Abstract
Cyanuric acid (CYA) is a chlorine stabilizer used in swimming pools to limit UV degradation of chlorine, thus reducing chlorine use and cost. However, CYA has been shown to decrease the efficacy of chlorine disinfection. In the event of a diarrheal incident, CDC recommends implementing 3-log10 inactivation conditions for Cryptosporidium (CT value = 15 300 mg·min/L) to remediate pools. Currently, CYA’s impact on Cryptosporidium inactivation is not fully determined. We investigated the impact of multiple concentrations of CYA on C. parvum inactivation (at 20 and 40 mg/L free chlorine; average pH 7.6; 25 °C). At 20 mg/L free chlorine, average estimated 3-log10 CT values were 17 800 and 31 500 mg·min/L with 8 and 16 mg/L CYA, respectively, and the average estimated 1-log10 CT value was 76 500 mg·min/L with 48 mg/L CYA. At 40 mg/L free chlorine, 3-log10 CT values were lower than those at 20 mg/L, but still higher than those of free chlorine-only controls. In the presence of ~100 mg/L CYA, average 0.8- and 1.4-log10 reductions were achieved by 72 h at 20 and 40 mg/L free chlorine, respectively. This study demonstrates CYA significantly delays chlorine inactivation of Cryptosporidium oocysts, emphasizing the need for additional pool remediation options following fecal incidents.
Graphical abstract
INTRODUCTION
Cryptosporidium spp. are obligate, intracellular, protozoan parasites that infect the gastrointestinal tracts of humans and a wide variety of vertebrate mammalian hosts. Cryptosporidium is transmitted via ingestion of thick-walled, environmentally stable oocysts that are shed in the feces of infected persons or animals. In immunocompetent individuals, infection with Cryptosporidium can cause diarrhea which typically resolves in 2–3 weeks.1 Severe diarrhea can lead to dehydration, which is of particular concern in vulnerable populations, such as young children and pregnant women. Immunocompromised patients, such as HIV-infected persons, might experience chronic, severe diarrhea, which can lead to life-threatening malabsorption and wasting.2
During 2001–2010, Cryptosporidium emerged as the leading etiologic agent of outbreaks associated with recreational water use in the United States. Due to the parasite’s extreme chlorine tolerance, reported cryptosporidiosis outbreaks have largely occurred in chlorine-treated venues such as pools, interactive fountains, spas (or hot tubs), and waterparks.3 Currently, the U.S. Centers for Disease Control and Prevention (CDC) recommends hyperchlorination following a diarrheal incident (i.e., high-risk Cryptosporidium contamination event) or in response to at least a suspected recreational water-associated outbreak of cryptosporidiosis to achieve a CT value [chlorine concentration (mg/L) × time (min)] of 15 300 mg·min/L for 3-log10 Cryptosporidium oocyst inactivation in the absence of cyanuric acid (CYA). This CT value can be achieved in pool water at pH 7.5 and 25 °C by increasing the free chlorine concentration to 20 mg/L and maintaining it at that concentration for 12.75 h.4 However, operational questions remain about the impact of additives, such as chlorine stabilizers, on the inactivation of Cryptosporidium by hyperchlorination.
CYA is a common chlorine stabilizer that has been used in many U.S. swimming pools since the late 1950s.5 CYA forms weak bonds with free chlorine in water, stabilizing the measured free chlorine level in the presence of UV light (e.g., sunlight). This stabilization reduces the amount of additional disinfectant required to maintain regulated levels during peak summertime use, which can provide substantial cost savings to pool operators. CYA can be added to chlorinated pools as a stabilizer in discrete amounts but is more typically added continuously as a chlorinated isocyanurate (e.g., dichloroisocyanuric or trichloroisocyanuric acid). CYA, including the cyanurate moiety of chlorinated isocyanurate disinfectants, is quite stable, and as it is added to a pool, it will accumulate to high levels unless the pool water is exchanged or diluted.
Research beginning in the 1960s has demonstrated that CYA decreases the disinfection efficacy of free chlorine against bacteria and viruses when compared with free chlorine alone.6–10 Shields11 used a cell culture infectivity assay to provide the first evidence of reduced efficacy of free chlorine against C. parvum in the presence of CYA; however, their study reported CYA effects for a 1-log10 inactivation at pH 6.5, not the 3-log10 inactivation at pH 7.5 recommended by CDC in response to diarrheal incidents and suspected or confirmed cryptosporidiosis outbreaks. Data achieving 3-log10 or more inactivation of Cryptosporidium over various CYA concentrations typically found in public swimming pools are needed in order to revise current hyperchlorination recommendations.
Currently, the Model Aquatic Health Code advises maintaining CYA levels at or below 100 mg/L,12 but many pools likely exceed this level with unknown impact on the efficacy of Cryptosporidium hyperchlorination inactivation. The lack of 3-log10 Cryptosporidium inactivation data in the presence of CYA, the wide range of CYA concentrations likely found in public pools, and the unknown impact of increasing levels of CYA on Cryptosporidium inactivation pose challenges to providing evidence-based updates to existing diarrheal incident and cryptosporidiosis outbreak response recommendations for public swimming pools. As a result, this study was initiated to better understand the impact of increasing CYA concentrations on C. parvum inactivation to inform revision of existing recommendations.
METHODS
C. parvum and Madin Darby Canine Kidney (MDCK) Stocks
C. parvum (Maine isolate) oocysts were produced at the CDC as previously described13,14 and stored at 4 °C. Madin Darby Canine Kidney (MDCK) cells (CDC Scientific Resources Program, Atlanta GA) were routinely subcultured and, for infectivity assays, were inoculated onto 2.1 cm2 cover glass-bottom culture chambers (Nunc Lab-Tek, Rochester, NY), and incubated at 37 °C with 5% CO2 to achieve confluent monolayers at 96 h. Dulbecco’s Modified Eagle Medium (DMEM), High Glucose media supplemented to contain 0.1 mM MEM nonessential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 2 mM L-Glutamine (Gibco, Long Island, NY), and 10% heat-inactivated fetal bovine serum (Atlas Biologicals, Fort Collins, CO) was used for subculturing and infectivity assays.
Preparation of Oxidant Demand Free (ODF) Water and Glassware
Laboratory grade 5.65% sodium hypochlorite solution was used throughout the study. ODF water was prepared by buffering water treated by reverse osmosis to pH 7.5 using 1 M monobasic and dibasic sodium phosphate (final concentration 10 mM), then adding sodium hypochlorite to at least 5 mg/L free chlorine. Water was covered and remained at room temperature for at least 48 h before exposure to ultraviolet light in a biosafety cabinet to remove free chlorine. ODF glassware and stir bars were prepared by soaking them in deionized water containing at least 10 mg/L free chlorine for a minimum of 3 h, then rinsing with ODF water.15 Both ODF glassware and water were sterilized by autoclaving at standard sterilization conditions.
Preparation of Experimental Flasks
Each round of oocyst experimentation involved: (A) a flask containing only ODF water to measure natural oocyst die-off; (B) a flask containing ODF water and 20 or 40 mg/L free chlorine to measure oocyst inactivation in the absence of CYA; and (C and D) duplicate flasks containing ODF water; 20 or 40 mg/L free chlorine; and 8, 16, 50, or 100 mg/L CYA to measure oocyst inactivation in the presence of CYA. To prepare the CYA stock solution, 0.27 g of CYA (TCI America, Portland, OR) was added to 100 mL of ODF water, dissolved completely, then filter-sterilized through a 0.2 μm filter. Once sodium hypochlorite and CYA were added to flasks to achieved target concentrations, water was allowed to stabilize for at least 30 min. Initial pH (Accumet AR25 Benchtop Meter, Fisher Scientific, Pittsburgh, PA), oxidation–reduction potential (ORP) (Orion APlus, Thermo Fisher Scientific, Waltham, MA), free chlorine concentration of a sample dilution in deionized water (DPD Free Chlorine Method, Hach, Loveland, CA), and CYA concentration (melamine turbidimetric method, Hach, Loveland, CA) were measured. Probes were calibrated or standardized according to manufacturer’s instructions prior to each experiment.
EXPERIMENTAL SECTION
The appropriate volume of C. parvum Maine isolate oocyst stock was added to each flask to achieve a final concentration of approximately 105 oocysts/mL. Flask openings were covered with foil and remained in an environmental chamber (Espec North America Inc., Hudsonville, MI) at 25 °C with magnetic stir bars stirring at 150 rpm, for the duration of the experiment. At select time points over the duration of experiments, a water sample was removed and pH, ORP, and free chlorine concentration were measured. Probes were rinsed thoroughly in deionized water between samples. Neither pH nor disinfectant concentrations were adjusted during the duration of the experiment. At set time points, 4-, 40-, or 120-mL (as three 40 mL aliquots) samples were taken and immediately quenched in 50 mL polypropylene conical tubes containing either 1 or 10 mL 0.01 M phosphate buffered saline (PBS; pH 7.4) plus 0.1% bovine serum albumin (BSA), respectively, and sodium thiosulfate (Fisher Scientific, Pittsburgh, PA) at 50 mg/L per 1 mg/L disinfectant. Samples were stored at 4 °C until all samples could be concentrated simultaneously. As previously described,12 samples were centrifuged at 3290g for 10 min at 4 °C to pellet oocysts. Supernatant was carefully removed by aspiration and the pellet was resuspended and transferred into a nonstick 1.5 mL microcentrifuge tube (Phenix Research Products, Candler, NC) which was then centrifuged at 15 800g for 3 min at 4 °C. Supernatant was carefully removed by aspiration and rinsate from a 1 mL PBS (0.01 M, pH 7.2) rinse of the respective conical tube was layered onto the pellet before a final centrifugation step at 15 800g for 3 min at 4 °C. Supernatant was carefully removed by aspiration down to the 0.1 mL demarcation on the microcentrifuge tube and 0.9 mL of DMEM, High Glucose plus 0.75% synthetic sodium taurocholate was added. The solution was triturated and then incubated at ambient temperature (approximately 22 °C) for 10–15 min to initiate excystation (i.e., sporozoite release).
C. parvum Infectivity Assay
The DMEM/taurocholate suspension was inverted three times to mix and 150 μL was inoculated in duplicate onto confluent MDCK cell monolayers in culture chambers containing 1.5 mL fresh DMEM medium. Slides were incubated at 37 °C and 5% CO2 for 48–60 h. To visualize individual meronts and gamonts (life cycle stages that develop following sporozoite excystation and infection of MDCK cells if oocysts were viable and infectious), cell layers were fixed and labeled as previously described,13 with minor modification. Briefly, culture medium was removed and monolayers were washed three times with sterile 0.01 M PBS (pH 7.2), then fixed in Bouin’s solution (Ricca Chemical, Arlington, TX) for 30 min. Bouin’s solution was removed and monolayers were decolorized with five 10 min washes using 70% aqueous ethyl alcohol (prepared from anhydrous ethyl alcohol and deionized water), followed by overnight incubation in PBS with 0.1% BSA at 4 °C. The Cryptosporidium-specific monoclonal antibody C3C3 was bound to meronts and gamonts during a 1-h incubation. Unbound C3C3 was removed and monolayers were washed three times with sterile PBS and then fluorescently labeled by a 1-h incubation with FITC-Goat Anti-Mouse IgG (H + L) antibody (Invitrogen, Frederick, MD) diluted 1/100 (1.0%) in PBS/BSA supplemented with 2 mM sodium azide, followed by three washes with sterile PBS. All rinses and incubation periods were performed with gentle rocking at ambient temperature. Steps following use of fluorescent antibodies used covered chambers to protect the fluorochrome from quenching by exposure to light. After the final PBS wash, monolayers in each well were sealed using three drops of poly(vinyl alcohol) mounting medium with DABCO16 under an 18 mm2 glass coverslip and stored covered at 4 °C.
Microscopy
Zeiss AxioVision software (Carl Zeiss, Thornwood, NY) systematically captured 72 adjacent immunofluorescent microscopical fields for each culture chamber at 100× magnification, representing approximately 20% of each monolayer. Images were captured using a Zeiss HRm digital camera (Carl Zeiss) on an AxioVert 200 M microscope (Carl Zeiss). Zeiss Vision Image (zvi) files were converted to JPEG files. More than 82 000 cell culture images were then visually assessed for defects (i.e., antibody labeling quality, lack of focus, missing monolayer), then analyzed by ImageJ software17 customized to enumerate individual meronts and gamonts based on size (~3–5 μm), shape (circularity), and labeling by fluorescent antibody (see Figure 1a,b). Microsoft Excel (Redmond, WA, U.S.A.) was used to calculate average number and size of developing life cycle stages per microscopical field. The number of developing stages in culture is directly related to the number of inoculated oocysts which contain infectious sporozoites.13 For each sample, an average count of developing stages in acceptable fields of duplicate wells was divided by the average number of microscopical fields counted; back-calculation provided expected counts for an entire well consisting of 350 microscopical fields. The original sample volume concentrated (e.g., 4 mL) and the volume of the concentrate applied to each well (i.e., 0.15 mL) were used to determine developing stages per mL in flasks. All in vitro parasite concentrations were log10 transformed and compared to control flasks to determine log10 inactivation.
Figure 1.
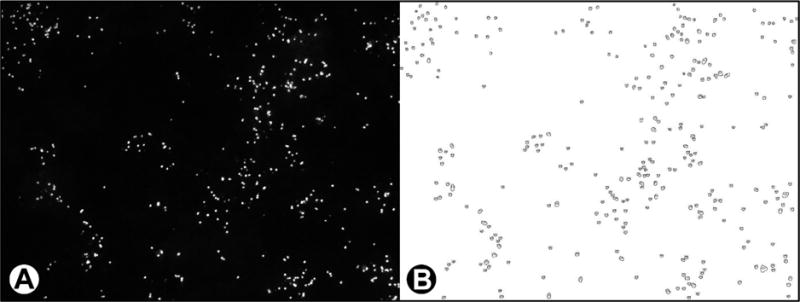
(a) Microscopical field with C. parvum developing stages labeled with a parasite-specific fluorescent monoclonal antibody; (b) counterpart image with C. parvum life stages enumerated by ImageJ Software.
CT Calculation
Free chlorine decay was defined as ≥20% decay in the free chlorine concentration from that at time 0 h; all C. parvum inactivation data associated with ≥20% decay in free chlorine were excluded. CT values were calculated for experiments that reached target log10 inactivation; data from experiments that did not achieve the target level of C. parvum inactivation were excluded from CT calculation to avoid extrapolation. Free chlorine concentrations in each individual flask throughout the experimental contact time were arithmetically averaged to provide the overall concentration for that flask. C. parvum inactivation throughout the experimental contact time in each individual flask was determined by subtracting log10[C. parvum] at each experimental time point from log10[C. parvum] at time 0 h. A linear trend line was fit to a scatter plot of time versus log10 inactivation; if R2 values were ≥0.9, then the resulting equation was used to determine time required for the target level of log inactivation. Assuming first order kinetics, the CT value for each individual flask was calculated by multiplying the average free chlorine concentration by the time required to achieve the target level of inactivation. For each condition studied, these CT values were arithmetically averaged.
Statistical Analysis
To assess the association between CT values and oocyst age, the Pearson correlation coefficient was calculated between 3-log10 CT and oocyst age. To investigate water parameter measurements (e.g., pH, ORP, free chlorine concentration) between and across experimental groups, and log10 inactivation across time, linear mixed models were implemented to account for the random effects arising from replicates with respect to the same CYA and free chlorine settings using SAS 9.3 (Cary, NC). Specifically, from modeling log10 inactivation by time, linear trends and their corresponding 95% confidence intervals were estimated for each group and plotted in R software (Vienna, Austria).18 As these models focus on the inactivation trend rather than the 3-log10 CT value, experiments that did not achieve 3-log10 reduction but had minimal free chlorine decay were included in these analyses. The LOWESS (locally weighted scatterplot smoothing) method was also implemented to graphically illustrate a smooth trend line across time for CYA = 100 mg/L and ODF control groups. Comparisons of the log10 inactivation among control and experimental groups were performed at selected time points. For all comparisons, Bonferroni correction, in which the original significance level is divided by the number of comparisons to derive the adjusted significance level for each individual comparison, was applied to adjust for the multiple comparisons.
RESULTS
ODF Water Control
The average ORP in ODF water control flasks (n = 12) was 275 mV (range 178–390 mV) (Table 1). In ODF water at 25 °C, natural oocyst die-off was <0.5 log10 through approximately 96 h; there was an approximate 1-log10 reduction in viability by 172 h and at least a 2.4-log10 reduction by 360 h (Figure 2c).
Table 1.
Descriptive Characteristics for Oxidant Demand-Free (ODF) Water Control, Free Chlorine (FC) Controls, and Low and High Concentration Cyanuric Acid (CYA) Experiments
| experimental type | no. experiments | avg oocyst age (days) | avg pHa | avg FC (mg/L)a | avg ORP (mV)a,c | avg CYA (mg/L)b |
|---|---|---|---|---|---|---|
| ODF control | 12 | 74 | 7.6 | 0.0 | 275 | 0 |
| 21.6 mg/L FC | 11 | 72 | 7.6 | 20.9 | 770 | 0 |
| 21.1 mg/L FC8 + mg/L CYA | 4 | 52 | 7.6 | 21.1 | 770 | 8 |
| 19.1 mg/L FC + 16 mg/L CYA | 4 | 90 | 7.6 | 19.5 | 757 | 16 |
| 21.2 mg/L FC + 48 mg/L CYA | 4 | 70 | 7.6 | 20.7 | 653 | 48 |
| 21.2 mg/LFC + 98 mg/L CYA | 4 | 40 | 7.5 | 20.3 | 652 | 98 |
| 40.6 mg/L FC | 8 | 75 | 7.6 | 40.3 | 796 | 0 |
| 40.9 mg/LFC + 9 mg/L CYA | 4 | 107 | 7.6 | 41.0 | 773 | 9 |
| 38.3 mg/L FC + 15 mg/L CYA | 4 | 86 | 7.6 | 37.4 | NDd | 15 |
| 38.5 mg/L FC + 46 mg/L CYA | 4 | 70 | 7.6 | 38.5 | 737 | 46 |
| 39.4 mg/L FC + 90 mg/L CYA | 4 | 40 | 7.5 | 38.7 | 692 | 90 |
Average of measurements taken at each sampling time point (generally 4–5 time points per experiment).
Average measurement at time 0 h.
ORP: oxidation reduction potential.
ND: no data.
Figure 2.
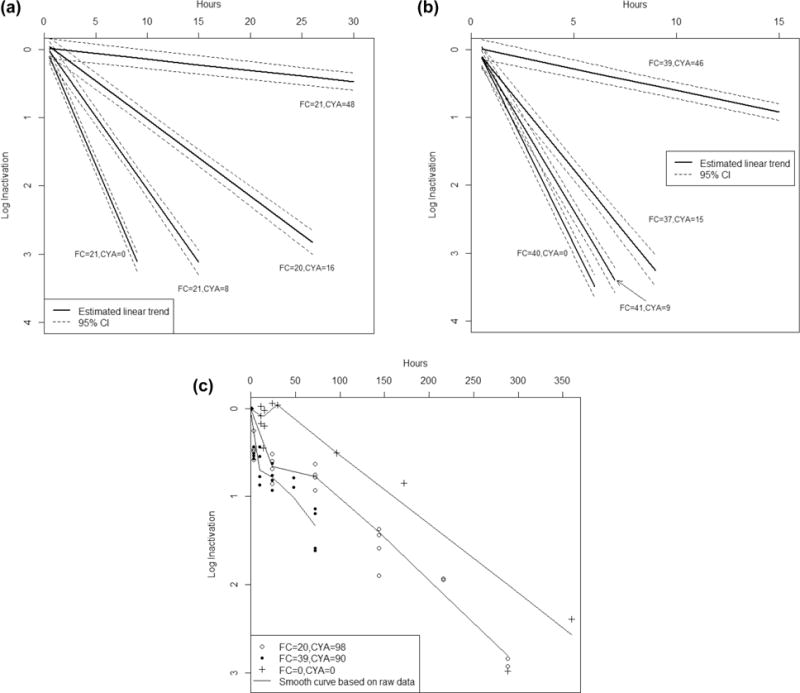
(a) Time (hr) versus log10 inactivation of C. parvum (95% confidence interval) at 20 mg/L free chlorine (FC) for cyanuric acid (CYA) concentrations of 0–48 mg/L. Note: solid lines represent the linear trend estimated by a linear mixed model; graphical representations of log10 inactivation utilize linear interpolation to communicate experimental results and should not be viewed as suggesting a kinetic model for the actual oocyst disinfection process. (b) Time (hr) versus log10 inactivation of C. parvum (95% confidence intervals) at 40 mg/L FC for CYA concentrations 0–46 mg/L. Note: solid lines represent the linear trend estimated by a linear mixed model; graphical representations of log10 inactivation utilize linear interpolation to communicate experimental results and should not be viewed as suggesting a kinetic model for the actual oocyst disinfection process. (c) Time (hr) versus log10 inactivation of C. parvum (95% confidence intervals) at 20 and 40 mg/L FC for 100 mg/L CYA and oxidant demand-free (ODF) water control. Note: points represent the observed raw data and solid lines represent the approximated smooth curved based on raw data.
20 mg/L Free Chlorine Control
The average free chlorine concentration in all 20 mg/L free chlorine control flasks (n = 11) was 20.9 mg/L (range 17.2–24.8 mg/L) and the average ORP was 770 mV (range 724–819 mV). A 3-log10 inactivation of oocysts was achieved in six 20 mg/L free chlorine control experiments after an average contact time of 8.2 h (range 6.9–9.5 h) (Figure 2a). The average estimated CT value for a 3-log10 inactivation of oocysts was 10,500 mg·min/L (range 9200–12 000 mg·min/L) (Table 2).
Table 2.
Average Estimated 3-log10 Inactivation CT Values for C. parvum in Free Chlorine (FC) Controls and Low Concentration Cyanuric Acid (CYA) Experiments; Average pH 7.6
| average FC conc. (mg/L) | average CYA conc. (mg/L) | average time 3-log10 inactivation (hr) | average estimated 3-log10 CT value (mg·min/L) (range) |
|---|---|---|---|
| 21.6 | 0 | 8.2 | 10 500 (9200–12 000) |
| 21.1 | 8 | 14.1 | 17 800 (16 000–20 300) |
| 19.1 | 16 | 27.5 | 31 500 (30 900–32 100) |
| 40.6 | 0 | 5.1 | 12 400 (10 300–15 200) |
| 40.9 | 9 | 6.2 | 15 300 (14 500–16 100) |
| 38.3 | 15 | 8.4 | 19 400 (19 300–19 500) |
40 mg/L Free Chlorine Control
The average free chlorine concentration in all 40 mg/L free chlorine control flasks (n = 8) was 40.3 mg/L (range 36.6–43.8 mg/L) and the average ORP was 796 mV (range 753–831 mV). A 3-log10 inactivation of oocysts was achieved in seven 40 mg/L free chlorine control experiments after an average contact time of 5.1 h (range 4.2–6.3 h) (Figure 2b). The average estimated CT value for a 3-log10 inactivation of oocysts was 12 400 mg·min/L (range 10 300–15 200 mg·min/L).
Low CYA Concentration Experiments
8 mg/L CYA Experiments
At 20 mg/L free chlorine (average 21.1 mg/L, range 19.6–24.0 mg/L) and with an average of 8 mg/L CYA (range 7–9 mg/L) (n = 4 flasks), the average ORP was 770 mV (range 744–792 mV). A 3-log10 inactivation of oocysts was achieved in three experiments after an average contact time of 14.1 h (range 13.0–16.3 h) (Figure 2a). The average estimated CT value for a 3-log10 inactivation of oocysts was 17 800 mg· min/L (range 16 000–20 300 mg·min/L).
At 40 mg/L free chlorine (average 41.0 mg/L, range 34.8–43.2 mg/L) and with an average of 9 mg/L CYA (range 8–9 mg/L) (n = 4 flasks), the average ORP was 773 mV (range 750–786 mV). A 3-log10 inactivation of oocysts was achieved in all four experiments after an average contact time of 6.2 h (range 6.1–6.4 h) (Figure 2b). The average estimated CT value for a 3-log10 inactivation of oocysts was 15 300 mg·min/L (range 14 500–16 100 mg·min/L).
16 mg/L CYA Experiments
At 20 mg/L free chlorine (average 19.5 mg/L, range 17.3–21.6 mg/L) and with an average of 16 mg/L CYA (range 16–17 mg/L) (n = 4 flasks), the average ORP was 757 mV (694–777 mV). A 3-log10 inactivation of oocysts was achieved in two experiments after an average contact time of 27.5 h (range 27.0–27.9 h) (Figure 2a). The average estimated CT value for a 3-log10 inactivation of oocysts was 31 500 mg·min/L (range 30 900–32 100 mg·min/L).
At 40 mg/L free chlorine (average 37.4 mg/L, range 33.2–40.6) and with an average of 15 mg/L CYA (range 14–16 mg/L) (n = 4 flasks); no ORP data are available due to technical difficulties. A 3-log10 inactivation of oocysts was achieved in two experiments after an average contact time of 8.4 h (range 8.4–8.5 h) (Figure 2b). The average estimated CT value for a 3-log10 inactivation of oocysts was 19 400 mg·min/L (range 19 300–19 500 mg·min/L).
High CYA Concentration Experiments
50 mg/L CYA Experiments
At 20 mg/L free chlorine (average 20.7 mg/L, range 16.2–28.6 mg/L) and with an average of 48 mg/L CYA (range 45–52 mg/L) (n = 4 flasks), the average ORP was 653 mV (range 546–728 mV). A 1-log10 inactivation of oocysts was achieved in all four experiments after an average contact time of 61.9 h (range 43.5–79.4 h) (Figure 2a). The average estimated CT value for a 1-log10 inactivation of oocysts was 76 500 mg· min/L (range 58 500–91 000 mg·min/L) (Table 3).
Table 3.
Average Estimated 1-log10 Inactivation CT Values for C. parvum in Free Chlorine (FC) Controls and 50 mg/L Cyanuric Acid (CYA) Experiments; Average pH 7.6
| average FC conc. (mg/L) | average CYA conc. (mg/L) | average time 1-log10 inactivation (hr) | average estimated 1-log10 CT value (mg·min/L) (range) |
|---|---|---|---|
| 21.6 | 0 | 2.7 | 3500 (3100–4000) |
| 21.2 | 48 | 61.9 | 76 500 (58 500–91 000) |
| 40.6 | 0 | 3.7 | 4100 (3400–5100) |
| 38.5 | 46 | 17.2 | 40 000 (31 100–55 300) |
At 40 mg/L free chlorine (average 38.5 mg/L, range 34.6–42.6 mg/L) and with an average of 46 mg/L CYA (range 41–52 mg/L) (n = 4 flasks), the average ORP was 737 mV (range 682–771 mV). A 1-log10 inactivation of oocysts was achieved in two experiments after an average contact time of 17.2 h (range 13.5–23.4 h) (Figure 2b). The average estimated CT value for a 1-log10 inactivation of oocysts was 40 000 mg·min/L (range 31 100–55 300 mg·min/L).
100 mg/L CYA Experiments
At both 20 and 40 mg/L free chlorine, 1-log10 CT values could not be calculated for 100 mg/L CYA experiments due to the nonlinearity of the data. Instead, log10 reduction values are provided.
At 20 mg/L free chlorine (average 20.3 mg/L, range 15.6–24.2 mg/L) and with an average of 98 mg/L CYA (range 90–100 mg/L) (n = 4 flasks), free chlorine concentrations did not decay ≥20% (compared with free chlorine concentration at time 0 h) up to a minimum of 144 h. The average ORP was 652 mV (range 614–688 mV). There was an average 0.8-log10 reduction of oocysts at 72 h and 1.6-log10 inactivation at 144 h (Figure 2c).
At 40 mg/L free chlorine (average 38.7 mg/L, range 30.4–42.6 mg/L) and with an average of 90 mg/L CYA (n = 4), the free chlorine concentration did not decay ≥20% up to a minimum of 72 h. The average ORP was 692 mV (range 660–729 mV). There was an average 0.8-log10 reduction of oocysts at 24 h and 1.4-log10 inactivation at 72 h (Figure 2c).
Statistical Comparisons
Oocyst Age
Across all experiments, the average age of oocysts at the time of experiment was 74 days (range 22–110 days). No systematic pattern was detected on a scatter plot between oocyst age and CT value in 20 and 40 mg/L free chlorine control groups (data not shown). Although a similar examination of oocyst age versus CT value among CYA experimental groups is restricted due to small sample sizes, this result suggests that oocyst age within the range tested was not associated with altered CT values for CYA experimental groups, as free chlorine controls utilizing the same oocyst lot were always conducted in parallel.
pH
Across all experimental conditions, the average pH over the duration of all experiments was 7.6 (range 7.4–7.7). pH was not statistically different between free chlorine controls and low CYA and ~50 mg/L CYA experimental groups (p > 0.03; adjusted significance level = 0.005); however, pH in ~100 mg/L CYA experiments was practically the same (average pH 7.5), but statistically lower (p < 0.001) in comparison.
Free Chlorine and ORP
Average free chlorine concentrations were consistent across flasks containing 20 mg/L free chorine or flasks containing 40 mg/L. ORP was not statistically different between free chlorine controls and low concentration CYA experimental groups (p > 0.02; adjusted significance level = 0.005), however ORP in ODF controls and high concentration CYA experiments was statistically lower (p < 0.001 for all) in comparison.
Log10 Inactivation
Comparisons of the control and experimental groups performed at select time points indicated that at both 20 and 40 mg/L free chlorine, log10 inactivation was not significantly different early in the disinfection process (up to 1 h for 20 mg/L and up to 0.5 h for 40 mg/L). However, as early as 2 h, log10 inactivation was significantly lower in the presence of low CYA concentrations and ~50 mg/L CYA (Table 4).
Table 4.
Statistical Comparisons of Log10 Inactivation at Different Cyanuric Acid (CYA) Concentrations and Select Time Points
| FCc = 20 mg/L |
p value
|
||||
|---|---|---|---|---|---|
| CYA (mg/L) | 0.5 h | 1 h | 2 h | 3+ hr | |
| 0 vs 8 | 0.355 | 0.080 | <0.001a | <0.001a | |
| 0 vs 16 | 0.268 | 0.056 | <0.001a | <0.001a | |
| 0 vs 48 | 0.837 | 0.328 | 0.012b | <0.001a | |
| 8 vs 16 | 0.369 | 0.108 | 0.003a | <0.001a | |
| 8 vs 48 | 0.910 | 0.424 | 0.031b | <0.001a | |
| 16 vs 48 | 0.946 | 0.528 | 0.044b | <0.001a | |
| p value | |||||
| FCc = 40 mg/L | CYA (mg/L) | 0.5 h | 1 h | 2 h | 3+ hr |
| 0 vs 9 | 0.282 | 0.011b | <0.001a | <0.001a | |
| 0 vs 15 | 0.569 | 0.037b | <0.001a | <0.001a | |
| 0 vs 46 | 0.893 | 0.072 | <0.001a | <0.001a | |
| 9 vs 15 | 0.362 | 0.024b | <0.001a | <0.001a | |
| 9 vs 46 | 0.590 | 0.050b | <0.001a | <0.001a | |
| 15 vs 46 | 0.306 | 0.015b | <0.001a | <0.001a | |
significant (p ≤ 0.008) (adjusted significance level).
marginally significant (p = 0.009–0.05).
FC: free chlorine.
DISCUSSION
Fecal incidents in public pools are relatively common and have the potential to transmit infectious pathogens among swimmers.19 The extreme chlorine tolerance of Cryptosporidium led to public health recommendations to treat a diarrheal release as a potential high-risk Cryptosporidium contamination event requiring hyperchlorination remediation as well as in response to suspected or confirmed recreational water-associated outbreak of cryptosporidiosis. As a result, CDC recommends increasing the free chlorine concentration to 20 mg/L for 12.75 h (CT value = 15 300 mg·min/L) for a 3-log10 inactivation of Cryptosporidium oocysts in the absence of CYA.4 In this study, we report increased CT values for inactivation of the C. parvum Maine isolate in the presence of low and high concentrations of CYA, at both 20 and 40 mg/L free chlorine (average pH 7.6, 25 °C). These data indicate that addition of CYA substantially delays Cryptosporidium inactivation as CYA concentration increases. Changes in disinfection efficacy are also suggested by the modest but statistically significant decrease in ORP readings observed between free chlorine controls and high CYA concentration experiments.
At 20 mg/L free chlorine, the presence of 8 and 16 mg/L CYA resulted in average estimated 3-log10 CT values (17 800 and 31 500 mg·min/L, respectively), nearly 2–3X higher than CT values calculated for the 20 mg/L free chlorine control (10 500 mg·min/L). At 9 and 15 mg/L CYA, doubling the free chlorine concentration to 40 mg/L resulted in average estimated 3-log10 CT values (15 300 and 19 400 mg·min/L, respectively); these CT values were lower than those calculated at 20 mg/L free chlorine, but still higher than that calculated for the 40 mg/L free chlorine control (12 400 mg·min/L). At 20 mg/L free chlorine, the presence of 48 mg/L CYA resulted in a nearly 22× increase in the average estimated 1-log10 CT value (76 500 mg·min/L) as compared with the 20 mg/L free chlorine control (3500 mg·min/L). At 40 mg/L free chlorine and 46 mg/L CYA, the average estimated 1-log10 CT value increase was nearly 10X that of the 40 mg/L free chlorine control (40 000 and 4100 mg·min/L, respectively). At both low CYA concentration and ~50 mg/L CYA, log10 inactivation was significantly less than 20 and 40 mg/L free chlorine controls within an exposure time of 2 h.
In the presence of ~100 mg/L CYA, 1-log10 CT values could not be calculated due to nonlinearity of the data; however, at 72 h, average 0.8- and 1.4-log10 reductions were achieved with 20 and 40 mg/L free chlorine concentrations, respectively. ODF water control data indicate that natural oocyst die-off was 0.5-log10 by 96 h and continued to increase to ≥2.4-log10 by 360 h. This suggests that natural oocyst die-off played an increasingly substantial role in the reduction of infectious oocysts over extended experimental time periods when CYA was present.
As in this study, Shields11 reported a significant reduction in C. parvum inactivation in the presence of CYA. However, several notable differences exist between the Shields study and the current study. Shields extrapolated data and suggested that in the presence of 50 mg/L CYA, increasing the free chlorine concentration to 40 mg/L and decreasing the pH to 6.5 would be needed to achieve a 3-log10 inactivation (extrapolated CT value of 67 000 mg·min/L, based on a 2.7-log10 inactivation). Such a response protocol would require substantial amendment of pool chemistries, which could pose challenges to pool operators depending on their level of training. In the current study, experiments were conducted at pH 7.5 (considered a more realistic option for typical pool operation and remediation) and lower CYA concentrations were investigated to enable estimation of CT values based on measurable 3-log10 reductions. Data from this study showed that 62 h were required to inactivate 90% (1-log10) of Cryptosporidium in the presence of approximately 50 mg/L CYA at 20 mg/L free chlorine; however, Shields reported a 0.7-log10 reduction of C. parvum Iowa oocysts at 10 h under these same conditions. These differences might be due to this study’s use of improved methodological and analytical procedures, such as automated image analysis. In addition, the improved methods used in this study suggest that the lower CT value derived for Cryptosporidium inactivation in the absence of CYA (10 500 mg·min/L), when compared to the Shields data (15 300 mg·min/L),14 should be evaluated for inclusion in future updates to diarrheal fecal incident remediation recommendations.
This study was subject to several notable limitations. First, water in recreational water venues can contain materials introduced by bathers and the environment, including nitrogenous wastes (e.g., sweat, urine), sunscreen and other personal care products, organic debris, disinfection byproducts, and other chemicals that make it a complex and ever-changing chemistry. Furthermore, uncovered outdoor pools receive exposure to ultraviolet (UV) light which affects free chlorine chemistry both in the presence and absence of CYA. This study was conducted in oxidant-demand-free water under ideal swimming pool water conditions without exposure to UV light to minimize and control experimental variability. Further research is warranted to evaluate disinfection differences that might exist in waters more representative of actual recreational swimming venues. Second, due to the complexity, labor-intensity, and high cost of experiments, the sample size for each CYA concentration was low. Data for additional replicates and CYA concentrations might help further elucidate relationships between CYA, free chlorine concentrations, C. parvum inactivation, and resulting CT values. Additional research evaluating variations in temperature and pH as well as research further evaluating the complex chemistry of these matrices,20 including the relationship between molar ratios and disinfection efficacy, is also warranted. Third, 3-log10 CT values were not able to be calculated in high CYA concentration experiments due to excessive free chlorine decay over extended experimental time periods (up to 360 h). Maintaining the target free chlorine concentration throughout entire experimental time periods, perhaps by means of an automated chlorine feed system, would have provided improved data for calculating CT values.
This study demonstrates that cyanuric acid significantly reduces the efficacy of chlorination for inactivating Cryptosporidium oocysts. Data from low CYA experiments indicate that hyperchlorination can still be effective in achieving 3-log10 inactivation of Cryptosporidium when CYA concentrations are low (~8 mg/L), but higher chlorine concentrations (e.g., 30–40 mg/L) might be needed to achieve such reductions within a time frame typically used for hyperchlorination (e.g., 12.75 h) (Figure 2). On the basis of the results of this study, existing responses to diarrheal fecal incidents and suspected or confirmed recreational water-associated outbreaks of cryptosporidiosis that rely on hyperchlorination are inadequate when used in pools that contain higher levels of cyanurate (50–100 mg/L), including concentrations currently acceptable in many state pool codes and the Model Aquatic Health Code. Such pools would need to reduce CYA levels to achieve 3-log10 inactivation conditions or employ an alternate disinfectant technique for remediation. While data for alternatives to hyperchlorination are not robust, some scientific literature is available that suggests that using disinfection technologies such as chlorine dioxide,21 UV irradiation systems,22 or ozonation23 could be effective for achieving 3-log10 inactivation of Cryptosporidium in aquatics facilities that use CYA-based water treatment products. In addition, facility operators also have the option of removing Cryptosporidium oocysts from affected pool systems by enhanced filtration or removing and replacing contaminated water in the systems. These findings suggest that implementable changes or alternatives to hyperchlorination remediation following diarrheal fecal incidents should be further investigated for use in recreational water venues that utilize CYA-based chlorine stabilization products.
Acknowledgments
The authors wish to thank Gordana Derado for assistance with statistical analyses. This study was supported in part by funding provided by Arch Chemicals, Inc (now Lonza Group Ltd). The use of trade names and names of commercial sources is for identification only and does not imply endorsement by the CDC or the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the CDC.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15(1):145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fayer R, Xiao L, editors. Cryptosporidium and Cryptosporidiosis. 2nd. CRC Press; Boca Raton, Fl: 2008. [Google Scholar]
- 3.Hlavsa MC, Roberts VA, Kahler AM, Hilborn ED, Wade TJ, Backer LC, Yoder JS. Recreational Water-Associated Disease Outbreaks—United States, 2009–2010. MMWR. 2014;63(1):6–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Notice to readers: Revised recommendations for responding to fecal accidents in disinfected swimming venues. MMWR. 2008;57(6):151–152. [Google Scholar]
- 5.Canelli ED. Chemical, bacteriological, and toxicological properties of cyanuric acid and chlorinated isocyanurates as applied to swimming pool disinfection: A review. Am J Public Health. 1974;64(2):155–162. doi: 10.2105/ajph.64.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JR. A study of the influence of cyanuric acid on the bactericidal effectiveness of chlorine. Am J Public Health N. 1965;55(10):1629–1637. doi: 10.2105/ajph.55.10.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald GP, DerVartanian ME. Factors influencing the effectiveness of swimming pool bactericides. Appl Microbiol. 1967;15(3):504–509. doi: 10.1128/am.15.3.504-509.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golaszewski G, Seux R. The kinetics of the action of chloroisocyanurates on three bacteria – Pseudomonas aeruginosa, Streptococcus faecalis, and. Staphylococcus aureus Water Res. 1994;28(1):207–217. [Google Scholar]
- 9.Robinton ED, Mood EW. An evaluation of the inhibitory influence of cyanuric acid upon swimming pool disinfection. Am J Public Health N. 1967;57(2):301–310. doi: 10.2105/ajph.57.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita T, Sakae K, Ishihara Y, Isomura S, Inoue H. Virucidal effect of chlorinated water containing cyanuric acid. Epidemiol Infect. 1988;101(3):631–639. doi: 10.1017/s0950268800029502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields JM, Arrowood MJ, Hill VR, Beach MJ. The effect of cyanuric acid on the disinfection rate of Cryptosporidium parvum in 20-ppm free chlorine. J Water Health. 2009;7(1):109–114. doi: 10.2166/wh.2009.008. [DOI] [PubMed] [Google Scholar]
- 12.The Model Aquatic Health Code. 1st. Aug, 2014. http://www.cdc.gov/healthywater/pdf/swimming/pools/mahc/Complete-First-Edition-MAHC-Code.pdf (accessed: Jan. 21, 2015)
- 13.Arrowood MJ. vitro cultivation of cryptosporidium species. Clin Microbiol Rev. 2002;15(3):390–400. doi: 10.1128/CMR.15.3.390-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields JM, Hill VR, Arrowood MJ, Beach MJ. Inactivation of Cryptosporidium parvum under chlorinated recreational water conditions. J Water Health. 2008;6(4):513–520. doi: 10.2166/wh.2008.068. [DOI] [PubMed] [Google Scholar]
- 15.American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. 21st. American Public Health Association; Washington, DC: 2005. [Google Scholar]
- 16.Arrowood MJ, Mead JR, Xie L, You W. In vitro anticryptosporidial activity of dinitroaniline herbicides. FEMS Microbiol Lett. 1996;136(3):245–249. doi: 10.1111/j.1574-6968.1996.tb08056.x. [DOI] [PubMed] [Google Scholar]
- 17.Rasband WS. ImageJ. http://imagej.nih.gov/ij/ (accessed Jan. 21, 2015)
- 18.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org/ (accessed Jan. 21, 2015) [Google Scholar]
- 19.Centers for Disease Control and Prevention. Prevalence of parasites in fecal material from chlorinated swimming pools—United States, 1999. MMWR. 2001;50(20):410–412. [PubMed] [Google Scholar]
- 20.O’Brien JE, Morris JC, Butler JN. Equilibria in Aqueous Solutions of Chlorinated Isocyanurate. In: Rubin AJ, editor. Chemistry of Water Supply, Treatment, and Distribution. Vol. 333. Ann Arbor Science Publishers Inc.; Ann Arbor, MI: 1974. p. 358. [Google Scholar]
- 21.Murphy JL, Haas CN, Arrowood MJ, Hlavsa MC, Beach MJ, Hill VR. Efficacy of chlorine dioxide tablets on inactivation of Cryptosporidium oocysts. Environ Sci Technol. 2014;48:5849–5856. doi: 10.1021/es500644d. [DOI] [PubMed] [Google Scholar]
- 22.Clancy J, Bukhari Z, Hargy T, Bolton J, Dussert BW, Marshall MM. Using UV to inactivate Cryptosporidium. J Am Water Works Assoc. 2000;92:97–104. [Google Scholar]
- 23.Donofrio RS, Aridi S, Saha R, Bechanko R, Schaefer K, Bestervelt LL, Hamil B. Laboratory validation of an ozone device for recreational water treatment. J Water Health. 2013;11(2):267–276. doi: 10.2166/wh.2013.198. [DOI] [PubMed] [Google Scholar]


