Abstract
Some of coronavirus disease 2019 (COVID-19) patients died after being hospitalized and early mortality is a matter of concern during the pandemic; therefore, it is critical to determine which patients are the most vulnerable of having early mortality. The aim of this study was to determine the risk factors for early mortality among hospitalized COVID-19 patients in Indonesia. A retrospective cohort study was conducted on hospitalized COVID-19 patients from July 2020 to September 2021 at Dr. Zainoel Abidin Hospital, Banda Aceh, Indonesia. Demographic data, clinical characteristics, laboratory findings, and mortality were collected. Early mortality was defined as a death before seven days of the hospitalization. Multivariate regression analysis was employed to determine the risk factors associated with early mortality. We included the data of 624 COVID-19 patients who died during the study period. More than half of the patients were male and aged over 50 years old. The average hospitalization period was 10 days and most patients had more than two comorbidities. Chronic lung disease was the most common comorbidity (46.0%) followed by respiratory disease (26.8%) and heart disease (14.3%). Multiple comorbidities and elevated D-dimers exceeding 3376.92 ng/mL were associated with early mortality with OR: 7.029; 95%CI: 2.02–24.43 and OR: 1.000085, 95%CI: 1.000028–1.000142, respectively. In conclusion, early mortality in COVID-19 patients was associated with having multiple comorbidities and elevated D-dimer level. Therefore, it is crucial to assess the presence of comorbidities and routine laboratory test while managing COVID-19 patients in order to prevent the early mortality.
Keywords: COVID-19, mortality, D-dimer, risk factor, early mortality
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic by the World Health Organization (WHO) and has spread worldwide including in Indonesia [1,2]. SARS-CoV-2 affects respiratory tract and causes clinical manifestations ranging from fatigue, fever, and cough to severe pneumonia, acute respiratory distress syndrome (ARDS), sepsis, septic shock, and death [3].
Apart from non-communicable diseases such as cardiovascular disease, malignancy, chronic lung disease, diabetes mellitus (DM), and others, Indonesia also has a heavy burden for other communicable diseases such as malaria, tuberculosis, human immunodeficiency virus, and other tropical infections [4]. Limited healthcare funds in low- and middle-income countries, including Indonesia, pose challenges for the population to access proper medical care [5]. This certainly can worsen patients’ clinical outcomes and have a detrimental impact on other diseases.
The risk factors of COVID-19 mortality remain incompletely understood. Limited capacity of healthcare system leads to overwhelming challenges amid critical and unprecedented conditions, resulting in widespread crises [5]. Optimal early care can improve the prognosis in high-risk patients and reduce the number of mortalities significantly [6]. Therefore, a reliable biomarker or risk assessment model is essential for predicting patient prognosis and aiding appropriate treatment [7]. Additionally, clinical and laboratory screening can enhance treatment option and effectively preventing mortality [7].
Studies on COVID-19 mortality in Asia, particularly in low-resource settings are limited [8-10]. Although having the highest case and mortality rate in Asia, research on mortality-related risk factors remains limited in Indonesia [11]. The aim of this study was to determine the risk factors associated with early mortality among hospitalized COVID-19 patients in a provincial referral hospital in Aceh, Indonesia.
Methods
Study setting
A cross-sectional study was conducted among COVID-19 patients treated in the respiratory intensive care unit (RICU), respiratory high care unit (RHCU), and new-emerging and re-emerging infectious disease special ward at Dr. Zainoel Abidin Hospital, Banda Aceh, Indonesia. All COVID-19 patients who were 18 years old, confirmed COVID-19 by polymerase chain reaction (PCR) test and hospitalized, and death between July 2020 to September 2021 were considered eligible. All patients were treated in accordance with established international and national protocols [12]. A total sampling was employed.
Data collection and variables
Demographic data including age and gender, and pre-existing medical conditions (hypertension, DM, heart disease, chronic lung disease, and other co-morbidities) were collected. Furthermore, laboratory and imaging results were also collected. Respiratory supports given to patients including low-flow nasal cannula, nonrebreathing mask, high flow nasal cannula, non-invasive mechanical ventilation, and endotracheal tube were recorded.
Clinical data were collected at the time of death while the PaO2/FiO2 ratio was calculated from initial arterial blood gas analysis during hospital admission in the emergency department. Blood counts and biochemical data were determined within the first 24 hours of admission [12]. ARDS severity, based on Berlin criteria of PaO2/FiO2 were categorized into normal (>300 mmHg), mild (201–300 mmHg), moderate (101–200 mmHg), and severe (100 mmHg) [13]. The International Society of Thrombosis and Hemostasis (ISTH) guideline was used for risk stratification of coagulopathy of the patients [14].
Outcome
The outcome measured in this study was COVID-19 mortality. It was defined as the duration of time for the COVID-19 patient hospitalized in the hospital before death. The mortality was divided into two categories: early mortality, which occurred before seven days of hospitalization, and late mortality which occurred seven days of the hospitalization or longer.
Statistical analysis
Multivariate regression model was used to determine risk factors associated with early mortality of COVID-19 patients. The regression model results were expressed as odds ratio (OR) with 95% confidence intervals (95%CIs) to identify mortality risk factors. The SPSS version 25.0 software was used for the statistical analyses (IBM SPSS, Chicago, IL, USA).
Results
Demographic and clinical characteristics
A total of 624 COVID-19-associated deaths were included. Out of total patients, fast majority of them were civil servants or followed Self-employed; only while 0.3% worked as health workers of which one of them was a pulmonologist (Table 1). Males were more dominant than females (56.6% vs 43.4%) and more majority of patients aged more than 50 years (73.1%). The mean hospitalization time was 10.7±30.4 days. About 15% of the patients had more than two comorbidities. Severe ARDS was documented in 87.2% patients, and approximately 30% experienced respiratory failure. More than 80% of the patients received oxygen therapy either nonrebreathing mask or high-flow nasal cannula (Table 1).
Table 1. COVID-19 patients’ characteristics included in the study (n=624).
| Variable | Frequency (%) |
|---|---|
| Occupation | |
| Unemployed | 23 (3-6) |
| Health worker | 2 (0.3) |
| Civil servant | 316 (50.8) |
| Self-employed | 283 (45-3) |
| Gender | |
| Female | 271 (43-4) |
| Males | 353 (56.6) |
| Age | |
| 17-30 years | 35 (5.6) |
| 31-50 years | 133 (21.3) |
| >50 years | 456 (73.1) |
| Length of hospitalization (mean±SD) | io.78± 30.4 |
| Comorbidity | |
| ≤2 | 563 (90.2) |
| >2 | 61 (9.8) |
| Severity of acute respiratory distress syndrome (ARDS) | |
| Mild | 2 (0.4) |
| Moderate | 76 (12.2) |
| Severe | 546 (87.5) |
| Respiratory failure | |
| Yes | 186 (29.8) |
| No | 438 (70.2) |
| Oxygen therapy | |
| Endotracheal tube | 56 (9.0) |
| Noninvasive ventilation | 52 (8.3) |
| Nonrebreathing mask | 247 (39.6) |
| High-flow nasal cannula | 245 (39.3) |
| Simple mask | 12 (1.9) |
| Nasal cannula | 12 (1.9) |
Comorbidity characteristics
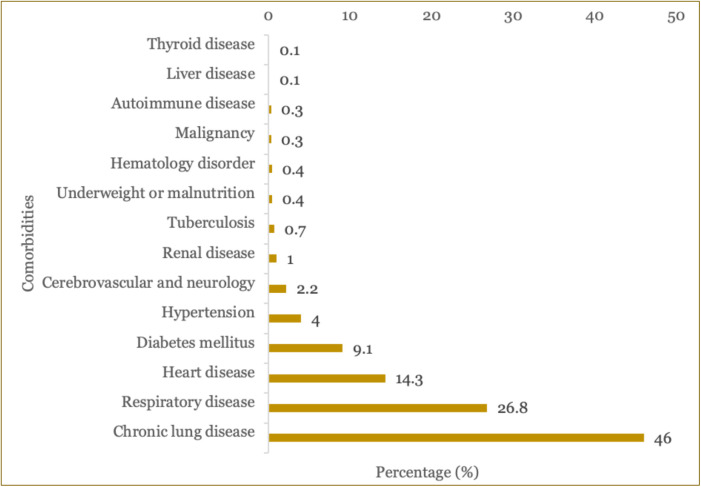
Out of total patients, most patients had comorbid, with the most common was chronic lung disease (46.0%) followed by other respiratory disease (26.8%), heart disease (14.3%), DM (9.1%), hypertension (4.0%), cardiovascular and neurology disorder (2.2%), renal disorder (1%), and tuberculosis (0.7%) (Figure 1). The least comorbid was thyroid disease followed by liver disease and autoimmune disease (Figure 1).
Figure 1. Types of comorbidities in COVID-19 patients included in the study.
Laboratory characteristics
Laboratory test results revealed elevated levels of D-dimer (mean 3376.92 mg/L), fibrinogen (601.17 mg/dL), ferritin (2681.39 mcg/L), and overt disseminated intravascular coagulation (DIC) (2.979) as shown in Table 2. The mean of initial SaO2 during the hospital admission of the patients was 94.21% (Table 2).
Table 2. Laboratory results of the COVID-19 patients included in this study (n=624).
| Parameter | Minimum | Maximum | Mean±SD |
|---|---|---|---|
| Partial pressure of oxygen (PaO2) (mmHg) | 22 | 203 | 63.80±25.93 |
| PaO2/FiO2 | 22 | 508 | 69.52±34.34 |
| Oxygen saturation (SaO2) (%) | 32 | 100 | 94.21±9.33 |
| D-dimer (mg/L) | 173 | 20000 | 3376.92±4637.90 |
| Prothrombin time | 0 | 201 | 26.2750±36.84 |
| International normalized ratio (INR) | 0.80 | 145 | 10.15±29.04 |
| Activated partial thromboplastin time (aPTT) | 13.3 | 715 | 66.31±104.27 |
| Fibrinogen (mg/dL) | 0.93 | 1024 | 601.17±173.01 |
| Ferritin level (mcg/L) | 34 | 26240 | 2681.39±4316.11 |
| Overt disseminated intravascular coagulation (DIC) | 1 | 7 | 2.979±1.18 |
| Fraction of inspired oxygen (FiO2) (%) | 0.4 | 1 | 0.941±0.10 |
Factors associated with early mortality
Multivariate logistic regression analysis of 624 patients showed that early mortality was higher among those with more than two comorbidities (OR: 7.029, 95%CI: 2.02-24.43) compared to those who having 2 or less comorbid (Table 3). This indicates that COVID-19 who had more than two comorbid had a chance to have mortality seven times higher compared to patients who had two or less comorbid. Our data also suggested that COVID-19 patients who had elevated D-dimer also associated with early mortality with a weaker OR (OR: 1.000085, 95%CI: 1.000028– 1.000142) (Table 3).
Table 3. Multivariate logistic regression showing risk factors of early mortality among COVID-19 patients included in the study (n=624).
| Risk factor | Mortality time | OR | 95%CI | |
|---|---|---|---|---|
| <7 days (n=444) n (%) | ≥7 days (n=180) n (%) | |||
| Gender | 0.895 | 0.62–1.29 | ||
| Female | 249 (39.9%) | 104 (17%) | ||
| Male | 195 (31.3%) | 76 (12%) | ||
| Diabetes mellitus | 1.288 | 0.56–2.95 | ||
| Yes | 390 (62.5%) | 171 (27%) | ||
| No | 54 (10.1%) | 9 (1.4%) | ||
| Hypertension | 1.939 | 0.39–9.43 | ||
| Yes | 418 (67%) | 178 (29%) | ||
| No | 26 (4.2%) | 2 (0.3%) | ||
| Respiratory failure | 1.166 | 0.68–1.99 | ||
| Yes | 314 (50.3%) | 124 (20 %) | ||
| No | 130 (20.8%) | 56 (9%) | ||
| Number of comorbidities | 7.029 | 2.02–24.43 | ||
| ≤2 | 386 (61.9%) | 177 (28%) | ||
| >2 | 58 (9.3%) | 3 (0.5%) | ||
| Age (year) (mean±SD) | 56.71±15.04 | 57.35±13.62 | 0.997 | 0.98– 1.01 |
| D-dimer (mg/L) (mean±SD) | 3749.42±5099.61 | 2458.08±3051.29 | 1.000085 | 1.000028– |
| 1.000142 | ||||
| PaO2 (%) (mean±SD) | 63.87±26.21 | 63.64±25.32 | 1.005 | 0.98–1.02 |
| Ratio PaO2/FiO2 (mean±SD) | 69.09±35.38 | 70.61±31.71 | 1.003 | 0.99–1.01 |
| Fibrinogen (mg/dL) (mean±SD) | 598.48±173.99 | 607.83±170.87 | 1.001 | 0.99–1.00 |
| Ferritin (mcg/L) (mean±SD) | 2686.42±4222.76 | 2668.98±4550.31 | 1.001 | 0.99–1.03 |
| Overt DIC (mean±SD) | 3.01±1.21 | 2.91±1.11 | 0.942 | 0.79–1.122 |
| Occurrence ARDS (mean±SD) | 2.88±0.349 | 2.84±0.369 | 2.173 | 0.84–5.62 |
Discussion
In this retrospective study, we investigated risk factors associated with early mortality in COVID-19 patients admitted to a provincial referral hospital in Aceh, Indonesia. Our data indicated that sex was not a significant risk factor for early mortality. The finding contradicts with a recent meta-analysis demonstrated that males exhibit a higher proportion of unfavorable clinical outcomes and mortality associated with COVID-19 [15]. This might be because the population included in the studies varied depending on time frame, region, and length of study. Furthermore, in the present study, most patients who died were over 50 years old. Consistent with prior reports, the present study found no significant association between age and COVID-19 mortality [16,17]. This is presumably due to the majority of patients in this study were >50 years, in contrast to other studies that included a higher proportion of individuals >65 years.
The present study identified prevalent comorbidities among the patients such as chronic pulmonary disease (46%), respiratory disease (26.8%), cardiovascular disease (14.3%), DM (9.1%), and hypertension (4.0%). Furthermore, our study indicated that multiple comorbidities significantly increased the risk factor for early mortality as reported by previous studies [18,19]. A recent meta-analysis reported a nearly 6-fold higher probability of mortality for patients with chronic obstructive pulmonary disease and a 2.5-fold increase for those with DM, potentially due to underlying immune and pulmonary dysfunction [20].
The present study also found that elevated D-dimer level was significantly associated with early mortality. D-dimer is a product aiding thrombotic disorder diagnosis [21]. A retrospective study conducted in the United States found that 1065 hospitalized COVID-19 patients showed that D-dimer was a predictor of death, intubation, and venous thromboembolism [22]. Elevated D-dimer exceeding 3376 mg/dL had a higher risk of early mortality, thus, can be considered as a poor prognostic marker in COVID-19 patients. Recent studies found that elevated D-dimer at admission had a higher relative risk for severe disease and had worse clinical outcomes, including all-cause mortality, ICU admission, and ARDS among COVID-19 patients [23,24]. Another study reported that disease severity can be predicted by D-dimer level of which 3.55 µg/ml predicting severe cases [25]. A meta-analysis found a relative risk of mortality at 4.60 (95%CI 2.72–7.79), taking 0.5 µg/ml as a cut-off value [26].
Hospitalized COVID-19 patients might experience acute respiratory failure due to compromised gas exchange, requiring oxygen supplementation and assisted ventilation. Initially, oxygen therapy is frequently given through an air-entrainment mask or a disposable oxygen catheter. However, COVID-19 patients typically have substantial oxygen deficits, requiring greater oxygen flows, such as high-flow nasal cannula oxygen, invasive mechanical ventilation, continuous positive airway pressure (CPAP), or bilevel positive airway pressure (BiPAP) [27]. Because of the chaotic condition at the time, where many patients had difficulty breathing and limited access to high-oxygen concentrations like high-flow nasal cannula oxygen, the patients in this study typically received oxygen support using a nonrebreathing mask, therefore making it more challenging to treat seriously ill COVID-19 patients.
One of the limitations is that this study was conducted in one center. Future study should consider several urban hospitals in Aceh to provide a clearer overview of mortality. Second, the patient data were collected retrospectively with a potency of incomplete computerized records of which only complete medicals were used in this investigation.
Conclusion
Our data suggest that having multiple co-morbidities and elevated D-dimer levels are significantly associated with early mortality in hospitalized COVID-19 patients. Therefore, mortality risk indicators must be considered to make proper triage decisions and resource allocation given the growing burden of care for critically ill patients and prevent early mortality.
Acknowledgments
We appreciate the support provided by medical personnel at the new-emerging and re-emerging infectious diseases special ward at Dr. Zainoel Abidin Hospital, Banda Aceh, Indonesia for providing care for the COVID-19 patients included in this study.
Ethical approval
This study was approved by the Health Research Ethics Committee of Dr Zainoel Abidin Hospital and the Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia (315/EA/FK-RSUDZA/2020).
Competing interests
The authors have no conflicts of interest.
Funding
This study received no external funding.
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Ismayana V, Yanti B, Kurniawan FD, et al. Risk factors of early mortality in COVID-19 patients in Indonesia: A retrospective cohort study in a provincial referral hospital of Aceh. Narra J 2023; 3 (3): e185 - http://doi.org/10.52225/narra.v3i3.185.
References
- 1.Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 2020;55(3):105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nada KM, Hsu E, Seashore J, et al. Determining cause of death during coronavirus disease 2019 pandemic. Crit Care Explor 2021;3(4):e0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta OP, Bhandari P, Raut A, et al. Coronavirus Disease (COVID-19): Comprehensive review of clinical presentation. Front Public Health 2021;8:582932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damasceno A. Noncommunicable disease. Heart of Africa: Clinical profile of an evolving burden of heart disease in Africa. Geneva: World Health Organization; 2016. p. 155–157 [Google Scholar]
- 5.Surendra H, Elyazar IR, Djaafara BA, et al. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: A hospital-based retrospective cohort study. Lancet Reg Health West Pac 2021;9:100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020;81(2):e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakka M, Connors JM, Hékimian G, et al. Association between D-Dimer levels and mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and pooled analysis. J Med Vasc 2020;45(5):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science 2020;370(6517):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsunaga N, Hayakawa K, Terada M, et al. Clinical epidemiology of hospitalized patients with coronavirus disease 2019 (COVID-19) in Japan: Report of the COVID-19 registry Japan. Clin Infect Dis 2021;73(11):e3677–e3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung HK, Kim JY, Heo J, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January-May 2020. J Korean Med Sci 2020;35(30):e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worldometer. Worldometer COVID-19 Data 2023. Available from: https://www.worldometers.info/coronavirus (2023). Accessed: 15 April 2023.
- 12.World Health Organization. Clinical management of COVID-19. Available from: https://app.magicapp.org/#/guideline/6915. Accessed: 7 April 2023
- 13.Santus P, Radovanovic D, Saderi L, et al. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: A prospective observational multicentre study. BMJ Open 2020;10(10):e043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayed M, Borahmah AA, Yazdani A, et al. Assessment of clinical characteristics and mortality-associated factors in COVID-19 critical cases in Kuwait. Med Princ Pract 2021;30(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salamanna F, Landini MP, Fini M.. Overt and non-overt disseminated intravascular coagulation and the potential role of heparin in the COVID-19 pandemic outbreak. Ther Adv Hematol 2020;11:2040620720951655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbadage T, Peterson BM, Awada J, et al. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med 2020;7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46(5):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Tang J, Wei F.. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol 2020;92(4):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ 2013;347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poudel A, Poudel Y, Adhikari A, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS One 2021;16(8):e0256744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naymagon L, Zubizarreta N, Feld J, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res 2020;196:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rostami M, Mansouritorghabeh H.. D-dimer level in COVID-19 infection: A systematic review. Expert Rev Hematol 2020;13(11):1265–1275. [DOI] [PubMed] [Google Scholar]
- 24.Bansal A, Singh AD, Jain V, Aggarwal M.. The association of D-dimers with mortality, intensive care unit admission or acute respiratory distress syndrome in patients hospitalized with coronavirus disease 2019 (COVID-19): A systematic review and metaanalysis. Heart Lung 2021;50(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gungor B, Atici A, Faruk Baycan O, et al. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am J Emerg Med 2021;39:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simadibrata DM, Lubis AM. D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: A meta-analysis. Epidemiol Infect 2020;148:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnesen B, Jensen JUS, Jeschke KN, et al. Management of COVID-19-associated acute respiratory failure with alternatives to invasive mechanical ventilation: High-flow oxygen, continuous positive airway pressure, and noninvasive ventilation. Diagnostics 2021;11(12):2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on request.