Abstract
Since adipocytes play a crucial role in pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection due to their interaction with angiotensin-converting enzyme 2 (ACE2) and interleukin 6 (IL-6), obesity is associated with an increased risk of coronavirus disease 2019 (COVID-19) mortality. Discovery of ACE2 as a SARS-CoV-2 receptor raises a controversy about whether to use ACE inhibitors (ACEIs) could be an optional therapy to prevent cytokine storms. Studies assessing the expressions of ACE2 and IL-6 upon exposure to SARS-CoV-2 is therefore important as a basis for therapeutical trials in the future. The aim of this study was to determine the effect of SARS-CoV-2 spike protein exposure on the production of ACE2 and IL-6 in adipocyte cells. Adipocytes were collected from abdominal adipose tissues of healthy and obese 45-year-old male donor having neither a history of SARS-CoV-2 infection nor COVID-19 vaccination. After being stained using the oil red O protocol, the viable adipocytes were then exposed to S1 subunit of SARS-CoV-2 spike protein. The levels of ACE2 and IL-6 were then examined using the enzyme-linked immunosorbent assay (ELISA). The results showed significant increase of ACE2 (90.22 µg/mL) and IL-6 level (60.01 µg/mL) in human adipocytes upon exposure compared to unexposed control cells (ACE2 13.33 µg/mL; IL-6 21.33 µg/mL), both comparisons had p<0.001). This study provides insight into the basic mechanism of severe COVID-19 symptoms in obese patients and provides a basic information of the potential of ACE inhibitors as an optional therapy for COVID-19 patients with obesity.
Keywords: ACE2, obesity, interleukin 6, SARS-CoV-2, adipocyte
Introduction
Adipocytes or lipocytes play a crucial role in the pathogenesis of coronavirus disease 2019 (COVID-19), as they mediate the spread, replication, and release of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Adipocytes, which are highly found in obese individuals, trigger the production of cytokines such as interleukin 6 (IL-6). The level of IL-6 increases during SARS-CoV-2 infection [2] and one of the pathological pathways is via the activation of the mitogen-activated protein kinase-nuclear factor kappa B (MAPK-NF-κB) pathway as a result of the SARS-CoV-2 spike protein attachment to angiotensin-converting enzyme 2 (ACE2) receptors [3]. This combined pathophysiology of IL-6 increase led to a hypothesis that cytokines are the cause of severe symptoms in COVID-19 patients with obesity [1].
The relationship between adipocytes and the pathogenesis of COVID-19 has been associated with ACE2 receptors, in which SARS-CoV-2 exclusively infects cells with the expression of ACE2 receptors. A recent study showed that the SARS-CoV-2 infection occurs due to the viral binding with the host cell membrane via the ACE2 receptors and viral glycoprotein molecules [4]. This indicates how ACE2 levels are increased in COVID-19 patients with severe symptoms. ACE2 receptors present at the cell membranes of some tissues and organs such as the lung, kidney, and heart. In adipose tissues, the levels of ACE2 are even higher than in the lung tissues [4]. Therefore, obesity is an independent risk factor for COVID-19 due to the high level of adipocytes. COVID-19 patients with obesity have been reportedly associated with an increased risk of intensive care admission in hospitals and mortality [5,6].
Since the discovery of ACE2 as the SARS-CoV-2 receptor, there has been controversy of whether to use of cardiovascular drugs, such as ACE inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), or aminoglycosides, as an optional therapy of COVID-19. The therapy aims to prevent the cytokine storms and ACE2 elevation to prevent severe of COVID-19. However, not all types of ARBs, ACEIs, and aminoglycosides show consistent effects on the expression of ACE2 [7]. Perindopril and losartan, among ACEI and ARB drugs respectively, have been suggested to increase the expression of ACE2 [8,9]. Further studies are needed to assess the expression of ACE2 and IL-6 in adipose tissues upon exposure to SARS-CoV-2, as a basis of therapeutical clinical trials in the future. The aim of this study was to determine the effect of SARS-CoV-2 S1 spike protein exposure on the production of ACE2 and IL-6 proteins in human adipocytes.
Methods
Study design and setting
An in vitro experimental study with a post-test-only was conducted at the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia. Lipocytes were obtained from abdominal adipose tissues of a 45-year-old healthy and obese male donor who had no history of HIV, hepatitis B virus (HBV), and hepatitis B virus (HCV) infections; no history of acute myocardial infarction, valvular heart disease, peripheral arterial disease, heart failure, malignant arrhythmias transient ischemic attack, stroke, diabetes mellitus, and kidney failure; no history of COVID-19 (confirmed by history taking and a negative PCR swab); and had never received any COVID-19 vaccines. The donor underwent echocardiography to ensure the heart structure was normal.
Collection and preparation of adipocytes
Subcutaneous fat was collected using skin incision (elliptical) technique according to the established method previously [10]. Briefly, adipocytes were isolated from adipose tissues using 0.1% collagenase type 1 solution and grown in a medium consisted alpha-minimum essential medium (α-MEM) supplemented with penicillin (10o U/I), streptomycin (100 µg/ml), and gentamycin (50 µg/ml) and platelet-rich plasma (PRP) (1%). The cells were incubated at 37°C with 5% CO2 for seven days. On each 10 cm culture dish, 1x106 cells were stained using oil red O to identify viable isolated adipocytes after a period of incubation following a previously published method [11].
SARS-CoV-2 S1 spike protein exposure
The adipocytes were divided into two groups: (1) untreated or unexposed cells as a control; and (2) exposed to S1 spike protein from SARS-CoV-2 as a treatment group. Each group consisted of three replications. In treatment group, 1 ml of 10 µM SARS-CoV-2 S1 spike protein was added to the 1 ml adipocytes culture medium, incubated for 30 min at room temperature then washed using PBS.
Measurement of ACE2 and IL-6 levels
The levels of ACE2 and IL-6 were measured using ELISA after 24 hours of S1 spike protein exposure. The level of ACE2 was measured using Abcam Human ACE2 ELISA following the manufacturer protocol (Abcam, Cat. Ab235649) while Human IL-6 ELISA kit (Elabscience Biotechnology, E-EL-H0102) was used to measure the levels of IL-16. The levels of ACE2 and IL-6 were measured in triplicates.
Statistical analysis
Independent Student t-test was used to analyze the mean of ACE2 and IL-6 between groups. A p-value of <0.05 was considered statistically significant. All data analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp, Armonk, NY, USA).
Results
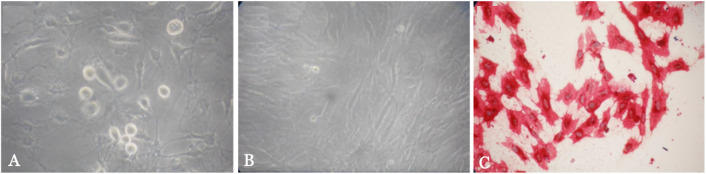
Identification of isolated adipocytes was conducted using a light microscope. The cells were identified as adipocytes if they were adherent, spindle-shaped, and positive oil red O staining (Figure 1). The observations on day seventh indicated that the cells were confluence and viable for further intervention. The result independent Student t-test indicated a significantly higher of ACE2 (90.22 µg/mL) and IL-6 (60.01 µg/mL) levels in the adipocytes of the treatment group compared to those of the control group (ACE2: 13.33 µg/mL; IL-6: 21.33 µg/mL) (Table 1 and Table 2). These data indicating that SARS-CoV-2 S1 spike protein exposure significantly increased the expression of both ACE2 and IL-6 in human adipose tissue (both had p<0.001).
Figure 1. Morphology of the adipocytes culture. (A) Day 1 of adipocytes culture; (B) day 7 of adipocytes culture; and (C) adipocytes stained with oil red O.
Table 1. Level of angiotensin-converting enzyme 2 (ACE2) in adipocytes with and without exposure to SARS-CoV-2 S1 spike protein.
| Group | ACE2 (pg/mL) | ||
|---|---|---|---|
| Mean ± SD | Min-Max | p-value* | |
| Control | 13.34 ± 1.513 | 11.83-14.85 | <0.001 |
| Exposure to SARS-CoV-2 S1 spike protein | 90.22 ± 4.725 | 85.50-94.95 | |
Analyzed using independent Student t-test
Table 2. Level of interleukin 6 (IL-6) in adipocytes with and without exposure to SARS-CoV-2 S1 spike protein.
| Group | IL-6 (pg/mL) | ||
|---|---|---|---|
| Mean ± SD | Min-Max | p-value* | |
| Control | 21.34 ± 2.566 | 18.77-23.90 | <0.001 |
| Exposure to SARS-CoV-2 S1 spike protein | 60.01 ± 1.327 | 58.68-61.34 | |
Analyzed using independent Student t-test
Discussion
We did the study to determine the effect of SARS-CoV-2 spike protein exposure on the expression of ACE2 and IL-6 in adipocyte cells. Our data suggested a significantly increased of ACE2 expression in adipose tissues upon exposure to S1 spike protein. This finding was in line with that reported in a previous study suggesting a seven-fold increase in ACE2 expression in severe COVID-19 conditions [12]. This high level of ACE2 in the present study is assumingly associated with a consequent increase in activity of the metalloproteinase domain 17 (ADAM17) activity due to the attachment of S1 spike protein to ACE2 receptor on adipocytes. ADAM17 causes the release of the ACE2 ectodomain to the extracellular medium as a biologically active form of the soluble angiotensin-converting enzyme 2 (sACE2), therefore causing an elevation in ACE2 levels [13]. A previous study confirmed that various pro-inflammatory cytokines can regulate ACE2 expression [14], and another study found that ACE2 expression was positively correlated with the expression of genes encoding transcription factors that control lipid synthesis, adipogenesis, and sterol response element-binding proteins (SREBP) 1 and 2 [15].
In addition to its function as SARS-CoV and SARS-CoV-2 receptor, ACE2 involves in the activation of renin-angiotensin system (RAS) by converting angiotensin I and angiotensin II into angiotensin (Ang) 1-9 and Ang 1-7 [13]. The ACE2/Ang 1-7 axis works by offsetting the ACE/Ang II-I axis by decreasing the levels of Ang II and activating the angiotensin type 1 receptor (AT1R). The activated receptor serve as an alternative protective measure to counteract the effects of dysregulation of RAS enzyme activity due to the attachment of the SARS-CoV-2 spike proteins to the ACE2 domain [16]. This homeostatic process is thought to stimulate increased ACE2 levels on the surface of adipocyte cell. A study indicated that the SARS-CoV-2 S1 spike protein exposure on adipocytes caused a rise in several transmembrane proteinase enzymes, enabling the SARS-CoV-2 S1 spike protein to bind to the ACE-2 surface receptor [17]. Transmembrane protease serine 2 (TMPRSS2) is one of the transmembrane proteinases causing cleavage of ACE2 to promote viral uptake [17].
The attachment of SARS-CoV-2 S1 spike protein to ACE2 receptors upregulates angiotensin type 1 (AT1) signaling, leading to IL-6 synthesis through activation of the MAPK-NF-κB pathway [3]. AT1 overexpression, owing to the impaired compensatory mechanisms of the ACE2/Ang 1-7 axis, leads to an increased ADAM17, which is responsible for the cleavage of the IL-6 receptor to its soluble form. IL-6 binds to its receptor agonist, which exists in both trans-membrane (gp80 or IL-6R) and soluble (gp50 or sIL-6R) forms [18]. Furthermore, classical signaling or transsignaling pathways through the gp130 membrane receptor spread throughout all tissues and organs, ultimately causing a hyperinflammatory response and resulting in a cytokine storm [3,18]. A study reported that M1-like macrophages can produce more pro-inflammatory cytokines such as IL-6 and TNF-α in obese patients [19]. TNF-α production by M1 macrophages is positively related to adipose tissue mass size [19]. A significant increase of TNF-α in critically ill patients with confirmed COVID-19, followed by further increase of IL-6 levels, has also been reported [20].
IL-6 is been known to have a clinical significance involving the pathomechanism of cytokine storms in COVID-19. Adipocytes are one of the primary sources of IL-6 and IL-6 receptor (IL-6R) and become a reservoir that continues to produce high amounts of IL-6 during viral infection [21]. Our finding suggested a significantly higher IL-6 levels in adipocytes after being exposed to SARS-CoV-2 S1 spike protein. This finding was in accordance with the study of Patra et al. reporting a significant increase in IL-6 upon exposure to SARS-CoV-2 S1 spike protein as compared to the control [3]. Our result also justifies those adipocytes of obese COVID-19 patients are at a high risk of immune system activation associated with the ACE2 expression as a result of the SARS-CoV-2 infection.
Conclusion
SARS-CoV-2 S1 spike protein exposure significantly increased the expression of ACE2 and IL-6 in human adipocytes. These increases, associated with a significant number of adipocytes in obese individuals, might link to the severity and mortality of COVID-19. The combined pathophysiology of escalation in both cytokines (the increase is due to both infection and obesity) in a setting of SARS-CoV-2 infection is believed to be an important pathological pathway. This study can provide insight into the basic mechanism of severe COVID-19 symptoms in obese patients, and provides a potential basis to promote the use of ACE inhibitors as an optional therapy for obese COVID-19 patients.
Acknowledgments
We would like to express our gratitude to Satuman and the head of the Physiology Laboratory, Faculty of Medicine, Universitas Brawijaya.
Ethics approval
This study has been approved by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (No. 198/EC/KEPK/07/2021).
Competing interests
No competing interests were disclosed.
Funding
This study was supported by the Ministry of Research, Technology, and Higher Education of Indonesia to I Gde Rurus Suryawan (279/UN3.15/PT/2021).
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Ardiana M, Suryawan IGR, Hermawan HO, et al. Effect of SARS-CoV-2 spike protein exposure on ACE2 and interleukin 6 productions in human adipocytes: An in-vitro study. Narra J 2023; 3 (3): e284 - http://doi.org/10.52225/narra.v3i3.284.
References
- 1.Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity 2020;28(7):1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 2020;71(8):1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patra T, Meyer K, Geerling L, et al. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PloS Pathog 2020;16(12):e1009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med 2020;19:100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020; 369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020; 28(7): 1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian J, Li Z.. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm Sin B 2021;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Ml, Li X, Meng Y, et al. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol 2010;37(1):e1–e6. [DOI] [PubMed] [Google Scholar]
- 9.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 10.Carswell KA, Lee M-J, Fried SK. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol Biol 2012:806;203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao N, Tan H, Wang L, et al. Palmitate induces fat accumulation via repressing FoxO1-mediated ATGL-dependent lipolysis in HepG2 hepatocytes. Plos One 2021;16(1):e0243938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reindl-Schwaighofer R, Hödlmoser S, Eskandary F, et al. ACE2 elevation in severe COVID-19. Am J Respir Crit Care Med 2021;203(9):1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipeto D, JdF Palmeira, Argañaraz GA, et al. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol 2020;11:576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler CG, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020;181(5):1016–1035: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Heialy S, Hachim MY, Senok A, et al. Regulation of angiotensin-converting enzyme 2 in obesity: implications for COVID-19. Front Physiol 2020;11:555039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126(10):1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Circ Care 2020;24(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGonagle D, Sharif K, O’Regan A, et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demeulemeester F, de Punder K, van Heijningen M, et al. Obesity as a risk factor for severe COVID-19 and complications: a review. Cells 2021;10(4):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Spigna G, Cernia DS, Vargas M, et al. Drastically elevated levels of Interleukin-6 and its soluble receptor complex in COVID-19 patients with acute respiratory distress. Clin Med Investig 2020;5:1–4. [Google Scholar]
- 21.Sindhu S, Thomas R, Shihab P, et al. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: Significance for metabolic inflammation. PloS One 2015; 10(7): e0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on request.