepression).
Do other domains of Sir3p have the same effect as Sir3N? To test this, we overexpressed the C-terminal half of Sir3p (Sir3C [Fig. 2]). In contrast to the effect of Sir3N, elevated levels of Sir3C lead to derepression of the telomere-proximal URA3 (Table 2). This derepression phenotype was confirmed by monitoring ADE2 expression at the VR telomere (Fig. 3) and in a variety of strain backgrounds (Table 2). Sir3C-mediated derepression, although weaker than that provoked by the overexpression of Sir4C, probably reflects the presence of essential homo- and heterodimerization motifs, as well as the Rap1 binding sites, in these two C-terminal domains (29, 43). Sir3C may also interfere with propagation of the Sir complex along nucleosomes by competing for the N termini of histones H3 and H4 (15).
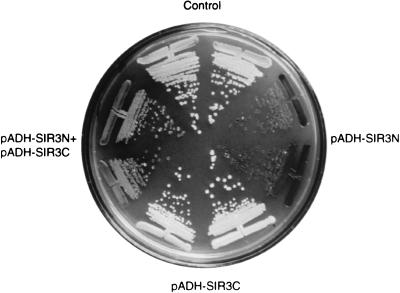
FIG. 3.
Overexpression Sir3N counteracts the effect of overexpression of Sir3C at telomeres. The strain UCC3107, which carries ADE2 adjacent to the VR telomere, was transformed with plasmids as indicated as well as a second control plasmid. The control plasmids are the backbone vectors without SIR gene inserts, namely, pAAH5 and p423ADH (see Materials and Methods). Plasmids with SIR3 gene inserts are pADH-SIR3N and pADH-SIR3C (pMG17). In each case, two independent transformants were streaked onto medium lacking histidine and leucine to ensure maintenance of the plasmids and with limiting adenine concentrations. The cells were allowed to grow for 3 to 5 days at 30°C, and the plates were stored at 4°C to enhance the pigmentation of the cells.
The effects of Sir3N and Sir3C overexpression on the repression of the telomere-proximal URA3 gene were confirmed by using a telomere-proximal ADE2 gene, which gives a red-sectored phenotype in SIR+ cells. Sir3N overexpression results in completely red colonies, indicating full repression of the telomeric marker, while Sir3C-expressing cells are white (Fig. 1e and 3). Using this assay, we determined that a shorter N-terminal fragment, which stops at aa 440 and lacks the Rap1p-interacting domain (Fig. 2), also improves TPE. The combined overexpression of Sir3N and Sir3C is discussed below.
Expression in trans of the N- and C-terminal domains of Sir3p restores HML repression in a strain with SIR3 deleted.
Do the N and C termini of Sir3p perform different and independent functions within the silencing complex? To test this, the N- and the C-terminal domains of Sir3p were overexpressed from separate vectors in a strain carrying a complete deletion of SIR3 (sir3::TRP1), and both TPE and mating-type silencing were scored. Although no red sectoring of the white colonies was detected with an ADE2-marked telomere in the UCC3107 background (data not shown), we did observe a 100-fold increase in mating efficiency when both Sir3N and Sir3C were expressed from separate plasmids in this sir3::TRP1 strain (Table 4). The expression of either domain individually does not restore mating in the absence of Sir3p (Table 4). Restriction mapping of plasmids recovered from these yeast cells rules out the possibility that the two plasmids might have recombined to create an intact SIR3 gene (data not shown).
TABLE 4.
Sir3N and Sir3C expression in trans complements a sir3::TRP1 allelea
| Strain | Relevant genotype | Plasmids | Mating efficiency (%)b |
|---|---|---|---|
| UCC3107 | SIR3 | Control | 90 (8.2 × 10−1) |
| pADH-SIR3N | 91 (8.3 × 10−1) | ||
| pADH-SIR3C | 103 (9.4 × 10−1) | ||
| pADH-SIR3N + pADH-SIR3C | 95 (8.7 × 10−1) | ||
| pADH-SIR3 | 100 (9.1 × 10−1) | ||
| GA822 | sir::TRP1 | Control | <0.002 (<2 × 10−5) |
| pADH-SIR3N | <0.004 (<4 × 10−5) | ||
| pADH-SIR3C | <0.003 (<3 × 10−5) | ||
| pADH-SIR3N + pADH-SIR3C | 0.2 (1.9 × 10−3) | ||
| pADH-SIR3 | 100 (9.1 × 10−1) |
Host strains UCC3107 and GA822, which are isogenic and Mata, were transformed with plasmids as indicated as well as a second control plasmid. Thus, all the strains contained two plasmids and mating was performed under selective conditions. The control plasmids are the backbone vectors without SIR3 gene inserts, namely, pAAH5 and p423ADH. The control contained both vectors. Plasmids with SIR3 inserts are pADH-SIR3N, pADH-SIR3C (also called pMG17), and pADH-SIR3 (pA-SIR3 [26]) (see Materials and Methods for a description of all plasmids). Mating efficiencies were determined by a quantitative mating assay as described in Materials and Methods.
Actual measured values are shown in parentheses.
We cannot conclude that there is no complementation of TPE whatsoever when the two Sir3p domains are expressed in trans in a sir3 deletion strain, yet, as we never see red sectors in the sir3::TRP1 strain used (GA822), any complementation must be below the limit of detection with the TelVR::ADE2 reporter. Importantly, in a SIR3+ background we do observe that Sir3N expression counteracts the disruptive effect of Sir3C overexpression at telomeres. This is visualized as a high frequency of red colonies, due to ADE2 repression, in contrast to the white colonies of strains overexpressing Sir3C (Fig. 3). The ability of Sir3N to counteract the derepression mediated by Sir3C may reflect a direct neutralization of Sir3C by Sir3N or simply a net improvement of TPE, due to the strong increase in silencing mediated by Sir3N. This might be able to balance an independent disruptive effect of Sir3C. Finally, the ability of Sir3p domains to substitute more efficiently at HMLα than at telomeres in the absence of Sir3p may be due to the redundancy of silencer organization (reviewed in reference 32). We propose that the additional nucleation sites provided by ORC and Abf1p at silencers may be critical to allow the separate Sir3p domains to function in trans.
Silenced chromatin extends inward from the telomere upon Sir3N overexpression.
A unique feature of Sir3p is its ability to propagate a repressed chromatin state inwards from the core of silent chromatin adjacent to the TG1–3 repeat. Indeed, upon overexpression of SIR3, silent chromatin and Sir3p itself spreads as far as 20 kbp from the telomere (16, 33). Although extended silencing requires SIR2 and SIR4, these proteins do not propagate stoichiometrically with Sir3p over the extended silencing domain (43). Since overexpression of Sir3N, like that of Sir3p, improves repression of a telomere-proximal gene, we next tested whether the N-terminal domain is sufficient to promote the spread of TPE.
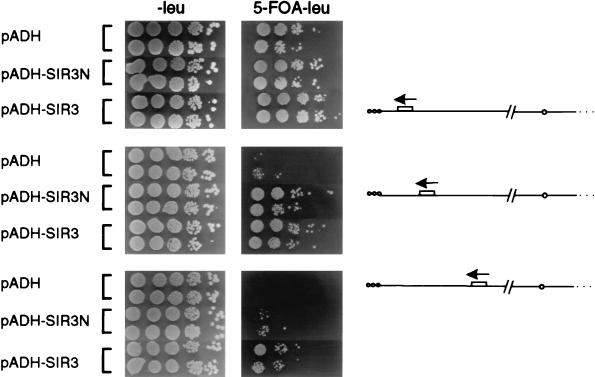
In a set of isogenic ppr1-deficient strains carrying URA3 at various distances from the right end of chromosome V (Ppr1p is a trans-activator that enhances URA3 expression [33, 36]), we monitored the efficiency of growth on 5-FOA (FOA R [Fig. 4]). Overexpression of Sir3N improves silencing of the most proximal URA3 gene (promoter at 2 kb; 5.9 × 10−1 ± 1.7 × 10−1 FOAR compared to 5.6 × 10−2 ± 0.8 × 10−2 FOAR of cells transformed with the vector alone). At 4 kb from the chromosomal end, URA3 repression is increased about 20-fold (5.9 × 10−3 ± 4.2 × 10−3 compared to 3.0 × 10−4 ± 3.76 × 10−4), and at 6 kb it is increased 10-fold (1.4 × 10−4 ± 1.8 × 10−4 compared to 1.6 × 10−5 ± 0.9 × 10−5), over the rate in control cells (Fig. 4). Although full-length Sir3p increases silencing more efficiently at the most internal site (4.6 × 10−3 ± 3.6 × 10−3 FOAR at 6 kb), the ability of Sir3N to promote propagation of silent chromatin is highly reproducible and statistically significant (Fig. 4). Other Sir proteins, such as Sir2p and Sir4p, do not extend silencing, although at low levels Sir2p can improve the efficiency of repression within the 4 kb of core heterochromatin (6).
FIG. 4.
Sir3N overexpression promotes TPE spreading. Strains UCC518, UCC520, and UCC522 were transformed with pADH (pAAH5), pADH-SIR3N, and pADH-SIR3 (p2μ-ASIR3 [26]). Colonies were grown for 3 to 5 days at 30°C on medium lacking leucine, to ensure maintenance of the plasmids, and resuspended in H2O, and 10-fold serial dilutions were plated onto medium lacking leucine (−leu) and medium lacking leucine and containing 1 mg of 5-FOA/liter (5-FOA −leu), as described in Materials and Methods. Two independent transformants for each case are shown. On the right is a schematic representation of the URA3-marked telomere of strain UCC518 (top; URA3 at 2 kb from the telomere), UCC520 (middle; URA3 at 4 kb), and UCC522 (bottom; URA3 at 6 kb). The frequency of resistance to 5-FOA was calculated from eight independent transformants, each scored in three independent assays. The means and standard deviations are given in the text.
Sir3N overexpression derepresses rDNA silencing.
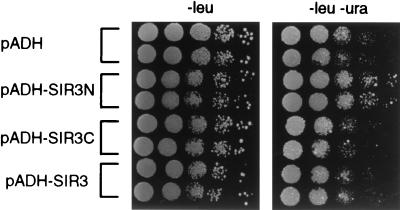
Recently it has been shown that the variegated expression of PolII genes inserted in the rDNA requires SIR2, but not SIR3 and SIR4 (4, 40). Consistently, in logarithmically growing wild-type cells, Sir2p, but not Sir3p and Sir4p, is found bound to the rDNA repeat within the nucleolus (10). Intriguingly, Sir3p becomes localized to the nucleolus in sir4 mutants and in aging cells (10, 22). In view of this, we next tested whether Sir3N affects the efficiency of silencing of a URA3 gene inserted at the rDNA (40), as assayed by the colony growth rate on medium lacking uracil. Wild-type cells form small, slow-growing colonies in the absence of uracil (Fig. 5, compare −leu with −leu −ura for pADH), while the same cells overexpressing Sir3N grow significantly faster (Fig. 5, pADH-SIR3N). This indicates derepression of the URA3 promoter in the rDNA, similar to the derepression observed when SIR2 is deleted (6, 40). Interestingly, overexpression of either full-length Sir3p or Sir3C does not lead to derepression of the URA3 reporter. We conclude that Sir3N is able to partially relieve transcriptional repression within the rDNA locus, achieving the opposite of its effect at telomeres.
FIG. 5.
Sir3N derepresses rDNA silencing. Strain JS231 carrying the URA3 gene inserted at the rDNA was transformed with plasmids pADH (pAAH5), pADH-SIR3N, pADH-SIR3C, and pADH-SIR3 (p2μ-ASIR3). Colonies were grown for 3 to 5 days at 30°C on medium lacking leucine, to ensure maintenance of the plasmids, and resuspended in H2O, and 10-fold serial dilutions were plated onto medium lacking leucine (−leu) and medium lacking leucine and uracil (−leu −ura), as described in Materials and Methods. Eight independent transformants for each plasmid were tested, of which two are shown.
Sir3N localizes to the nucleolus in a Sir2p-dependent manner.
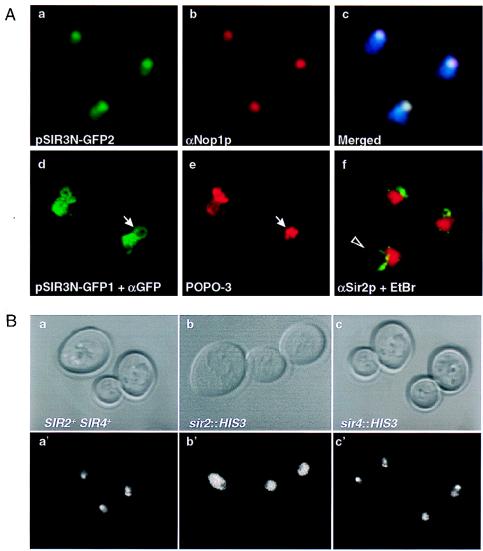
Does Sir3N act directly at both the rDNA and telomere-proximal sites, or are these effects indirect? To help address this question, we created a Sir3N C-terminal fusion with the green fluorescent protein, allowing us to follow the distribution of Sir3N in vivo. The fusion protein confers the same silencing phenotypes as Sir3N alone (data not shown). When carried on a 2μ plasmid (pSIR3N-GFP2), the fusion protein produces an intense but diffuse nuclear green fluorescence visible by direct fluorescence microscopy (Fig. 6A, view a). In many cells we can identify a portion of the nucleus that is significantly brighter than the rest of the nucleoplasm, reminiscent of the nucleolar staining observed for Sir2p (10). The fixed spheroplasts carrying pSIR3N-GFP2 were then stained with anti-Nop1 antibodies, producing red fluorescence (Fig. 6A, view b) which was superimposed on the green GFP fluorescence. The coincidence of the signals (Fig. 6A, view c) indicates that Sir3N-GFP is indeed enriched in the nucleolar compartment, although it is not excluded from the rest of the nucleoplasm.
FIG. 6.
(A) SIR3N-GFP localizes to the nucleolus. The haploid strain UCC3107 was transformed with either a 2μ-based plasmid (a, b, and c) or a centromeric plasmid (d and f) expressing Sir3N fused to the green fluorescent protein under the control of the ADH promoter (pSIR3N-GFP2 and pSIR3N-GFP1, respectively). The direct fluorescence of Sir3N-GFP (green) (a), anti-Nop1p staining on the same cells visualized with a Cy3-conjugated secondary antibody (red) (b), and the merge of the two stainings (c) are shown. The blue area represents the nucleus. Colocalization of Sir3N-GFP and Nop1p is white. Cells transformed with pSIR3N-GFP1 and stained with anti-GFP antibodies (the kind gift of K. E. Sawin, Imperial Cancer Research Fund, London, England) visualized by a DTAF-conjugated secondary antibody (green) (d); the DNA staining of the same cells with POPO-3, which preferentially stains the nucleolar domain (red) (e); and anti-Sir2p immunofluorescence on a fixed wild-type diploid strain (GA229) that had been washed in 1% Triton–0.02% sodium dodecyl sulfate as described previously (10) (f) are also shown. Sir2p staining is visualized by a DTAF-conjugated secondary antibody (green), and the DNA is counterstained with ethidium bromide. Immunofluorescence assays were performed with affinity-purified antibodies as described in Materials and Methods. The arrows indicate an apparent looped body, while the arrowhead indicates the same loop extended. (B) SIR2 but not SIR4 is necessary for the enrichment of Sir3N-GFP in the nucleolus. The haploid strains UCC3107 (SIR+) (a and a′), UCC3203 (sir2::HIS3) (b and b′), and UCC3207 (sir4::HIS3) (c and c′) were transformed with plasmid pSIR3N-GFP1. The phase-contrast image (a to c) and the direct fluorescence (a′, b′, and c′) of Sir3N-GFP are shown. Results identical to those shown in c and c′ were obtained for a sir3::HIS3 strain.
To see if Sir3N, like Sir2p, is associated with the rDNA (10), we used an anti-GFP antibody to detect the fusion protein expressed from a centromere plasmid (pSIR3N-GFP1). We observe diffuse green fluorescence in the nucleoplasm, but also along an apparently looped structure within the strongly POPO-3-stained nucleolus (Fig. 6A, views d and e). This loop was previously shown to be the rDNA of chromosome XII (13), and it stains brightly with anti-Sir2p antibodies (Fig. 6A, view f). When spheroplasts are gently lysed in detergents the rDNA loop extends, yet still maintains anti-Sir2p staining, consistent with cross-linking data that show that Sir2p precipitates with the rDNA repeats (Fig. 6A, view f). The fact that Sir3N-GFP produces a similar fluorescence both in living cells and in fixed spheroplasts suggests that Sir3N is also associated with rDNA.
We have previously shown that Sir3p accumulates in the nucleolus in a sir4 deletion strain, and that it requires both SIR2 and UTH4 gene products for this localization (10). To see if Sir2p and Uth4p are required for Sir3N localization, we scored for the localization of the Sir3N-GFP fusion expressed from a centromere plasmid in strains that lack either SIR2, SIR3, SIR4, or UTH4. The direct fluorescence of Sir3N-GFP is shown in Fig. 6B, below the phase images of the corresponding living yeast cells. Whereas the fluorescence is localized in a nucleolar subdomain in SIR+ strains, the signal is dispersed throughout the nucleoplasm in a sir2::HIS3 strain (Fig. 6B, views b and b′). On the other hand, in strains carrying gene disruptions for SIR4 (Fig. 6B, views c and c′), SIR3, or UTH4 (data not shown), Sir3N-GFP is still enriched in the nucleolus. Thus, the only known silencing factor required for the nucleolar accumulation of Sir3N is Sir2p. These results make it likely that derepression of the URA3 reporter in the rDNA repeat reflects a direct action of Sir3N.
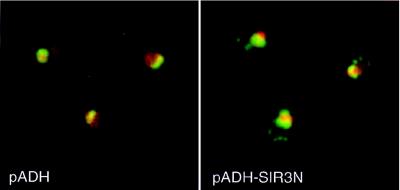
What happens to Sir2p when Sir3N accumulates in the nucleolus? We have recently observed that increasing the gene dosage of SIR2 by a single copy leads to increased telomeric silencing (reference 6; see Discussion below). This led to the hypothesis that Sir2p might be released from the rDNA upon Sir3N overexpression and therefore be free to increase silencing at telomeres. To test this possibility, we performed immunofluorescence assays with anti-Sir2p antibodies on yeast cells transformed with the vector alone or with the plasmid overexpressing Sir3N. Although the majority of the detectable Sir2p is found in the nucleolus in control cells, weak foci of staining can be detected at the nuclear periphery, colocalizing with telomeres (10, 43). When cells expressing Sir3N are stained with anti-Sir2p, we observe a higher fluorescent signal in the nucleoplasm and more intense telomeric spots than are observed in the pADH control (Fig. 7). By performing Western blotting on extracts from cells carrying either the pADH vector or pADH-SIR3N we can rule out the possibility that Sir2p levels increase in the Sir3N-overexpressing cells (data not shown). This and our immunofluorescence data are consistent with the idea that Sir3N overexpression causes a partial redistribution of Sir2p from the rDNA to telomeres, which could explain how Sir3N overexpression can have opposite effects on these two sites of repression.
FIG. 7.
Sir2p is enriched at telomeric foci upon overexpression of Sir3N. The wild-type haploid strain UCC3107, transformed with plasmids pADH (pAAH5) and pADH-SIR3N, was stained with anti-Sir2p, detected by a DTAF-conjugated secondary antibody (green). The DNA was counterstained with POPO-3 (red).
Overexpression of Sir3p and Sir4p restores telomeric silencing and telomeric foci.
The redistribution of Sir2p from the rDNA to telomeres cannot completely account for the effect of Sir3N at telomeres, since Sir2p overexpression does not extend silencing beyond 4 kb, which both Sir3p and Sir3N can do (Fig. 4) (6). Indeed, the many parallels between the effects of overexpressing either Sir3p or Sir3N suggested to us that perhaps Sir3N was able to upregulate or activate the nuclear pool of full-length Sir3p, resulting in phenotypes similar to those obtained when SIR3 is overexpressed. We could rule out the possibility that Sir3N increases the amount of Sir3p in the nucleus by performing Western blotting on cells carrying either the pADH vector or pADH-SIR3N. Within the twofold range of accuracy afforded by chemiluminescence, we conclude that there is no variation in total cellular Sir3p when Sir3N is overexpressed (data not shown).
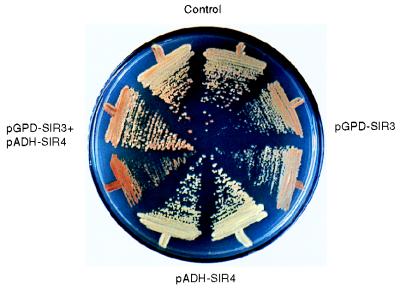
If Sir3N were to “activate” or allow recruitment of an inactive population of Sir3p, we would predict that the overexpression of full-length Sir3p would restore TPE in a strain overexpressing Sir4C, like Sir3N does. As shown in Figure 1c, this is indeed the case. Moreover, whereas raising the dosage of full-length Sir4p derepresses a telomere-proximal ADE2 gene, repression can be restored, and even enhanced above background levels, by balancing the higher levels of Sir4p with elevated levels of Sir3p (Fig. 8). This is visualized as an enhancement in the frequency of dark-red colonies when both pGPD-SIR3 and pADH-SIR4 are introduced into a strain carrying an ADE2-marked telomere (Fig. 8). These results demonstrate that the disruptive effect of Sir4C or Sir4p overexpression can be compensated for by increasing the amounts of Sir3p available for the assembly of silent chromatin.
FIG. 8.
Overexpression of full-length Sir3p restores silencing in a strain that overexpress full-length Sir4p. The strain UCC3107, which carries ADE2 adjacent to the VR telomere, was transformed with plasmids as indicated as well as a second control plasmid. The control plasmids are the backbone vector without SIR gene inserts, namely, pAAH5 and p2HG (see Materials and Methods). The plasmids with SIR gene inserts are pADH-SIR4 and pGPD-SIR3. Colonies (two independent transformants in each case) were streaked onto medium lacking histidine and leucine to ensure maintenance of the plasmids and allowed to grow for 3 to 5 days at 30°C. Following incubation, the plates were stored at 4°C for 1 week to enhance the pigmentation of the cells.
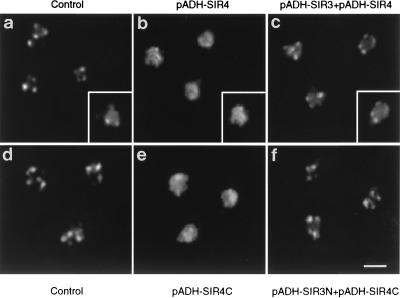
Loss of silencing correlates tightly with the delocalization of Rap1p and Sir proteins from telomeric foci (7, 9, 10, 31). We have previously shown that in strains overexpressing either Sir4C or full-length Sir4p telomeric silencing is relieved and Rap1p and Sir3p are delocalized from telomeric foci (7, 26). Here we show that the simultaneous overexpression of both Sir3N and Sir4C (Fig. 9f), like the simultaneous and balanced overexpression of full-length Sir3p and Sir4p, restores the punctate staining of full-length Sir3p (Fig. 9c). This correlates with the restoration of repression at telomeres. Similarly, Rap1p and Sir4p foci are restored under conditions of balanced overexpression (6). Western blot analysis confirms that the levels of Sir4p and Sir4C remain high when Sir3p or Sir3N are overexpressed, ruling out a trivial effect of Sir3N or Sir3p on SIR4 expression or protein stability (reference 26 and data not shown). Thus, we extend the tight correlation between the restoration of TPE and balanced levels of Sir3p and Sir4p. In view of this and the fact that all phenotypes reported here for Sir3N both mimic and depend on full-length Sir3p, we propose that Sir3N acts by enhancing the pool of functional Sir3p.
FIG. 9.
Overexpression of Sir3N restores the focal staining pattern of Sir3p. Yeast cells were stained with anti-Sir3p antibodies detected by a DTAF-conjugated secondary antibody (white signal). All signals are within the yeast nuclei, as indicated in the insets, where the anti-Sir3p signals are superimposed on a DNA stain to reveal the nuclear shape (see Materials and Methods). Strain EG37 (26) transformed with the vectors pAAH5 and pRS316 (a), pC-ASir4 (26) and pAAH5 (b), and pC-ASir4 and p2μ-ASir3 (c) and strain AJL275-2AVIIL transformed with the vectors pAAH5 and p2HG (d), pADH-SIR4C (pFP340) and pAAH5 (e), and pADH-SIR4C (pFP340) and pADH-SIR3N (f) are shown.
DISCUSSION
Here we show that expression of the N-terminal 503 aa of Sir3p in an otherwise wild-type strain counteracts the derepression provoked by Sir4C overexpression. Moreover, expression of Sir3N alone improves and extends telomere-proximal repression. Both these effects require the presence of full-length Sir3p and can also be achieved by overexpression of full-length Sir3p. As with Sir3p, the Sir3N-dependent improvement of silencing requires SIR2 and SIR4 and the histone N-termini, although not SIR1. Thus, repression by Sir3N is not achieved by bypassing the normal pathway of telomere-proximal repression, nor does it function by targeting Sir1p to telomeric sites.
Surprisingly, we were unable to detect any interaction between Sir3N and the well-characterized components of the repression machinery; that is, Sir3N cannot dimerize, nor can we detect Sir3N interaction with either Sir2p, Sir4p, Rap1p, or the N termini of histones H3 and H4 by two-hybrid and/or coimmunoprecipitation assays (references 6, 12a, 15, and 38a and data not shown). In contrast, interaction between the C-terminal domain of Sir3p and the last four components has been well characterized (Fig. 2) (6, 15, 16, 29, 43). Indeed, the presence of multiple binding sites in the Sir3 C-terminal domain for components of repressed chromatin is consistent with its dominant-negative effect on TPE. The fact that the overexpression of Sir3N and that of Sir3C have opposite effects on silencing is consistent with the notion that they interact with different subsets of proteins.
There is conflicting data in the literature as to whether Sir2p interacts directly with Sir3p (28, 43). However, it is clear that Sir2p does not coimmunoprecipitate with Sir3p in strains with SIR4 deleted (43), and in our hands no Sir2p interactions could be detected with either the Sir3N or Sir3C termini in two-hybrid assays. It is possible that this is a regulated interaction or one requiring another factor.
Alternative models for Sir3N-dependent enhancement of repression.
The Sir3N fragment alone accumulates in the nucleolus and appears to provoke the release or relocalization of a fraction of Sir2p from the nucleolus to telomeric sites (see below). This effect cannot, however, account for the extension of silencing observed upon Sir3N overexpression, since increased Sir2p expression only improves repression up to 4 kb from the telomeric repeat (6). One simple explanation for the Sir3N-induced phenotypes would be that Sir3N expression results in elevated levels of Sir3p, and perhaps also of Sir2p, in the cell. However, quantitative Western blots show no significant variation in either of these components when Sir3N is overexpressed. A second possibility is that Sir3N itself can promote the propagation of silent chromatin. Recently, Strahl-Bolsinger and colleagues (16, 43) have shown that transcriptionally inert regions of the yeast genome can have two distinct forms. One of these, present as the “core” heterochromatin at telomeres in wild-type cells, contains the three Sir proteins and Rap1p as structural components of the repressed chromatin. The second type, which is induced by overexpression of Sir3p, can only be generated as an extension from a preexisting domain of the core heterochromatin but appears to require only the propagation of a Sir3p-histone complex (43). The N-terminal 503 aa of Sir3p may be able to promote propagation of a repressed chromatin structure from a preassembled core. However, this would suggest that the propagation can occur without direct interaction with histone N termini, since Sir3N is lacking the domain that binds histone tails. Moreover, it would require that Sir3N interact with other components of telomeric chromatin, which was not observed.
In view of the similarity of the effects at telomeres provoked by overexpression of Sir3N and Sir3p, we favor a third model, in which Sir3N increases the pool of full-length Sir3p available to the repression machinery. This might be achieved by exerting an allosteric effect on Sir3p, which would improve its ability to interact with other components of the silencing machinery. In its simplest form, this mechanism would imply a physical interaction between Sir3N and Sir3C; however, this could not be detected by a two-hybrid assay. Alternatively, the pool of active full-length Sir3p could be increased by releasing or activating a subpopulation of Sir3p that is normally sequestered in a silencing-incompetent form. This may reflect interaction with an unidentified third component which binds Sir3p through its N terminus, or it could reflect activation of Sir3p by posttranslational modification or a conformational change. Results currently cannot distinguish between these possibilities.
Genetic evidence supports the model in which the N terminus of Sir3p activates its C-terminal domain, namely, the N-terminal mutations SIR3R3 and SIR3R1, which suppress mutations in the Rap1 C terminus and in histone N termini (18, 24). In both cases, the mutations suppressed by SIR3R3 are expected to weaken interaction between either Rap1p or the histone N termini and the Sir3 C terminus (7, 15, 25). Thus, one explanation for the suppression data is that these Sir3 N-terminal mutations activate full-length Sir3p by improving the ability of Sir3C to bind other silencing factors, such as Rap1p and the histone tails. Consistently, Park et al. have shown that a central region of Sir3p inhibits the silencing initiation function of the C-terminal 144 aa, which, however, can be overcome by Sir3N (31a).
Sir3p is a target of regulatory mechanisms.
The ability of Sir3N and Sir3p overexpression to compensate for the derepression provoked by Sir4C and Sir4p overexpression underscores how important the balance between these factors is for proper transcriptional control. Consistently, Sir3p appears to be the target of multiple regulatory mechanisms, including one which may involve the sequestering of the full-length protein in an inactive form. Our model would suggest that overexpression of Sir3N overcomes this. We do not know whether this requires a yet-unidentified Sir3p ligand, but if there is a significant pool of full-length Sir3p that is not participating in a silencing complex, then it is likely to be localized at telomeric foci, since immunofluorescence does not reveal a significant fraction of Sir3p elsewhere in the nucleus (Fig. 9).
Recently it was shown that Sir3p is a phosphoprotein and that hyperphosphorylation by a mitogen-activated protein (MAP) kinase pathway leads to increased silencing (42). Interestingly, although the phosphorylation sites have not yet been mapped, nearly all consensus sites for MAP kinases (minimal consensus as PT/S or T/SP) are found in the N-terminal half of Sir3p, suggesting another means by which this domain might regulate the activity of full-length Sir3p. As well as being phosphorylated itself, Sir3N might target kinases to other components of the silencing complex to regulate silencing efficiency, protein assembly, or turnover.
Competition between domains for a limiting amount of Sir2p.
Although Sir3N improves telomeric repression, it antagonizes repression of a URA3 reporter gene inserted in the rDNA repeats (4, 8, 40). This repression, as well as the suppression of recombination between rDNA repeats, is mediated by Sir2p, which is associated with the rDNA (4, 10, 11, 22, 40). Upon deletion of SIR3 or SIR4 or overexpression of SIR2, silencing at the rDNA is increased, suggesting that there is a competition between the rDNA and telomeres for a limiting amount of Sir2p (40). Indeed, a moderate increase in Sir2p dosage also leads to increased repression at telomeres (6). Our data are consistent with the idea that overexpression of Sir3N causes the release or displacement of some fraction of the nucleolar Sir2p from the rDNA, leading to partial derepression of URA3 and enhanced repression at telomeres. Consistent with this hypothesis, telomeric foci, as detected by anti-Sir2p antibodies, are more intense when Sir3N is expressed than they are in the control (Fig. 7). This result corroborates increasing evidence that the different loci at which silencing occurs (HM loci, telomeres, and rDNA) compete for limiting amounts of silencing factors (5, 6, 14, 26).
The interference of Sir3N in the repression pathway of rDNA may reflect competition between Sir3N and Sir2p for a common third factor. Since rDNA repression is poorly characterized on a molecular level, it is impossible to say which nucleolar elements might be influenced by Sir3N. ORC subunits, Abf1p, Reb1p, and nucleosomes are all possible candidates (2, 19). Moreover, since a significant pool of Sir3N is found dispersed in the nucleoplasm, we cannot rule out the possibility that this domain acts indirectly to influence repression. The fact that Sir3N has opposite effects on TPE and rDNA repression supports the hypothesis that the two types of silencing, although related, make use of different molecular mechanisms.
The Sir3N terminus is highly enriched in the nucleolar compartment. We and others have previously found that the relocalization of Sir3p and Sir4p to the nucleolus occurs both in old mother cells and in mutants that show suppression of precocious-aging phenotypes (22, 39). The relocation of Sir3p requires Sir2p and the product of UTH4, a gene also implicated in yeast life span determination (22). The data presented here indicate that the first 503 aa of Sir3p contains information necessary and sufficient for nucleolar targeting. Since the nucleolar localization of Sir3N, unlike that of Sir3p, does not require Uth4p, it appears that Uth4p overcomes a negative element that impedes the nucleolar targeting of full-length Sir3p. Sir3N and Sir3p, on the other hand, both require Sir2p for their accumulation in the nucleolus, indicating that the presence of Sir2p itself provides or creates a binding site for Sir3N and Sir3p.
Whether Sir3N, Sir3p, and/or Sir4p has a unique function in the nucleolus is unclear. Although deletion of either SIR3 or SIR4, like deletion of SIR2, shortens life span, only deletion of SIR2 affects recombination rates and PolII expression in the rDNA (4, 11, 40). Additional studies are needed to determine why Sir2p is necessary for the nucleolar accumulation of Sir3N and what Sir3N and Sir3p achieve in the nucleolus. Although we are unable to detect Sir2p-Sir3N interaction by two-hybrid or coimmunoprecipitation assays (43), this does not conclusively rule out an interaction. Novel approaches are clearly required to resolve both this question and the mechanism by which Sir3N activates the Sir3p holoprotein.
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of T. Laroche. We thank K. Sawin for the anti-GFP antibodies, Ed Hurt for anti-Nop1 antibodies, H. Renauld for strains, and M. Cockell, H. Renauld, D. Shore, A. Lustig, and M. Grunstein for allowing us to cite unpublished results and for helpful discussions.
M.G. thanks ISREC for a Ph.D. fellowship. F.P. was supported by the Human Frontiers Science Program. Research in the Gasser laboratory is supported by the Swiss National Science Foundation, the Human Frontiers Science Program, and the Swiss League against Cancer.
REFERENCES
- 1.Aparicio O M, Billington B L, Gottschling D E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 2.Bell S P, Mitchell J, Leber J, Kobayashi R, Stillman B. The multidomain structure of Orc1p reveals similarity to regulators of DNA replication and transcriptional silencing. Cell. 1995;83:563–568. doi: 10.1016/0092-8674(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 3.Boeke J D, Trueheart J, Natsoulis G, Fink G R. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 4.Bryk M, Banerjee M, Murphy M, Knudsen K E, Garfinkel D J, Curcio M J. Transcriptional silencing of Ty elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- 5.Buck S W, Shore D. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 1995;9:370–384. doi: 10.1101/gad.9.3.370. [DOI] [PubMed] [Google Scholar]
- 6.Cockell, M., M. Gotta, F. Palladino, S. G. Martin, and S. M. Gasser. Targeting limiting pools of Sir proteins to sites of action: a general mechanism for regulated repression. Cold Spring Harbor Symp. Quant. Biol., in press. [DOI] [PubMed]
- 7.Cockell M, Palladino F, Laroche T, Kyrion G, Liu C, Lustig A J, Gasser S M. The carboxy termini of Sir4 and Rap1 affect Sir3 localization: evidence for a multicomponent complex required for yeast telomeric silencing. J Cell Biol. 1995;129:909–924. doi: 10.1083/jcb.129.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritze C E, Verschueren K, Strich R, Esposito R E. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, Gasser S M. The clustering of telomeres and colocalization with Rap1, Sir3 and Sir4 proteins in wild-type Saccharomyces cerevisiae. J Cell Biol. 1996;134:1349–1363. doi: 10.1083/jcb.134.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotta M, Strahl-Bolsinger S, Renauld H, Laroche T, Kennedy B K, Grunstein M, Gasser S M. Localization of Sir2p: the nucleolus as a compartment for silent information regulators. EMBO J. 1997;16:3243–3255. doi: 10.1093/emboj/16.11.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb S, Esposito R E. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 12.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 12a.Grunstein, M. Personal communication.
- 13.Guacci V, Hogan E, Koshland D. Chromosome condensation and sister chromatid pairing in budding yeast. J Cell Biol. 1994;125:517–530. doi: 10.1083/jcb.125.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy C F, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 15.Hecht A, Laroche T, Strahl-Bolsinger S, Gasser S M, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 16.Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- 17.Ivy J M, Klar A J S, Hicks J B. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:688–702. doi: 10.1128/mcb.6.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson L M, Kayne P S, Kahn E S, Grunstein M. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1990;87:6286–6290. doi: 10.1073/pnas.87.16.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J J, Yokoi T J, Holland M J. Binding sites for abundant nuclear factors modulate RNA polymerase I-dependent enhancer function in Saccharomyces cerevisiae. J Biol Chem. 1995;270:28723–28732. doi: 10.1074/jbc.270.48.28723. [DOI] [PubMed] [Google Scholar]
- 20.Karpen G H. Position-effect variegation and the new biology of heterochromatin. Curr Opin Genet Dev. 1994;4:281–291. doi: 10.1016/s0959-437x(05)80055-3. [DOI] [PubMed] [Google Scholar]
- 21.Kayne P S, Kim U J, Han M, Mullen J R, Yoshizaki F, Grunstein M. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell. 1988;55:27–39. doi: 10.1016/0092-8674(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy B K, Gotta M, Sinclair D A, Mills K, McNabb D S, Murthy M, Pak S M, Laroche T, Gasser S M, Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension in life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 23.Kyrion G, Liu K, Liu C, Lustig A J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Lustig A J. Genetic analysis of Rap1p/Sir3p interactions in telomeric and HML silencing in Saccharomyces cerevisiae. Genetics. 1996;143:81–93. doi: 10.1093/genetics/143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Mao X, Lustig A J. Mutational analysis defines a C-terminal tail domain of RAP1 essential for telomeric silencing in Saccharomyces cerevisiae. Genetics. 1994;138:1025–1040. doi: 10.1093/genetics/138.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maillet L, Boscheron C, Gotta M, Marcand S, Gilson E, Gasser S M. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 1996;10:1796–1811. doi: 10.1101/gad.10.14.1796. [DOI] [PubMed] [Google Scholar]
- 27.Marshall M, Mahoney D, Rose A, Hicks J B, Broach J R. Functional domains of SIR4, a gene required for position effect regulation in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:4441–4452. doi: 10.1128/mcb.7.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moazed D, Kistler A, Axelrod A, Rine J, Johnson A D. Silent information regulator protein complexes in Saccharomyces cerevisiae: a SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci USA. 1997;94:2186–2191. doi: 10.1073/pnas.94.6.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- 30.Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 31.Palladino F, Laroche T, Gilson E, Axelrod A, Pillus L, Gasser S M. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993;75:543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- 31a.Park, Y., J. Hanish, and A. J. Lustig. Sir3p domains involved in the initiation of telomeric silencing in Saccharomyces cerevisiae. Genetics, in press. [DOI] [PMC free article] [PubMed]
- 32.Pillus L, Grunstein M. Chromatin structure and epigenetic regulation in yeast. In: Elgin S C R, editor. Chromatin structure and gene expression. Oxford, England: IRL press; 1995. pp. 123–146. [Google Scholar]
- 33.Renauld H, Aparicio O M, Zierath P D, Billington B L, Chhablani S K, Gottschling D E. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 1993;7:1133–1145. doi: 10.1101/gad.7.7a.1133. [DOI] [PubMed] [Google Scholar]
- 33a.Renauld, H. Unpublished data.
- 34.Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose M D, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 36.Roy A, Exinger F, Losson R. cis- and trans-acting regulatory elements of the yeast URA3 promoter. Mol Cell Biol. 1990;10:5257–5270. doi: 10.1128/mcb.10.10.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 38a.Shore, D. Personal communication.
- 39.Sinclair D A, Mills K, Guarente L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science. 1997;277:1313–1316. doi: 10.1126/science.277.5330.1313. [DOI] [PubMed] [Google Scholar]
- 40.Smith J S, Boeke J D. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 41.Sprague G F. Assay of yeast mating reaction. Methods Enzymol. 1991;194:77–93. doi: 10.1016/0076-6879(91)94008-z. [DOI] [PubMed] [Google Scholar]
- 42.Stone E M, Pillus L. Activation of a MAP kinase cascade leads to Sir3p hyperphosphorylation and strengthens transcriptional silencing. J Cell Biol. 1996;135:571–583. doi: 10.1083/jcb.135.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strahl-Bolsinger S, Hecht A, Kunheng L, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 1997;11:83–93. doi: 10.1101/gad.11.1.83. [DOI] [PubMed] [Google Scholar]
- 44.Sussel L, Vannier D, Shore D. Epigenetic switching of transcriptional states: cis- and trans-acting factors affecting establishment of silencing at the HMR locus in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:3919–3928. doi: 10.1128/mcb.13.7.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson J S, Ling X, Grunstein M. Histone H3 amino terminus is required for telomeric and silent mating locus repression in yeast. Nature. 1994;369:245–247. doi: 10.1038/369245a0. [DOI] [PubMed] [Google Scholar]
- 46.Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 47.Tsukamoto Y, Kato J, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–903. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- 48.Weiler K S, Wakimoto B T. Heterochromatin and gene expression in Drosophila. Annu Rev Genet. 1995;29:577–605. doi: 10.1146/annurev.ge.29.120195.003045. [DOI] [PubMed] [Google Scholar]