Abstract
Curcumin, a dietary polyphenol derived from turmeric’s rhizome, exhibits a range of pharmacological activities, such as antioxidant, analgesic, antipyretic, and anti-inflammatory effects. It has been investigated for its therapeutic potential in chronic obstructive pulmonary disease (COPD) due to its ability to inhibit nuclear factor kappa B (NF-κB), modulate oxidative stress, impact cell viability, and regulate gene expression. However, most studies have been limited to in vitro conditions. To address this gap, we conducted in vivo experiments using the fruit fly Drosophila melanogaster to explore the antioxidant activities and biological significance of curcumin. Several parameters were assessed using different assays, including curcumin toxicity assay, fly survival, locomotor response to curcumin treatment (with or without cigarette smoke), trypan blue staining, larval crawling assays, and gene expression analysis. Our data revealed no significant differences in toxicity and locomotor tests across various curcumin concentrations. D. melanogaster tolerated curcumin at concentrations of 0.5 μM, 5 μM, 50 μM, and 500 μM, suggesting its safety without negatively impacting locomotor. Furthermore, curcumin at 5 μM extended the lifespan of D. melanogaster exposed to cigarette smoke, while reversing the negative effects of smoke exposure on gut cell viability and larval locomotor activity. In conclusion, curcumin administration appeared safe for D. melanogaster, with potential benefits for longevity and locomotory function. These findings support the idea that curcumin possesses in vivo antioxidant properties and may serve as a promising pharmacological agent. However, further study is needed to explore its potential applications in human health and disease management, particularly in the context of COPD.
Keywords: Antioxidant, curcumin, D. melanogaster, fruit fly, COPD
Introduction
Since 2015, chronic obstructive pulmonary disease (COPD), characterized by respiratory-related restrictions or severe airflow obstruction, has surpassed heart disease and cancer as the third leading cause of mortality worldwide [1]. The primary cause of COPD is environmental factors, particularly cigarette smoke that contains nicotine as well as other chemicals [2, 3]. Smoking releases free radicals into the body with each inhalation [4].
COPD is a result of oxidative stress, although the lung’s antioxidant levels typically maintain a balance between oxidants and antioxidants. In COPD, oxidative stress leads to inflammation when the oxidant load surpasses the body’s response [5, 6]. The presence of free radicals necessitates the presence of antioxidants, which are chemicals that can neutralize them [7]. Oxidative stress, primarily caused by external factors such as cigarette smoke and pollution, as well as internal factors like reactive oxygen species (ROS) generated by inflammatory and structural cells in the lung (with mitochondrial oxidative stress playing a crucial role), greatly influences the development of COPD [8].
Oxidative stress exacerbates chronic inflammation, induces fibrosis and emphysema, promotes corticosteroid resistance, accelerates lung aging, damages DNA, and triggers the formation of autoantibodies [9]. Managing oxidative stress through antioxidant supplementation or stimulating the production of endogenous antioxidants could potentially be an effective strategy in treating the underlying mechanisms of COPD. Understanding the significance of antioxidants highlights the potential similar mechanism of curcumin, suggesting its potential as a natural antioxidant in COPD treatment [10].
Studies have revealed that curcumin, a dietary polyphenol derived from the turmeric rhizome, offers therapeutic advantages in COPD by suppressing nuclear factor kappa B (NF-κB) [11]. Curcumin’s modulatory effects on oxidative stress, cell viability, and gene expression may benefit COPD therapy [12]. Due to its chemical contains, curcumin possesses a strong antioxidant potential that is almost three times greater than that of vitamin C and more than 1.5 times greater than that of vitamin E [13]. In addition to acting as an endogenous free radical scavenger, curcumin promotes the production of the antioxidant glutathione, which protects cells and tissues from free radical damage [13]. Moreover, curcumin suppresses and regulates pro-inflammatory cytokines such as interleukin-4 (IL-4), IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α) [13].
Fruit fly (Drosophila melanogaster) has been proposed as useful animal model for studying the physiological and pathological components of diseases or treatments, including those related to antioxidants [14, 15]. With a short lifespan of approximately 10 days from egg to adult, D. melanogaster is an ideal organism for toxicity testing and pharmacological efficacy assessments [16]. The brief life cycle of D. melanogaster makes them excellent subjects for evaluating toxicity parameters or assessing pharmacological effects [17, 18]. Given their numerous benefits, D. melanogaster warrants investigation as a rapid screening platform for developing novel pharmaceuticals and repurposing existing drugs. The aim of this study was to explore the antioxidant activities and biological significance of curcumin, particularly in the context of cigarette smoke exposure, using D. melanogaster as a model organism.
Methods
Drosophila melanogaster stock
Adult males of D. melanogaster Oregon R (wildtype) genotype were used as the model organism in this study. This fly line was obtained from the Laboratory of Host Defense and Responses (Kanazawa University, Japan) and have been maintained in the Laboratory of Pharmacology and Toxicology, Faculty of Pharmacy, Universitas Hasanuddin, Indonesia) for more than 20 generations under standard conditions. These conditions include a standard cornmeal-based food, a temperature of 25°C, and a 12-hour light and 12-hour darkness cycle [19].
Sample preparation
Curcumin powder, obtained from Sigma-Aldrich (Merck Pte. Ltd., Singapore), with analytical grade standard was dissolved in 96% ethanol (Merck Emsure Reag., Darmstadt, Germany) for analytical purposes.
Toxicity assay
The objective of this experiment was to identify the lowest effective dose of curcumin without any sign of toxicities. The toxicity assay was carried out by exposing second-instar larvae to curcumin at concentrations of 0.5 μM, 5 μM, 50 μM, and 500 μM. Curcumin was administered orally by mixing it into the feed, which was kept at a temperature of approximately 25°C in culture vials. Larvae were visually counted. A total of 250 larvae were prepared, divided into four curcumin-treated groups and one non-curcumin control group. Each group had five replications, with ten larvae in each replication. To ensure the non-toxic nature of curcumin on D. melanogaster, the life cycle was monitored from the larval stage to the pupal stage and from the pupal stage to the adult fly stage, observing no adverse effects [20].
Exposure to cigarette smoke, survival, and locomotor assay
A pool of second-instar larvae was prepared, and 90 larvae were randomly selected and assigned to six treatment groups using the online randomization software (https://www.graphpad.com/quickcalcs/randomSelect1/). Curcumin was administered orally by mixing it into the feed, which was kept at a temperature of approximately 25°C in culture vials. Every three days, the flies were transferred to new vials containing curcumin-treated fly food. Two control groups were included: one group was maintained in a typical diet without curcumin and one group was treated with cigarette smoke (negative control) without any curcumin treatment. The remaining four groups were exposed to cigarette smoke and maintained on curcumin-containing fly food at varying concentrations (0.5 μM, 5 μM, 50 μM, and 500 μM) with five replications. Each D. melanogaster vial (with a size of 25 × 95 mm) contained 10 flies and was exposed to cigarette smoke for 30 minutes, three times daily, for two days. Afterward, all groups were evaluated for survival and locomotor assay, totaling 250 flies.
The survival assay was conducted to examine the efficacy of curcumin following cigarette smoke exposure. This assay was carried out aiming to assess the effects of different treatments or conditions on the lifespan and survival of the flies. By measuring the survival of the flies over time, the impact of genetic or environmental factors on longevity, aging, and potential interventions or therapies that may improve lifespan or delay the onset of age-related diseases can be seen as phenotypical effects. Parameters such as age, diet, and treatment were considered.
The locomotor assay was performed on all fly groups using the negative geotaxis approach [21], with slight modifications. The primary objective of locomotor assays in D. melanogaster is to evaluate the impact of different treatments or conditions on the locomotor activity of the flies. These assays enable the investigation of curcumin’s influence on locomotor activity and motor function in D. melanogaster, thereby identifying potential therapeutic or toxic effects. For the experiment, the vial containing the flies to be tested was positioned vertically in front of a climbing wall. The flies were gently tapped down to ensure they remained at the bottom of the vial. Observers then observed the flies in the vial for a duration of up to 15 seconds, documenting any relevant events. The number of flies that successfully climbed to the marked finish line was recorded, and this process was repeated three times for accuracy.
Assessment of the effect of cigarettes on cellular damages
The objective of this study was to establish that cigarette smoke induces a specific biological response. Cell death, indicated by changes in staining intensity in the intestinal cells, can be detected using trypan blue staining in larvae exposed to cigarette smoke, while nerve injury can be identified using the crawling test. In our experiment, we stained the larvae with trypan blue to assess cell damage, which is indicated by a blue color. Second-instar larvae were utilized, and they were exposed to cigarette smoke three times a day for two days. Subsequently, the larvae were reared to their third instar, rinsed with a PBS solution, soaked in trypan blue solution for 30 minutes, cleaned again, and examined using a stereomicroscope [22]. Observation of color changes in the larvae’s midgut (middle intestine) served as a reliable indicator of cellular injury. Dead tissue or cells were stained with trypan blue [23], and a darker midgut color in D. melanogaster larvae was associated with higher levels of cellular damage [24].
Larval crawling assay
The purpose of this study was to assess whether cigarette smoke exposure elicits a specific phenotypical crawling response. Second-instar larvae were exposed to cigarette smoke and subsequently placed on a 0.8% agarose gel on graph paper. Their crawling speed was measured for one minute [25]. This experiment aimed to observe whether cigarette smoke affects larval locomotion, including crawling trajectory, turning angle, and overall crawling speed.
Gene expression assay
Total RNA isolation was performed using five live flies from each group. Flies that were exposed to cigarette smoke and treated with curcumin were placed into Treff tubes and crushed using a micropestle. Total RNA isolation was conducted using the PureLink RNA Mini Kit (Invitrogen, Thermo Fisher Scientific Inc., USA). Quantitative examination of total RNA levels was carried out using a nano spectrophotometer (BioDrop, USA). The expression levels of sod1 and sod2 were analyzed using the reverse transcriptase quantitative PCR (RT-qPCR) method in a reaction volume of 10 μl each. The SuperScript III Platinum SYBR Green One-Step RT-qPCR kit with ROX (Invitrogen, Thermo Fisher Scientific Inc., USA) was used according to the manufacturer’s instructions. The amplification of the expected products was validated using a post-amplification melt curve profile. As an internal reference control in the RT-qPCR assay, we utilized ribosomal RNA level of rp49. The rp49 gene encodes a ribosomal protein that is part of the large subunit of the ribosome and is responsible for protein synthesis. The rp49 gene is highly expressed in various tissues throughout development, making it a reliable tool for normalizing gene expression data in studies of gene regulation, genetic manipulation, and developmental biology in D. melanogaster. The use of rp49 as a reference control has been validated in numerous studies and is considered a reliable and stable internal reference control for gene expression studies in D. melanogaster. The Rotor-Gene Q thermal cycler (Qiagen, Germany) was employed with the following temperature profiles: 37°C for 15 minutes, 95°C for 10 minutes, and then 40 cycles at 95°C for 10 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, followed by a melt curve analysis from 60°C to 95°C. Data were analyzed using the relative quantification method. The list of primers used in the RT-qPCR is provided in Table 1 [26].
Table 1. Primers used in the RT-qPCR assay.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| sod1 | 5’ – AGG TCA ACA TCA CCG ACT CC – 3’ | 5’ – GTT GAC TTG CTC AGC TCG TG – 3’ |
| sod2 | 5’ – TGG CCA CAT CAA CCA CAC – 3 | 5’– TTC CAC TGC GAC TCG ATG – 3’ |
| rp49 | 5’ – GAC GCT TCA AGG GAC AGT ATC TG – 3’ | 5’ – AAA CGC GGT TCT GCA TGA G – 3’ |
Data processing
The survival data were depicted in a Kaplan-Meier graph and assessed for statistical significance using the log rank test. Conversely, the locomotor and gene expression data were statistically analyzed using the one-way analysis of variance (Anova) test, followed by post hoc analysis. In our study, we used Tukey’s honestly significant difference (Tukey’s HSD) test for the post hoc analysis, which compares all groups pairwise and provides a range of values indicating significant differences between each pair of groups. The results were presented in a bar graph format. Mean ± SD was used to report all statistical analyses, and p-values below 0.05 were considered statistically significant. The obtained data were analyzed using GraphPad Prism 8 software.
Results
Curcumin is relatively safe for Drosophila melanogaster
The aim of the toxicity assay was to assess the safety of curcumin when administered to D. melanogaster, specifically focusing on its insecticidal properties. Four different concentrations of curcumin (500 μM, 50 μM, 5 μM, and 0.5 μM) were employed in this study, along with an untreated control group. As depicted in Figure 1, the results indicated that curcumin exhibited no toxicity towards D. melanogaster larvae across all tested concentrations. The findings further demonstrated that curcumin did not disrupt the normal life cycle of D. melanogaster, as observed from the larval stage through the pupal stage and from the pupal stage to the adult fly stage.
Figure 1. Developmental profile of Drosophila melanogaster in the presence or absence of curcumin. D. melanogaster larvae successfully undergo pupation (A) and develop into adult flies (B) regardless of curcumin treatment at different concentrations. The differences observed were found to be non-significant (ns).
Cigarette smoke exposure provokes cell damage in Drosophila melanogaster larvae
Trypan blue staining assay
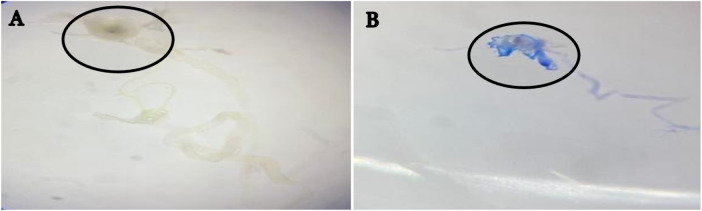
The results of the trypan blue staining test conducted on control (untreated) larvae revealed that the gut did not exhibit a blue/dark coloration, as depicted in Figure 2A. This finding indicates the absence of cell damage. In contrast, larvae exposed to cigarette smoke displayed a blue stain in their intestines, indicating the occurrence of cell damage (Figure 2B). The trypan blue dye used in the test selectively penetrates cells and cell membranes that have been compromised or damaged, thereby demonstrating the ability of cigarette smoke to induce cell damage in the exposed larvae [23].
Figure 2. Trypan blue staining on the Drosophila melanogaster larval gut. The gut was unstained with trypan blue (A) but stained as blue upon the introduction of cigarette smoke (B).
Larval crawling assay
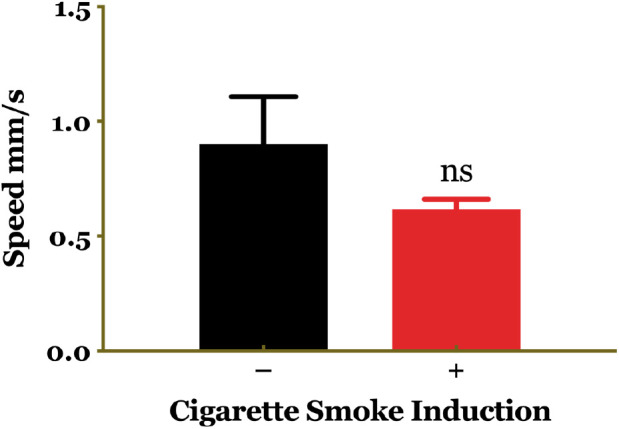
The crawling assay was performed on two groups: the control group and the group exposed to cigarette smoke, in order to compare their crawling patterns. The control group exhibited a straight and consistent crawling path, while the group exposed to cigarette smoke displayed irregular crawling paths with abrupt twists or circles. However, the results of statistical tests did not yield a significant difference between the two groups. D. melanogaster larvae demonstrate coordinated locomotor activities, including crawling and turning [25]. Figure 3 illustrates the crawling speed of the larvae, indicating that the larvae exposed to cigarette smoke had a slower crawling speed compared to the healthy controls, although this difference was not statistically significant.
Figure 3. Comparison of larval speed between the untreated control group and the group treated with cigarette smoke. Statistical analysis revealed no significant difference between the two groups. ns, non-significant.
Survival assay
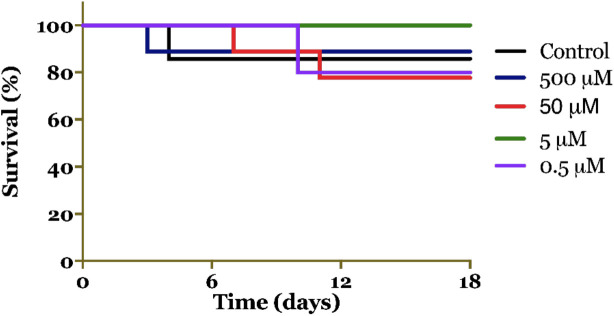
The survival assay is an important phenotypic parameter in antioxidant research as it assesses the ability of flies exposed to cigarette smoke to survive after treatment with curcumin. This assay provides a simple approach to evaluate the lifespan effects of drugs. The results revealed that treatment with curcumin at a concentration of 5 μM extended the lifespan of D. melanogaster induced by cigarette smoke compared to the control group (Figure 4). Our hypothesis is that the extract improves organ quality and prevents cell damage. The results from the survival assay clearly demonstrate the antioxidant activity of curcumin [26]. Notably, the observed effect was independent of the concentration.
Figure 4. Survival of Drosophila melanogaster exposed to cigarette smoke and treated with different concentrations of curcumin (500 μM, 50 μM, 5 μM, and 0.5 μM).
Locomotor of cigarette smoke-treated larvae was not affected in the presence of curcumin
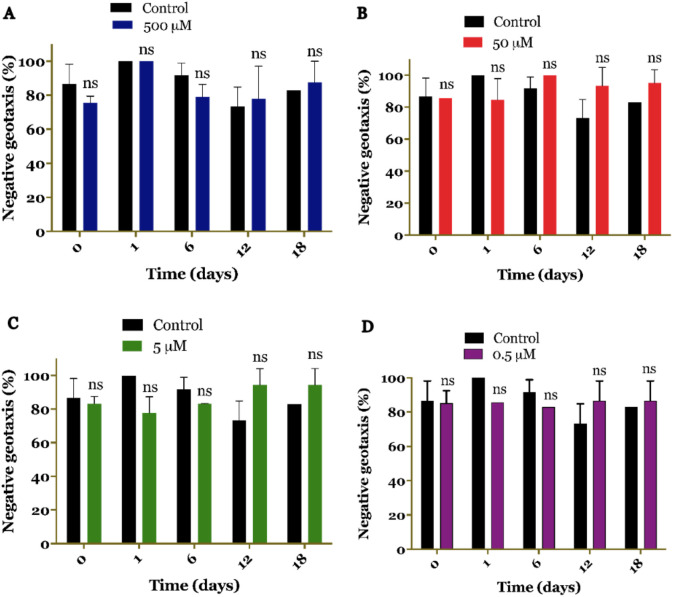
The impact of curcumin on the locomotor activity of cigarette smoke-treated larvae was investigated in this experiment. The negative geotaxis test method was employed to assess the locomotor behavior. The results demonstrated that all concentrations of curcumin had no significant effect on the locomotor activity of D. melanogaster at various testing times (day 0, 1, 16, 12, and 18). The locomotor behavior of the curcumin-treated larvae did not differ significantly from the control group without treatment. These findings indicate that curcumin does not alter the locomotor activity of D. melanogaster, suggesting that it does not interfere with motor function (Figure 5) [19, 26].
Figure 5. Locomotor activity of Drosophila melanogaster upon treatment with curcumin at different concentrations (A-D). Curcumin treatment at all concentrations did not impair fly locomotion. No significant (ns) differences were observed.
Gene expression analysis
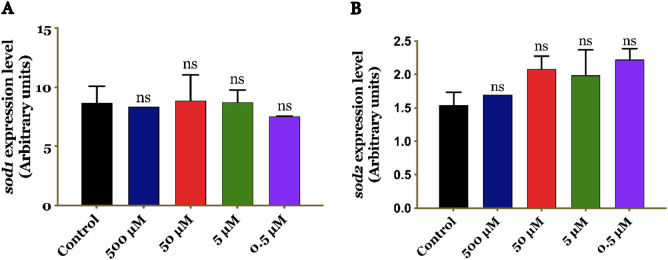
The RT-qPCR analysis revealed that the expression of sod1 and sod2 genes was not affected by any of the tested concentrations of curcumin (500 μM, 50 μM, 5 μM, or 0.5 μM) (Figure 6). This observation raises the question of whether the improvement in fly survival was not attributed to increased activity of antioxidant genes sod1 and sod2, but rather to the combined regulation of multiple genes.
Figure 6. Expression levels of sod1 (A) and sod2 (B) in Drosophila melanogaster in the presence or absence of curcumin, as determined by RT-qPCR relative to rp49. The results indicate no significant differences in the expression of sod1 and sod2 between the control and treatment groups. ns, non-significant.
Discussion
Turmeric is known to contain curcumin, the most commonly recognized active substance, which exhibits biological activities such as analgesic, anticancer, antidiabetic, antipyretic, and anti-inflammatory effects [26]. The objective of this study is to investigate the antioxidant effects of curcumin using D. melanogaster as a model organism. Animal models, including fruit flies, offer significant potential for studying the physiological and pathological components of diseases or treatments, particularly in the context of antioxidants [14, 15]. The life cycle of D. melanogaster, starting from egg to adult, spans approximately 10 days [16]. Due to their short life cycle, fruit flies can serve as a useful tool for toxicity assessment and evaluating the effectiveness of potential medications in experimental survival studies [17, 18].
Given the well-established status of fruit flies as a model organism with a short life cycle and the advantages of genetic manipulation, they have been frequently employed to examine the roles of antioxidants in vivo [27]. D. melanogaster is commonly used as a model for evaluating the harmful activity of chemicals due to its extreme sensitivity, even to minute concentrations of hazardous substances [28]. In this study, a D. melanogaster toxicity test was performed to confirm that the curcumin compound used did not possess pesticidal properties. The results demonstrated the non-toxic nature of curcumin to D. melanogaster larvae across all tested concentrations (500 μM, 50 μM, 5 μM, and 0.5 μM).
Oxidative stress, characterized by a shift in redox equilibrium favoring the generation of reactive oxygen species, plays a crucial role in cellular impacts, including cellular senescence, apoptosis, and altered cellular signaling. It is well accepted that reactive oxygen species can induce cellular death at supraphysiological doses while influencing survival at low concentrations [29]. The purpose of the survival experiment, a key phenotypic characteristic in antioxidant research, was to determine whether curcumin could increase the lifespan of D. melanogaster exposed to cigarette smoke compared to the control group. There is evidence suggesting that curcumin can enhance organ function and protect cells from harm. Results from the survival tests provide valuable insights into the antioxidant activity of curcumin. Previous studies have consistently reported similar findings, specifically an increase in survival even in the absence of cigarette smoke treatment [30].
The effect of curcumin on locomotor or motor activity was assessed using a negative geotaxis test. Inconclusive results from the geotaxis test [28] suggested potential compromise in the locomotor system, but further analysis indicated that curcumin did not affect normal locomotor function or motor disturbances. Cigarette smoke exposure was conducted in this study and evaluated through two tests: a trypan blue staining assay designed to detect cell damage in midgut larvae exposed to cigarette smoke (cells become discolored after being irritated by the smoke) and a crawling assay to compare the movement patterns of larvae in the control and exposure groups. Levels of trypan blue staining signal in the midgut served as an indicator of the extent of cell injury [24]. Larvae exposed to the trypan blue staining test exhibited cell death or successful exposure to cigarette smoke.
Neuronal injury was detected during the early stages of larval development using the crawling experiment [24]. The crawling test results showed the crawling speed of the larvae and revealed that, compared to the healthy controls, the larvae exposed to cigarette smoke had a slower crawling speed, although the difference was not statistically significant according to the results of the statistical analysis.
According to the results of the survival assay, the effect on survival was found to be independent of concentration. This observation is likely attributed to the hormetic effect of certain molecules. A low dose or concentration of a chemical may have a stimulatory or advantageous impact, whereas a high dose or concentration may have an inhibitory or harmful effect. In this case, the lack of effect at high concentrations does not necessarily indicate a lack of toxicity, as the compound may still have adverse effects on cellular or physiological functions. It is important to note that the hormetic effect is a complex and context-dependent phenomenon, and the exact mechanisms underlying it may vary depending on the specific compound, biological system, and experimental conditions. Further investigations, including dose-response studies and mechanistic analyses, are necessary to understand the hormetic effect and its implications for toxicity assessment and therapeutic applications.
Oxidative damage to biomolecules, including DNA, lipids, and proteins, can result from an excessive generation of reactive oxygen species. Antioxidants, which can be obtained from both endogenous sources and external sources, help reduce the levels of ROS and mitigate oxidative damage [26]. The primary antioxidant enzymes involved in ROS neutralization include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GRx). SOD, for instance, effectively scavenges free radicals by converting superoxide anion radicals (O2-) into hydrogen peroxide (H2O2). It is noteworthy that humans and the fruit fly D. melanogaster share a genetic similarity of approximately 75% [19], with the function of sod genes in humans resembling that in D. melanogaster.
Subsequently, we investigated gene expression changes following curcumin administration using RT-qPCR to conduct a molecular-level analysis. The expression levels of sod1 and sod2 genes did not exhibit noticeable differences between the control and treatment groups. This suggests that the improvement of survival of cigarette smoke-exposed flies in the presence of curcumin may not occur in a manner dependent on the expression of sod1 and sod2 but other genes. The collected data were then analyzed using GraphPad Prism software. Statistical analysis was performed for D. melanogaster survival, locomotor activity, and gene expression studies using RT-qPCR. Survival data were analyzed using the Kaplan-Meier graph, coupled with Log-Rank statistical analysis. On the other hand, locomotor activity and gene expression data were analyzed using the One-way ANOVA approach, followed by post hoc analysis. The results were presented using a bar graph. All statistical analyses used mean ± SD, and p-values less than 0.05 were considered significant. The acquired data revealed a novel finding D. melanogaster can be utilized as an in vivo platform for antioxidant testing, and the screening results demonstrated the antioxidant effect of curcumin, which could be evaluated phenotypically.
Evaluating the bioavailability of curcumin in a fly model can offer valuable insights into its absorption, distribution, metabolism, and excretion in living organisms. These factors are crucial for determining its therapeutic potential. However, despite much effort has been given in the field, it seems still difficult to carry out drug pharmacokinetics-related research using D. melanogaster. Nevertheless, it is not an impossible task. Flies are extensively utilized as model organisms in biomedical research due to their genetic and physiological similarities to humans, as well as their amenability to genetic manipulation and high-throughput screening [31]. Therefore, employing a fly model to assess the bioavailability of curcumin can provide significant understanding regarding its pharmacokinetics, pharmacodynamics, and potential therapeutic applications for various diseases. Overall, the evaluation of curcumin’s bioavailability in a fly model holds great importance for the development and optimization of curcumin-based therapies, expediting the translation of basic research into clinical applications.
Conclusions
This study concluded that administering curcumin was safe for the growth of D. melanogaster. Treatment of curcumin on D. melanogaster can extend lifespan without affecting cognitive function. Larvae exposed to cigarette smoke can damage cells. While curcumin has an antioxidant impact, it may not specifically target the sod1 and sod2 pathways. Overall, this study highlights the promising use of D. melanogaster model in the investigation of antioxidant effects both at the molecular and phenotypical levels.
Acknowledgments
The authors would like to express their sincere gratitude to Prof. Yoshinobu Nakanishi and Assoc. Prof. Takayuki Kuraishi from Kanazawa University, Japan, for their invaluable supports in providing the necessary materials and special consumables, particularly the wildtype strain of D. melanogaster used in this study. Furthermore, the authors extend their acknowledgments to Prof. Elly Wahyuddin (Biofarmaka Laboratory, Faculty of Pharmacy, Universitas Hasanuddin) and Dr. Isra Wahid (Department of Parasitology, Faculty of Medicine, Universitas Hasanuddin), for generously providing all the required instruments to carry out this research. Special thanks are also due to our dedicated students Andi Nur Aulia, Lia Gustiana Rahman, Putri Devi Natalia, Magfirah, Adinda Aisyah Rahayu, and Rizki Dahlan for their contributions to this project.
Ethics approval
Not required.
Competing interests
All the authors declare that there are no conflicts of interest.
Funding
This research received financial support from the Directorate General of Higher Education, Ministry of Education, Culture, Research, and Technology, Indonesia, through the PDP 2022 research grant awarded to N.R.R and colleagues (Decree Number 1:033/ES/PG.02.00/2022 and Agreement/Contract Number 203/LL9/PK.00.PG/2022;010/STIFA/LPPM/VI/2022). Research in FN’s lab is supported by PDUPT Research Grant 2022 (No.020/E5/ PG.02.00PT/2022) from the Directorate General of Higher Education, Ministry of Education, Culture, Research, and Technology, Indonesia and Penelitian Fundamental Kolaboratif 2023 grant (No.00323/UN4.22/PT.01.03/2023) from Universitas Hasanuddin.
Underlying data
Derived data supporting the findings of this study are available from the corresponding author on request.
How to cite
Rumata NR, Purwaningsih D, Asbah A, et al. Phenotypical and molecular assessments on the pharmacological effects of curcumin in Drosophila melanogaster. Narra J 2023; 3 (2): e117 - http://doi.org/10.52225/narra.v3i2.117.
References
- 1.Perez-Warnisher MT, De Miguel M, Seijo LM. Tobacco use worldwide: legislative efforts to curb consumption. Ann Glob Health 2018; 84(4):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu S, Zhang D, He Q, et al. Efficacy of Liuzijue Qigong in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Med 2022;65:102809. [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa R, Aggarwal T, Malyla V, et al. Identification of biomarkers and genetic approaches toward chronic obstructive pulmonary disease. J Cellular Physiology 2019; 234(10):16703–16723. [DOI] [PubMed] [Google Scholar]
- 4.Bitzer ZT, Goel R, Trushin N, et al. Free radical production and characterization of heat-not-burn cigarettes in comparison to conventional and electronic cigarettes. Chem Res Toxicol 2020; 33(7):1882–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dailah HG. Therapeutic potential of small molecules targeting oxidative stress in the treatment of chronic obstructive pulmonary disease (COPD): A comprehensive review. Molecules 2022; 27(17):5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih Y-M, Chang Y-J, Cooke MS, et al. Alkylating and oxidative stresses in smoking and non-smoking patients with COPD: implications for lung carcinogenesis. Free Radic Biol Med 2021; 164:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Admassu S, Kebede M.. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv Food Technol Nutr Sci 2019; 5:38–49. [Google Scholar]
- 8.Sharma V, Mehdi MM. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem Int 2023:105490. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biology 2020; 33:101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves PB, Romeiro NC. Multi-target natural products as alternatives against oxidative stress in Chronic Obstructive Pulmonary Disease (COPD). Eur J Med Chem 2019; 163:911–931. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Sun J, Mohammadtursun N, et al. Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARγ-NF-κB signaling pathway. Food Funct 2019; 10(12):7983–7994. [DOI] [PubMed] [Google Scholar]
- 12.Safari S, Davoodi P, Soltani A, et al. Curcumin effects on chronic obstructive pulmonary disease: A systematic review. Health Sci Rep 2023; 6(3):e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y-S, Chen T-H, Weng L, et al. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed Pharmacother 2021; 141:111888. [DOI] [PubMed] [Google Scholar]
- 14.Nainu F, Nakanishi Y, Shiratsuchi A.. Fruit fly as a model organism in the study of human diseases and drug discovery. J Cent Med Educ Sapporo Med Univ 2019; 10:21–32. [Google Scholar]
- 15.De Lazzari F, Sandrelli F, Whitworth AJ, et al. Antioxidant therapy in Parkinson’s disease: insights from Drosophila melanogaster. Antioxidants 2020; 9(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bovier TF, Cavaliere D, Colombo M, et al. Methods to test endocrine disruption in Drosophila melanogaster. J Vis Exp 2019(149):e59535. [DOI] [PubMed] [Google Scholar]
- 17.Sandner G, König A, Wallner M, et al. Alternative model organisms for toxicological fingerprinting of relevant parameters in food and nutrition. Crit Rev Food Sci 2022; 62(22):5965–5982. [DOI] [PubMed] [Google Scholar]
- 18.Lee S-H, Min K-J.. Drosophila melanogaster as a model system in the study of pharmacological interventions in aging. Transl Med Aging 2019; 3:98–103. [Google Scholar]
- 19.Asfa N, Mahfufah U, Khadafi M, et al. Imunosuppresive activity of Momordica charantia L. fruit extract on the NF-κB pathway in Drosophila melanogaster. Biointerface Res Appl Chem 2022; 12:6753–6762. [Google Scholar]
- 20.Halmenschelager PT, da Rocha JBT. Biochemical CuSO4 toxicity in Drosophila melanogaster depends on sex and developmental stage of exposure. Biol Trace Elem Res 2019; 189(2):574–585. [DOI] [PubMed] [Google Scholar]
- 21.Jaya A, Wahyudin E, Djabir YY, et al. Phenotypical Effect of phosphodiesterase 5 (PDE5) Inhibitor on behavioral activities of fruit fly Drosophila melanogaster. Biointerface Res Appl Chem 2021; 12:222–229. [Google Scholar]
- 22.Mishra PK, Ekielski A, Mukherjee S, et al. Wood-based cellulose nanofibrils: haemocompatibility and impact on the development and behaviour of Drosophila melanogaster. Biomolecules 2019; 9(8):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou K, Goodman CL, Ringbauer J, et al. Establishment of two midgut cell lines from the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). In Vitro Cell Dev Biol 2020; 56(1):10–14. [DOI] [PubMed] [Google Scholar]
- 24.Yang P, Yang X, Sun L, et al. Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae. Scientific Reports 2022; 12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solana-Manrique C, Moltó MD, Calap-Quintana P, et al. Drosophila as a model system for the identification of pharmacological therapies in neurodegenerative diseases. In: Insights into human neurodegeneration: Lessons learnt from Drosophila. Springer; 2019: 433–467. [Google Scholar]
- 26.Asbah A, Ummussaadah U, Parenden N, et al. Pharmacological effect of caffeine on Drosophila melanogaster: A proof-of-concept in vivo study for nootropic investigation. Arch Razi Inst 2021; 76(6):1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Ren Z, Zhang J, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol 2018; 9:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutsuddi M, Mukherjee A.. Insights into human neurodegeneration: Lessons learnt from Drosophila: Springer; 2019 [Google Scholar]
- 29.Kieliszek M, Edris A, Kot AM, et al. Biological activity of some aromatic plants and their metabolites, with an emphasis on health-promoting properties. Molecules 2020; 25(11):2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asfa N, Widianto AS, Pratama MKA, et al. Curcumin-mediated gene expression changes in Drosophila melanogaster. Pharm Educ 2023; 23(2):p. 84–91. [Google Scholar]
- 31.Ávila-Gálvez MÁ, Giménez-Bastida JA, González-Sarrías A, et al. New insights into the metabolism of the flavanones eriocitrin and hesperidin: A comparative human pharmacokinetic study. Antioxidants 2021; 10(3):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on request.