Abstract
Aging is a natural skin process that occurs due to intrinsic and extrinsic factors, such as excessive exposure to ultraviolet light (photoaging). The mechanism of damage involves the production of excess free radicals that trigger oxidative stress in the skin. Determining the natural products that have high antioxidant activities as antiaging is important. Cinnamomum burmannii and Michelia champaca are typical Aceh plants that are believed to have high antioxidant effects. The aim of this study was to determining the contents of C. burmannii and M. champaca as well as to determine the antioxidant and antiaging activities of either individually or combinations. The qualitative phytochemical and semi-quantitative analysis of the extracts were measured using gas chromatography-mass spectroscopy (GC-MS). The antioxidant activity was examined by radical scavenging using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical method while the antiaging activity was measured using the tyrosinase enzyme inhibition test. The phenolic and flavonoid contents of C. burmannii were higher than M. champaca (66.34 vs 24.71 mg gallic acid equivalent/gr and 80.52 vs 60.20 mg quercetin equivalent/gr, respectively. The inhibitory concentration (IC50) of M. champaca extract in inhibiting DPPH indicated that M. champaca had a better antioxidant activity than C. burmannii. The combination of C. burmannii and M. champaca extracts had a lower IC50 compared to M. champaca alone. C. burmannii and M. champaca extracts had a weak potential to inhibit tyrosinase activity (IC50 value ≥1000 μg/mL). In conclusion, this study indicates that M. champaca and C. burmannii have strong antioxidant activities and these might associate with polyphenol contents.
Keywords: Antioxidant, antiaging, champaca, cinnamon, flavonoid, anti-tyrosinase
Introduction
Aging is one of the main problems for individuals in middle age or older. Aging process causes the physical appearance to become less attractive and lowering self-confidence [1]. The process of skin aging involves deterioration in the structure and function of the skin system and this process is associated with the phenomenon of slowing down or stopping the process of skin growth with age [2]. One of the extrinsic factors that cause aging is exposure to sunlight (photo-aging), which results from reactions involving free radicals that trigger oxidative stress in the skin [3]. Free radicals damage the phospholipid membrane and reduce elasticity, making the skin look wrinkled and dry, which are the main signs of aging [4].
Reactive oxygen species (ROS) have been found to significantly increase tyrosinase activity, the enzyme that initiates skin pigmentation, leading to the upregulation of melanin synthesis [5]. The tyrosinase catalyzes the rate-limiting reaction in the melanin biosynthetic pathway, where L-tyrosine is hydroxylated to L-3,4-dihydroxyphenylalanine (L-DOPA), for further oxidized to o-quinone. Hydrogen peroxide (H2O2) and ROS which are produced by melanogenesis leads to exposing human melanocytes to high levels of oxidative stress [6] Therefore, it has been a trend recently to use antioxidants to protect the skin from the damaging effects of ultraviolet (UV) [7]. ROS scavengers and inhibitors of ROS generation may down-regulate UV-induced human melanogenesis [8]. Moreover, tyrosinase is also the main target in screening inhibitors for melanogenesis.
The discovery of antioxidants and anti-tyrosinase drugs that inhibit the catalytic activity of tyrosinase has received much attention, in particular in skin care products [9]. Study on the development of therapies to address the problem of premature ageing is urgently needed, one of which is by utilizing extracts of natural ingredients. Some of the compounds found in plants that can protect the skin include flavonoids, phenolics, and tannins. Flavonoids have strong antioxidant properties due to their ability to stabilize free radicals by donating hydrogen atoms or single electron transfers. [10,11]. Flavonoids also act as chelators for metals by incorporating copper ions from tyrosinase activity. Recent studies reported the significance of flavonoids as a promising whitening agent by inhibiting the tyrosinase enzyme, thereby controlling melanin production and having a low level of toxicity [5,12].
Various secondary metabolites in plants are synergistic in controlling multiple metabolism pathways in the body [13]. Flavonoids have the potential as potent antioxidants due to they have hydroxyl groups attached to the carbon of the aromatic ring, which can capture free radicals resulting from lipid peroxidation reactions to stabilize free radicals or ROS [14,15]. Michelia champaca and Cinnamomum burmannii are typical Aceh plants rich in flavonoids. M. champaca is often used as a medicinal plant for healing wounds and its metabolites such as gallic acid, phenolic, tannins, and essential oils have antioxidant activities [16,17]. These compounds exert antioxidant actions through their redox properties, which counteract and neutralize free radicals and decompose peroxide compounds [18]. The fragrant aroma of M. champaca is convenient to develop as a skin topical moisturizer and perfume.
C. burmannii is also extensively used by the industries of food, cosmetic, and pharmaceutical [19]. Metabolic compounds of C. burmannii such as cinnamaldehyde, eugenol, phenolic group, trans-cinnamic acid, tannins, catechins, proanthocyanidin, limonene, and α-terpineol also act as antioxidants [20]. Several studies reported the antioxidant activity of Indonesian C. burmannii extract from various regions and had inhibitory concentration (IC50) value of around 75.48–136.88 μg/mL examined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical method [21-24].
It is suggested that combination of C. burmannii and M. champaca extracts could be developed as ingredients for skin cosmetics [20]. The abundant availability of M. champaca and C. burmannii, fragrant aromas and empirical evidence in the community support the development of these two plant extracts as additives for skin care products. The aim of this study was to investigate the role of flavonoid extracts of M. champaca and C. burmannii as antiaging and antioxidant.
Methods
Plants and chemicals
C. burmannii were collected from plantations located at Simpang Teritit, Bener Meriah, Aceh, Indonesia and M. champaca were collected from Aceh Besar, Aceh, Indonesia. DPPH, L-ascorbic acid, gallic acid, quercetin, Folin-Ciocalteu reagent, Tween 80, L-Dopa, tyrosinase, dimethyl sulfoxide (DMSO), kojic acid were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Other used chemicals and reagents were all analytical grade and were purchased as indicated.
Extraction process
A total of 500 of C. burmannii and 500 g of M. champaca leaves were dried without direct exposure to sunlight and were crushed into fine powder. The fine powder was macerated in 70% ethanol with a ratio of extract and solvent of 1:10 for 24 h with three times maceration. The macerate was made into a viscous extract using a pressurized rotary evaporator. After the thick ethanol extracts of C. burmannii and M. champaca were obtained, the water content, water-soluble extract, ethanol-soluble extract, and ash content were measured. Furthermore, the extracts were subjected to qualitative phytochemical and semi-quantitative analysis using gas chromatography-mass spectroscopy (GC-MS) [25].
Gas chromatography-mass spectroscopy (GC-MS)
The phytochemical analysis of M. champaca and C. burmannii was conducted using a GC-MS Shimadzu - QP2010 Ultra (Shimadzu Corporation, Kyoto, Japan). The helium was used as carrier gas at a 1.2 mL/min flow rate. The gas chromatography column was Rtx-5MS (Shimadzu Corporation, Kyoto, Japan). The injector port was maintained at 270°C, with an oven temperature of 10°C/min from 30°C to 300°C. A total of 90 μL of extract was added with 10 μL of trimethyl silane hydrogen (TMSH), and 1 μL of the mixture was injected into the GC-MS. The metabolites were defined by comparing their mass spectra data with available database.
Experimental design
There were five experimental groups in this study: C. burmannii extract only, M. champaca extract only and three concentration combinations of the two extracts. Antioxidant and antiaging activities were determined on all extract groups and compared with positive and negative controls. The detailed of the groups as follows:
Negative control : solvent extract only (ethanol 70%)
Positive control : ascorbic acid (for antioxidant) and kojic acid (for antiaging)
CB group : C. burmannii extract
MC group : M. champaca extract
CB25MC75 group : combination of C. burmannii 25% + M. champaca 75% extract
CB50MC50 group: combination of C burmannii 50% + M. champaca 50% extract
CB75MC25 group : combination of C. burmannii 75% + M. champaca 25% extract.
Antioxidant and antiaging activities were analyzed three times (triplo).
Determination of total phenol content and total flavonoid content
To determine the total phenol content, the Folin-Ciocalteu reagent was used [7]. The mixture contained 100 μL extracts, 500 μL Folin-Ciocalteu reagent, and 1.5 mL 20% sodium carbonate. With pure water, the final volume was increased to 10 mL. The absorbance at 765 nm was measured after 2 h of reaction and used to calculate the phenolic content using gallic acid as a standard and therefore expressed as mg gallic acid equivalent (GAE)/g dry extract.
The total flavonoid content was measured. Briefly, an aliquot of 500 μL of each extract was diluted with 3.2 mL of water. Initially, 150 μL 5% NaNO2 solution was added to each sample, and after 5 min, 150 μL of 10% AlCl3 was added. After six minutes incubation, 1 mL 1.0M NaOH 1 M was added and mixed well. The absorbance was read at a wavelength of 510 nm, while quercetin was used as the standard for the calibration curve. The total content of flavonoid was calibrated using the calibration curve-based linear equation, and was stated as mg quercetin equivalent (QE)/g dry extract.
Antioxidant activity test with 2,2-diphenyl-1-picrylhydrazyl (DPPH)
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) replicates the previous study’s radical scavenging activity [26]. In brief, 50 μL extract with various concentrations (2, 4, 8, 16, 32 ppm) were mixed with 1 mL DPPH 0.4 mM and 3,950 μL ethanol. The mixture was then vortexed and left for 30 min. The absorbance of the solution was then measured at a wavelength of 517 nm against a blank (which consisted of 50 μL extract and 4,950 μL ethanol). The absorbance of the control, which consisted of 1 mL DPPH and 4 mL ethanol, was also measured. Vitamin C was used as a positive control. The concentration that gave an inhibitory concentration 50 (IC50) value was the concentration of the fraction that gave percentage radical scavenging activity by 50% compared to the control via a linear regression curve with the formula: Percent (%) radical capture =[(A0-A1)]÷A0×100%. A0 is the absorbance of the control (not containing the tested extract), and A1 is the absorbance in the presence of the test sample or reference (vit C).
Antiaging test through inhibition of tyrosinase activity
The activity of inhibiting the tyrosinase enzyme using the spectrophotometric method was used to measure the antiaging base on previous studies [27,28]. The l-3,4-dihydroxyphenylalanine (L-DOPA) and kojic acid were used as the substrate and positive control, respectively. Each extract was prepared with a concentration series of 50–1000 μg/mL and 30 μL extract was mixed with 125 μL phosphate buffer (0.1 M, pH 6.8) and 5 μL tyrosinase enzyme (2500 units/mL). After 30 min incubation at 37°C, 40 μL L-DOPA (2.5 mM) was added. The absorbance was observed at a wavelength of 515 nm, and measurements were also made for the solvent blank and the kojic acid as positive control. The inhibition percentage was obtained from the linear regression equation y = a + bx, where a and b are values from linear equation, x is the extract concentration and y is the % inhibition.
Statistical analysis
The antioxidant activity data and tyrosinase inhibition were articulated as the mean ± standard deviation (SD). One-way analysis of variance (Anova) was used to assess the differences between groups. Any differences between extract groups and control groups were assessed using the least significant difference (LSD) test. A p<0.05 was considered significant. All analyses were conducted using SPSS statistics for Windows, Version 20.0 (IBM Corp, NY, USA).
Results
Extract characterization
The C. burmannii extract was reddish-brown and viscous, while that of M. champaca was dark green and thick. The characteristics of the two extracts are presented in Table 1.
Table 1. Characteristics of the extracts of Cinnamomum burmannii and Michelia champaca.
| Parameter | Cinnamomum burmannii | Michelia champaca |
|---|---|---|
| Water content | 1.03% | 1.08% |
| Ash Content | 4.23% | 9.84% |
| Water soluble content | 64.77% | 66.37% |
| Ethanol soluble content | 36.75% | 48.24% |
| Phytochemical analysis | Alkaloids, flavonoids, polyphenols, tannin, quinones, saponins, and triterpenoids | Alkaloids, flavonoids, polyphenols, tannin, quinones, saponins, and steroids |
Total phenol content and total flavonoid content
This study quantitatively analyzed the levels of total phenol and flavonoid of the two extracts (Table 2). The total phenolic content in the extracts of C. burmannii and M. champaca was 66.34 mg GAE/g and 24.71 mg GAE/g, respectively. This mean that the content of total phenolic of every gram of C. burmannii extract was equivalent to 66.34 mg of gallic acid. The total flavonoid content in the extracts of C. burmannii and M. champaca was 80.52 mg QE/g and 60.20 mg QE/g, respectively.
Table 2. Quantitative analysis of the flavonoids, phenols, and total tannins levels in the extracts of Cinnamomum burmannii and Michelia champaca.
| Extract | Phenolic content (mg gallic acid equivalent (GAE)/gr) | Flavonoid content (mg quercetin equivalent (QE)/gr) |
|---|---|---|
| Cinnamomum burmannii | 66.34 | 80.52 |
| Michelia champaca | 24.71 | 60.20 |
Gas chromatography-mass spectroscopy (GC-MS) results
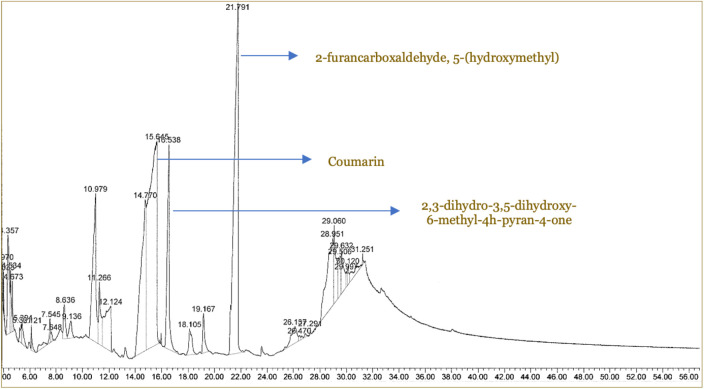
Analysis of semi-quantitative using GC-MS was conducted on the extracts of C. burmannii and M. champaca. The most abundant putative compounds in C. burmannii extract were 2-furan carboxaldehyde, 5-(hydroxymethyl), coumarin and 2,3-dihydro-3,5-dihydroxy-6-methyl-4h-pyran-4-one, with 35.17%, 19.34%, and 7.13% respectively (Figure 1). Detailed detected putative compounds, composition of metabolite in extract, and retention time are presented in Table 3.
Figure 1. Gas chromatography and mass spectroscopy (GC-MS) pattern of chemical compounds from Cinnamomum burmannii extract.
Table 3. The putative compounds of Cinnamomum burmannii based on gas chromatography and mass spectroscopy (GC-MS).
| Putative compound | Retention time (minute) | Percentage composition (%) |
|---|---|---|
| α-furole | 4.356 | 3.65 |
| 2,3 dihydro-3-5-dihydroxy-6-methyl-4H-pyran-4-one | 10.975 | 7.13 |
| Cinnamaldehyde | 11.265 | 4.92 |
| Glycerin | 12.127 | 4.19 |
| 2-furancarboxaldehyde-5-(hydroxymethyl-) | 15.643 | 35.17 |
| Cinnamyl alcohol | 16.540 | 6.43 |
| Imidazole,2-hydrxy-4-methyl- | 18.105 | 1.21 |
| Hydrocoumarin | 19.167 | 1.26 |
| Coumarin | 21.794 | 19.34 |
| 3,4-altrosan | 26.138 | 2.49 |
| 2-amino-5-guanidino-pentanoic acid | 28.951 | 6.52 |
| N-acetyl-D, L-norleucenine | 29.061 | 3.43 |
| D-glycero-D-galacto-heptose | 29.503 | 2.63 |
| 1,5-anhydro-D-lalitol | 29.643 | 1.63 |
| 100 |
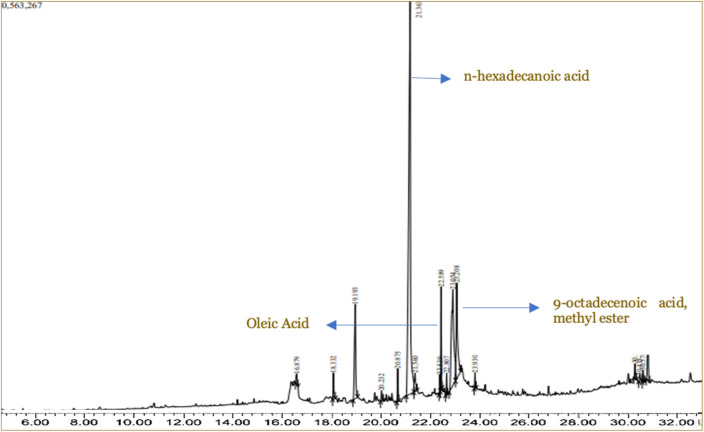
The n-hexadecanoic acid was the most common in the M. champaca extract, which was 38.23% (Figure 2). All putative compounds of M. champaca which detected based on GC-MS are presented in Table 4.
Figure 2. Gas chromatography and mass spectroscopy (GC-MS) pattern of chemical compounds from Michelia champaca extract.
Table 4. The putative compounds of Michelia champaca based on gas chromatography and mass spectroscopy (GC-MS).
| Putative compound | Retention time (minute) | Percentage composition (%) |
|---|---|---|
| Dodecanamide, n,n-bis(2-hydroxyethyl)- | 16.879 | 1.07 |
| 1(2h)-naphthalenone, octahydro-4a,8a-dim | 18.332 | 2.55 |
| Tetradecanoic acid | 19.193 | 9.18 |
| 9-octadecenoic acid (z)-(cas) oleic acid | 20.232 | 1.00 |
| Hexadecanoic acid, methyl ester (cas) me | 20.875 | 3.23 |
| N-hexadecanoic acid | 21.363 | 38.23 |
| Hexadecanoic acid, ethyl ester (cas) ethyl | 21.560 | 1.82 |
| 9,12-octadecadienoic acid (z,z)-, methyle | 22.539 | 1.76 |
| 9-octadecenoic acid, methyl ester, (e)- | 22.589 | 10.02 |
| Methyl stearate | 22.807 | 2.11 |
| Oleic acid | 23.054 | 9.33 |
| Octadecanoic acid | 23.208 | 9.29 |
| Hexadecanoic acid, 2-hydroxy-1-(hydroxym | 23.930 | 1.53 |
| Stigmasta-5,22-dien-3-ol, acetate, (3.beta.,2 | 30.254 | 1.44 |
| Stigmast-5-en-3-ol, (3.beta.)-(cas) 24.be | 30.435 | 0.59 |
| Cholesta-4,6-dien-3-ol, (3.beta.)- | 30.571 | 1.16 |
| Stigmast-5-en-3-ol, oleat | 30.765 | 5.68 |
| Total | 100 |
Antioxidant activity of C. burmannii and M. champaca
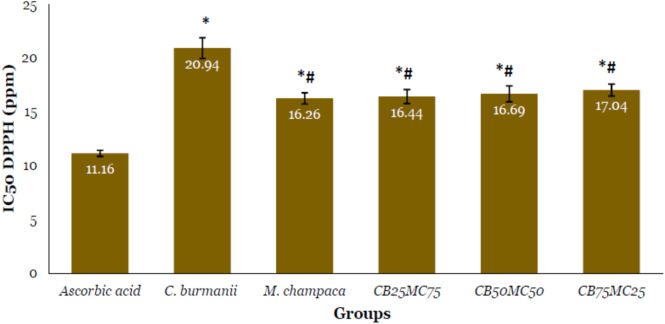
Antioxidant test using in vitro DPPH method indicated that the extracts of C. burmannii and M. champaca had strong antioxidant abilities. The IC50 of M. champaca extract in inhibiting DPPH was lower, indicating that M. champaca had a better antioxidant ability than C. burmannii (Figure 3). The combination of M. champaca and C. burmannii extracts showed a more significant IC50, suggesting that the combination with C. burmannii reduced the antioxidant capacity of M. champaca extract.
Figure 3. Antioxidant activity of Cinnamomum burmannii and Michelia champaca extracts based on scavenging radical of DPPH test. Data in mean ± standard deviation with three replications. CB25MC75: C. burmannii 25% and M. champaca 75%; CB50MC50: C. burmannii 50% and M. champaca 50%; CB75MC25: C. burmannii 75% and M. champaca 25%. *Statistically significant at p<0.05 compared to ascorbic acid group. #Statistically significant at p<0.05 compared to C. burmannii group.
Antiaging activity of C. burmannii and M. champaca
Tyrosinase inhibitory activity of M. champaca and C. burmannii extracts are presented in Table 5. With refers to the IC50 value of kojic acid, its activity potential can be categorized from strong to weak based on previous reference [29]. The IC50 value below 100 μg/mL indicates the most substantial potential for inhibiting tyrosinase activity, IC50 100–450 μg/mL indicates the weakest category, and IC50 450–700 μg/mL indicates the potential for inhibiting tyrosinase activity is very weak [29]. Our data suggested that C. burmannii extract has the potential to inhibit tyrosinase activity. However, it had weaker potential compared to the IC50 value of kojic acid which was 1,382.65 ppm (Table 5). On contrary, the value of IC50 of M. champaca extract was higher than that of C. burmannii extract suggested that its potency as an inhibitor of tyrosinase activity was weakest than C. burmannii and kojic acid.
Table 5. Antiaging activity of Cinnamomum burmannii and Michelia champaca extracts indicating by tyrosinase inhibition activity test.
| Group | Tyrosinase IC50 value (ppm) |
|---|---|
| Kojic acid | 15.418±0.11 |
| Cinnamomum burmannii, mean ± SD | 1,382.65±12.34a |
| Michelia champaca, mean ± SD | 4,258.23±21.24a, b |
Statistically significant at p<0.05 compared to kojic acid
Statistically significant at p<0.05 compared to C. burmannii
Discussion
The maceration process using 70% ethanol was performed to extract the active compounds M. champaca and C. burmannii. Furthermore, the phytochemical content of C. burmannii extract includes alkaloids, flavonoids, polyphenols, tannins, quinones, saponins, and triterpenoids, while M. champaca contains alkaloids, flavonoids, polyphenols, tannins, quinones, saponins, and steroids. Both extracts contain flavonoids and polyphenols. However, the levels of flavonoids and phenols in C. burmannii extract were higher than in M. champaca. According to studies, the compounds of phenolic have antioxidant activity since several their hydroxyl groups act as electron donors [13,24,30,31]. A study conducted on Thai medicinal plants reported that the higher the total phenolic content, the higher the DPPH free radical scavenging activity [24].
Flavonoids are a widespread group of plant phenolic compounds characterized by the benzo-γ-pyrone structure [31]. The compounds of phenolic are secondary metabolites which consist of an aromatic ring with one or more hydroxyl groups [32]. The role of phenolics as antioxidants is to neutralize lipid free radicals and prevent the decomposition of hydroperoxides into free radicals [31,33]. Our ethanol extracts of C. burmannii had lower total phenolic content compared reported by another study [34]. However, the flavonoid level in C. burmannii in this study were nearly identical to previous study [35]. The levels of flavonoids and phenols in the M. champaca extract were higher than those found in previous study [36]. This can be influenced by the growing conditions of C. burmannii that leads different number of phenolic groups [34].
The GC-MS results in this study indicated that 2-furan carboxaldehyde was the most putative compound of C. burmannii (43.22%). This differs from a where the highest content was cinnamaldehyde (20.61%) [37]. The cinnamaldehyde compound was identified in our C. burmannii extract but not the highest quantity of metabolite. Similarly, the highest putative compound quantity of M. champaca extract was n-hexadecanoic acid (38.23%) while the most abundant content reported by previous study was β-caryophyllene [38]. Again, this can be influenced by the growing conditions such as height above sea level, environment, light intensity, temperature, and water availability [34].
Not many studies have reported the antioxidant activity of M. champaca. The findings of this present study demonstrated that M. champaca extract had very strong antioxidant activity. However, a previous study revealed lower antioxidant activity [36]. Similarly, although lower than M. champaca, the antioxidant activity of C. burmannii extract was also strong and this has been reported previously [32,35,39]. Combining both extracts of C. burmannii and M. champaca also yielded similar strength of antioxidant activity. Therefore, the blends of these extracts have powerful antioxidant activity that potentially develops in pharmaceutical and skincare products. A previous study has proven that the bioactive compounds from C. burmannii extract could increase mRNA levels and trigger type I collagen biosynthesis in dermal fibroblasts without cytotoxicity. Cinnamaldehyde in C. burmannii is the main active component that triggers collagen expression and C. burmannii peel extract can reduce the signs of ageing caused by photo ageing [40].
This study suggested that the extracts of M. champaca and C. burmannii have weak potency as tyrosinase inhibitors compared to kojic acid. The principle of testing the activity of tyrosinase inhibitors is to prevent the formation of dopachrome products from the reaction of L-DOPA substrate and tyrosinase enzymes [41,42]. We used kojic acid as tyrosinase inhibitor as suggested previously [42]. Kojic acid exhibits inhibition competitively with its ability to chelate copper metal at the active site of the tyrosinase enzyme [43]. The flavonoids in M. champaca and C. burmannii are thought to act as a tyrosinase inhibitors. They could competitively inhibit L-DOPA oxidation by the tyrosinase enzyme, and the 3-hydroxy-4-keto part of the flavonoid structure acts as a copper (Cu) chelator of the tyrosinase enzyme structure. Furthermore, the primary consideration in selecting a compound as tyrosinase inhibitor is its safety in long-term use. The use of kojic acid is becoming more restricted due to skin irritation and its ability to enter the systemic bloodstream, which can cause thyroid gland problems. Therefore, the extracts of M. champaca and C. burmannii or combinations containing natural compounds, flavonoids, have the potential to be developed as tyrosinase inhibitors [29]. This can help develop new drugs or skin care products that are safe, efficacious, and effective for preventing as well as protecting against ageing skin disorders [44].
There are some of limitations that need to be discussed. First, our results suggested strong antioxidant activity of two extracts in biochemistry analysis using DPPH methods. Other in vitro studies to prove the antioxidant activity of the extracts could be carried out to support this result and confirm in appropriate animal models, which was not conducted in the present study. Second, although the phenolic content of the extracts was high and the antioxidant activity of these extracts was very strong, the activity of anti-tyrosinase of the extracts was very weak, both for C. burmannii and M. champaca. Therefore, it is suggested that further research examine the antiaging activity using other tests such as the anti-collagenase and anti-elastase tests on these two extracts.
Conclusion
This study suggests that extracts of C. burmannii and M. champaca, as well as their combination, are considered as possible sources of natural antioxidants due to their high polyphenol content. However, further research is needed to fully evaluate the potential benefits of these extracts for use in skincare and other applications, given that their anti-tyrosinase activity was relatively weak at concentrations greater than 1000 μg/mL.
Acknowledgments
The authors would like to thank the Ministry of Education, Culture, Research, and Technology Indonesia and the Institute of Research and Community Services, Universitas Syiah Kuala, Indonesia for providing financial support.
Ethics approval
Not required.
Conflict of interest
The authors declare no conflict of interest.
Funding
This study was funded by Institute of Research and Community Services, Universitas Syiah Kuala, Indonesia through Thesis Magister Research grant (145/E5/PG.02.00.PT/2022, 80/UN11.2.1/PT.01.03/DPRM/2022).
Underlying data
All data underlying the results can be requested from the corresponding author.
How to cite
Qarani W, Husna F, Yulia W, et al. Antioxidant and antiaging activities of Cinnamomum burmannii, Michelia champaca and their combinations. Narra J 2023; 3(2): e111 - http://doi.org/10.52225/narra.v3i2.111.
References
- 1.Yaar M, Gilchrest BA. Ageing and photoageing of keratinocytes and melanocytes. Clin Exp Dermatol. 2001;26(7):583–591. [DOI] [PubMed] [Google Scholar]
- 2.Cerimele D, Celleno L, Serri F.. Physiological changes in ageing skin. Br J Dermatol. 1990;122():13–20. [DOI] [PubMed] [Google Scholar]
- 3.Ray PD, Huang B-W, Tsuji Y.. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliwell B. Reactive species and antioxidants. Redox biology Is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J-W, Chiang H-M, Lin Y-C, Wen K-C.. Natural products with skin - Whitening effects. J Food Drug Anal. 2020;16(2):Article 8. [Google Scholar]
- 6.Meyskens FL, Chau H, Tohidian N, Buckmeier J.. Luminol-enhanced chemiluminescent response of human melanocytes and melanoma cells to Hydrogen peroxide stress. Pigment Cell Res. 1997;10(3):184–189. [DOI] [PubMed] [Google Scholar]
- 7.Sies H, Stahl W.. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24(1):173–200. [DOI] [PubMed] [Google Scholar]
- 8.Funasaka Y, Chakraborty A, et al. The depigmenting effect of α-tocopheryl ferulate on human melanoma cells. Br J Dermatol. 1999;141(1):20–29. [DOI] [PubMed] [Google Scholar]
- 9.Verschooten L, Claerhout S, Van Laethem A, et al. New strategies of photoprotection. Photochem Photobiol. 2006;82(4):1016–1023. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Chung CB, Kim JG, et al. Anti-wrinkle activity of Ziyuglycoside isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci Biotechnol Biochem. 2008;72(2):303–311. [DOI] [PubMed] [Google Scholar]
- 11.Wiedow O, Schröder JM, Gregory H, et al. Elafin: An elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990;265(25):14791–14795. [PubMed] [Google Scholar]
- 12.Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41(2):93. [PubMed] [Google Scholar]
- 13.Karim AA, Azlan A, Ismail A, et al. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement Altern Med. 2014;14(1):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pouillot A, Polla LL, Tacchini P, et al. Natural antioxidants and their effects on the skin. In: Formulating, packaging, and marketing of natural cosmetic products. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2011. p. 239–257. [Google Scholar]
- 15.Raja S, Koduru R.. A complete profile on Michelia champaca - Traditional uses, pharmacological activities and phytoconstituents. Int J Pharm Res Sch. 2014;1(2):496–504. [Google Scholar]
- 16.Maulida LF, Wahyuni ES. Upaya menurunkan radikal bebas dengan ekstrak bunga cempaka pada tikus model menopause. Gaster. 2018;16(1):6. [Google Scholar]
- 17.Ananthi T, Chitra M, Aruna B.. In-vitro anticancer activity of Michelia champaca L. flowers against ehrlich ascites carcinoma cell line. Int J Pharma Bio Sci. 2014;5:P357–363. [Google Scholar]
- 18.Rao PV, Gan SH. Cinnamon: A multifaceted medicinal plant. Evidence-Based Complement Altern Med. 2014;2014:642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Błaszczyk N, Rosiak A, Kałużna-Czaplińska J.. The potential role of cinnamon in human health. Forests. 2021;12(5):648. [Google Scholar]
- 20.Anggraini T, Novendra V, Novelina. Antioxidant activity of Archidendron pauciflorum, Syzygium oleana, Mangifera indica, Theobroma cacao and Cinnamomum burmannii young leaves and their application as jelly drink colourants. Pakistan J Nutr. 2018;17(10):492–499. [Google Scholar]
- 21.Abdelwahab SI, Mariod AA, Taha MME, et al. Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm. (Lauraceae). Arab J Chem. 2017;10(1):131–135. [Google Scholar]
- 22.Sana S, Arshad M, Saeed F, et al. Nutritional characterization of cinnamon and turmeric with special reference to their antioxidant profile. Int J Biosci. 2019;15(4):178–187. [Google Scholar]
- 23.Shahid MZ, Saima H, Yasmin A, et al. Antioxidant capacity of cinnamon extract for palm oil stability. Lipids Health Dis. 2018;17(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongkolsilp S, Pongbupakit I, Sae-Lee N, et al. Radical scavenging activity and total phenolic content of medicinal plants used in primary health care. SWU J Pharm Sci. 2004;9(1):32–35. [Google Scholar]
- 25.Soesilo S. Materia Medika Indonesia Jilid V. Jakarta: Departemen Kesehatan RI: 1989. 549–550 p. [Google Scholar]
- 26.Kikuzaki H, Hisamoto M, Hirose K, et al. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50(7):2161–2168. [DOI] [PubMed] [Google Scholar]
- 27.Chang CT, Chang WL, Hsu JC, et al. Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Bot Stud. 2013;54(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukitaningsih E. Bioactive compounds in bengkoang (Pachyrhizus erosus) as antioxidant and tyrosinase inhibiting agents. Indones J Pharm. 2014;25(2):68–75. [Google Scholar]
- 29.Batubara I, Darusman LK, Mitsunaga T, et al. Potency of Indonesian medicinal plants as Tyrosinase inhibitor and antioxidant agent. J Biol Sci. 2010;10(2):138–144. [Google Scholar]
- 30.Chandra S, Khan S, Avula B, et al. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evidence-Based Complement Altern Med. 2014;2014:253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arina N, Rohman A.. The phenolic contents and antiradical activity of Indonesian Phyllantus urinaria L. Int Food Res J. 2013;20(3):1119–1124. [Google Scholar]
- 32.Wijewardhana US, Gunathilaka UGSA, Navaratne SB. Determination of total phenolic content, radical scavenging activity and total antioxidant capacity of cinnamon bark, black cumin seeds and garlic. Int Res J Adv Eng Sci. 2019;4(2):55–57. [Google Scholar]
- 33.Rohman A, Riyanto S, Yuniarti N, et al. Antioxidant activity, total phenolic, and total flavonoid of extracts and fractions of red fruit (Pandanus conoideus Lam). Int Food Res J. 2010;17:97–106. [Google Scholar]
- 34.Chan KW, Khong NMH, Iqbal S, et al. Cinnamon bark deodorised aqueous extract as potential natural antioxidant in meat emulsion system: a comparative study with synthetic and natural food antioxidants. J Food Sci Technol. 2014;51(11):3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antasionasti I, Jayanto. Aktivitas antioksidan ekstrak etanol kayu manis (Cinnamomum burmani) secara In Vitro / Antioxidant activities of cinnamon (Cinnamomum burmani) In Vitro. J Farm Udayana. 2021;10(1):38–47. [Google Scholar]
- 36.Ruwali P, Adhikari M, Sharma S.. Phytochemical and antioxidant properties of various extracts of Michelia champaca leaves. Int J Pharm Pharm Sci. 2019;11(5):56–61. [Google Scholar]
- 37.Marzuki I, Hariroh S.. Karakteristik GC-MS Minyak Kayu Manis Asal Pulau Banda (GC-MS Characterictics of Banda Island’s Cinnamon Oils). 2021;5(2):1–7. [Google Scholar]
- 38.Lago JHG, Fávero OA, Romoff P.. Chemical composition and seasonal variation of the volatile oils from leaves of Michelia champaca L., Magnoliaceae. Rev Bras Farmacogn. 2009;19(4):880–882. [Google Scholar]
- 39.Ervina M, Lie HS, Diva J, et al. Optimization of water extract of Cinnamomum burmannii bark to ascertain its in vitro antidiabetic and antioxidant activities. Biocatal Agric Biotechnol. 2019;19:101152. [Google Scholar]
- 40.Takasao N, Tsuji-Naito K, Ishikura S, et al. Cinnamon extract promotes type I Collagen biosynthesis via activation of IGF-I Signaling in human dermal fibroblasts. J Agric Food Chem. 2012;60(5):1193–1200. [DOI] [PubMed] [Google Scholar]
- 41.Batubara I, Prastya ME. Potential use of Indonesian medicinal plants for cosmetic and oral health: A review. J Kim Val. 2020;6(1):120–134. [Google Scholar]
- 42.Chang TS. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials. 2012;5(9):1661–1685. [Google Scholar]
- 43.Chang TS. An updated review of tyrosinase inhibitors. International Journal of Molecular Sciences. 2009;10(6):2440–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Nashar HAS, El-Din MIG, Hritcu L, Eldahshan OA. Insights on the inhibitory power of flavonoids on tyrosinase activity: A survey from 2016 to 2021. Molecules. 2021;26(24):7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results can be requested from the corresponding author.