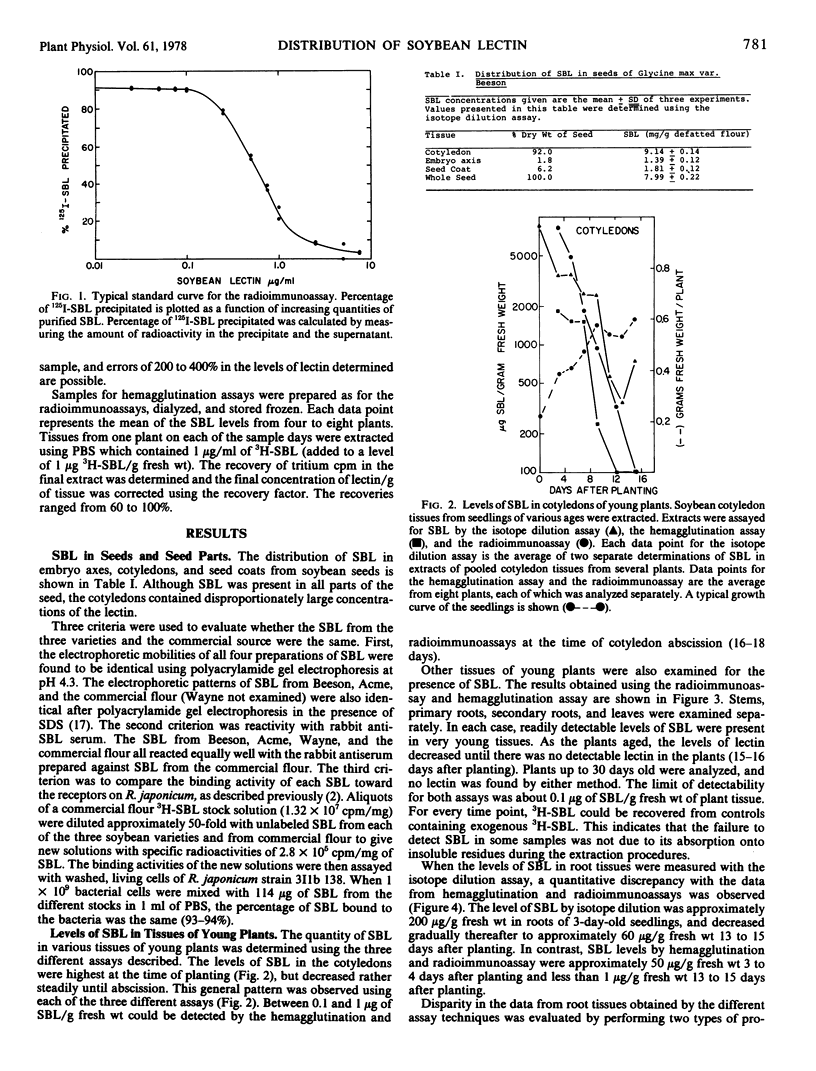
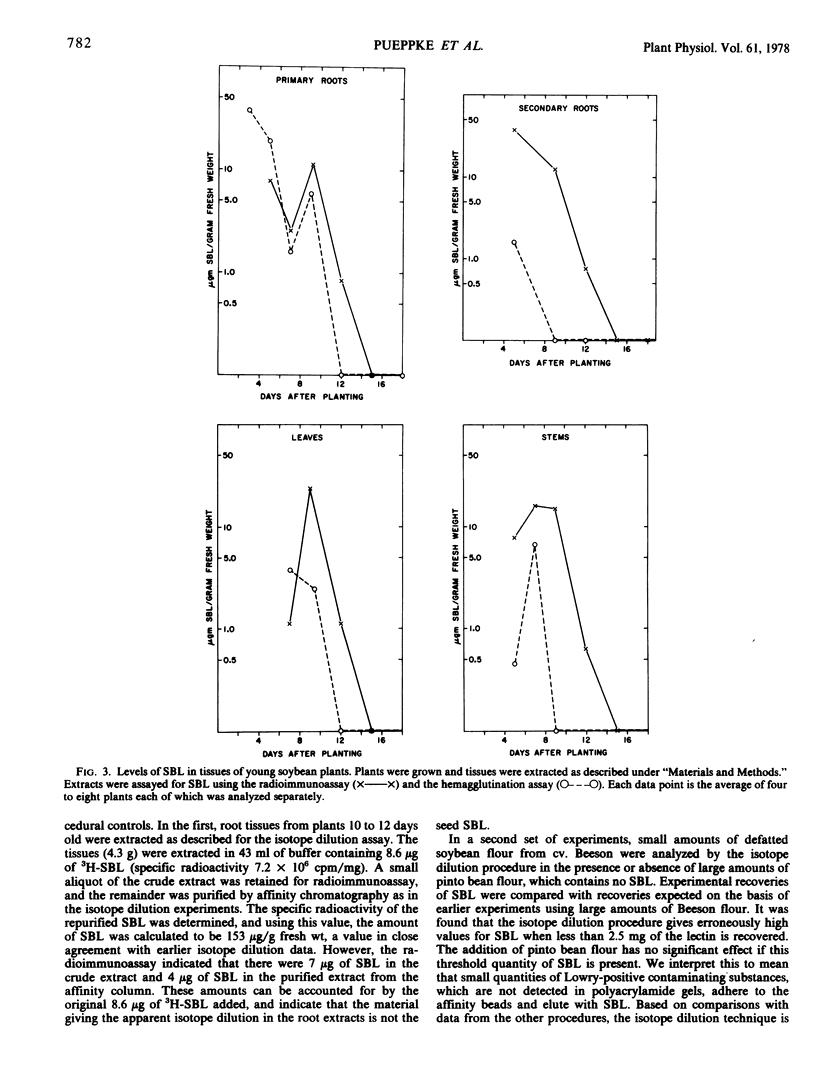
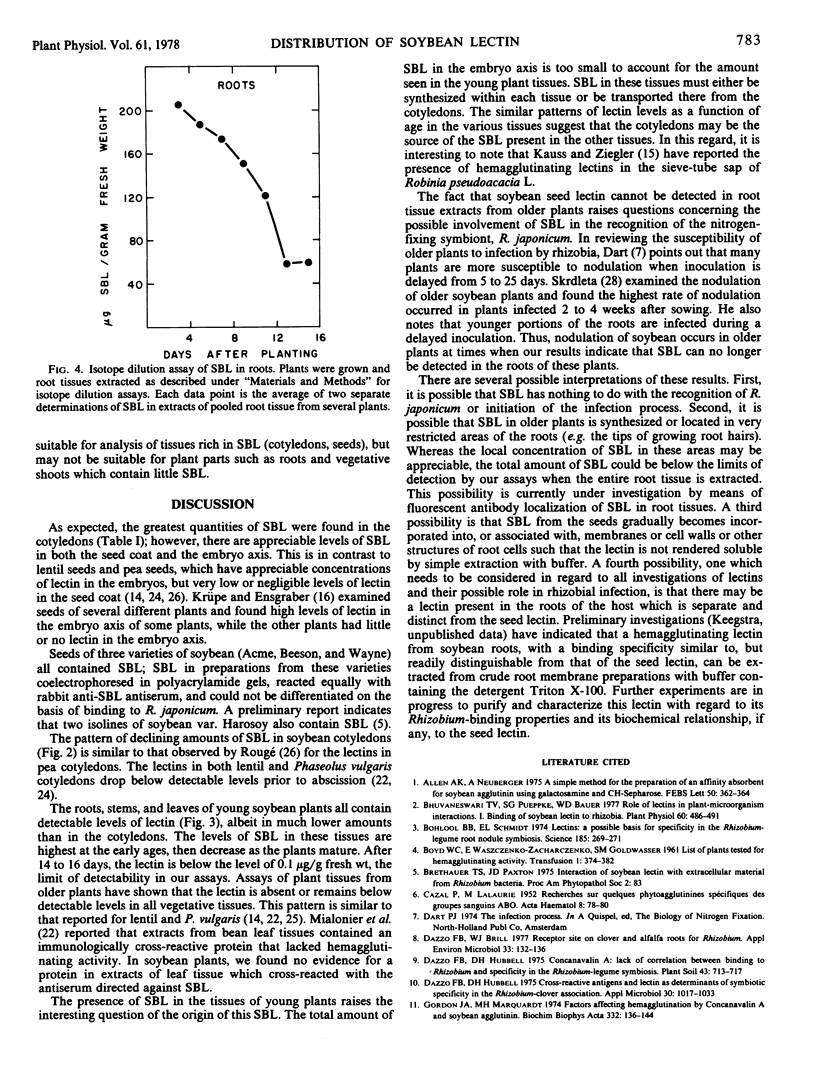
Abstract
Three different assay procedures have been used to quantitate the levels of soybean (Glycine max [L.] Merr.) lectin in various tissues of soybean plants. The assays used were a standard hemagglutination assay, a radioimmunoassay, and an isotope dilution assay. Most of the lectin in seeds was found in the cotyledons, but lectin was also detected in the embryo axis and the seed coat. Soybean lectin was present in all of the tissues of young seedlings, but decreased as the plants matured and was not detectable in plants older than 2 to 3 weeks. Soybean lectin isolated from seeds of several soybean varieties were identical when compared by several methods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A. A simple method for the preparation of an affinity absorbent for soybean agglutinin using galactosamine and CH-Sepharose. FEBS Lett. 1975 Feb 15;50(3):362–364. doi: 10.1016/0014-5793(75)80528-x. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- CAZAL P., LALAURIE M. Recherches sur quelques phyto-agglutinines spécifiques des groupes sanguins ABO. Acta Haematol. 1952 Jul-Aug;8(1-2):73–80. doi: 10.1159/000204150. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Receptor site on clover and alfalfa roots for Rhizobium. Appl Environ Microbiol. 1977 Jan;33(1):132–136. doi: 10.1128/aem.33.1.132-136.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin J., Kent S. P. Possible role of phytohaemagglutinin in Phaseolus vulgaris L. Nat New Biol. 1973 Sep 5;245(140):28–30. doi: 10.1038/newbio245028a0. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Horton C. B. Studies on the appearance and location of hemagglutinins from a common lentil during the life cycle of the plant. Arch Biochem Biophys. 1972 Mar;149(1):323–326. doi: 10.1016/0003-9861(72)90328-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Lotan R., Debray H., Cacan M., Cacan R., Sharons N. Labeling of soybean agglutinin by oxidation with sodium periodate followed by reduction with sodium [3-H]borohydride. J Biol Chem. 1975 Mar 10;250(5):1955–1957. [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rougé P. Devenir des phytohémagglutinines provenant des diverses parties de la graine dans les jeunes germinations du pois. C R Acad Sci Hebd Seances Acad Sci D. 1975 May 12;280(18):2105–2108. [PubMed] [Google Scholar]
- Sela B. A., Lis H., Sharon N., Sachs L. Quantitation of N-acetyl-D-galactosamine-like sites on the surface membrane of normal and transformed mammalian cells. Biochim Biophys Acta. 1971 Dec 3;249(2):564–568. doi: 10.1016/0005-2736(71)90132-5. [DOI] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]



