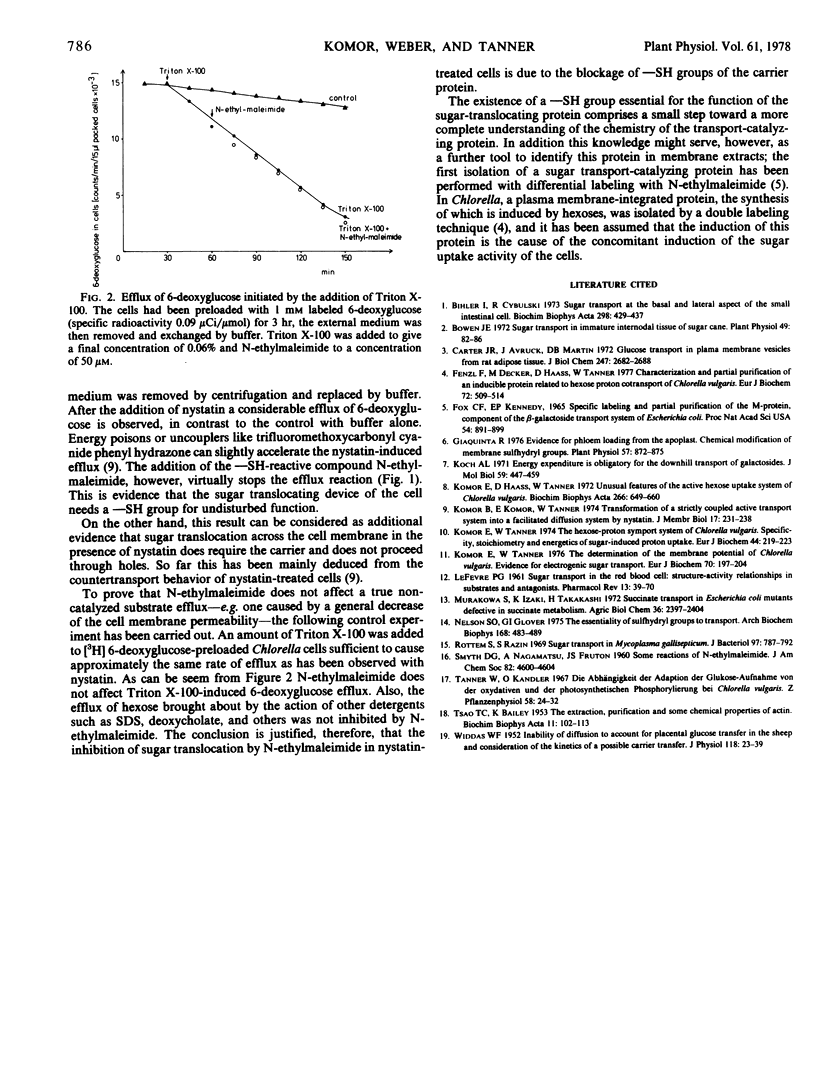
Abstract
The polyene antibiotic nystatin transforms the sugar-proton contransport system of Chlorella to a mere facilitated diffusion system. This experimental condition was used to test the sugar-translocating unit of the active uptake system for possible essential sulfhydryl groups. It could be shown that the catalyzed translocation of sugar is sensitive to the sulfhydryl-reactive compound N-ethylmaleimide. Sugar flow by passive leak as induced by the detergent Triton X-100 is not affected by sulfhydryl reagents. These results show that the sugar-translocating carrier protein possesses a sulfhydryl group, which is essential for its function.
Full text
PDF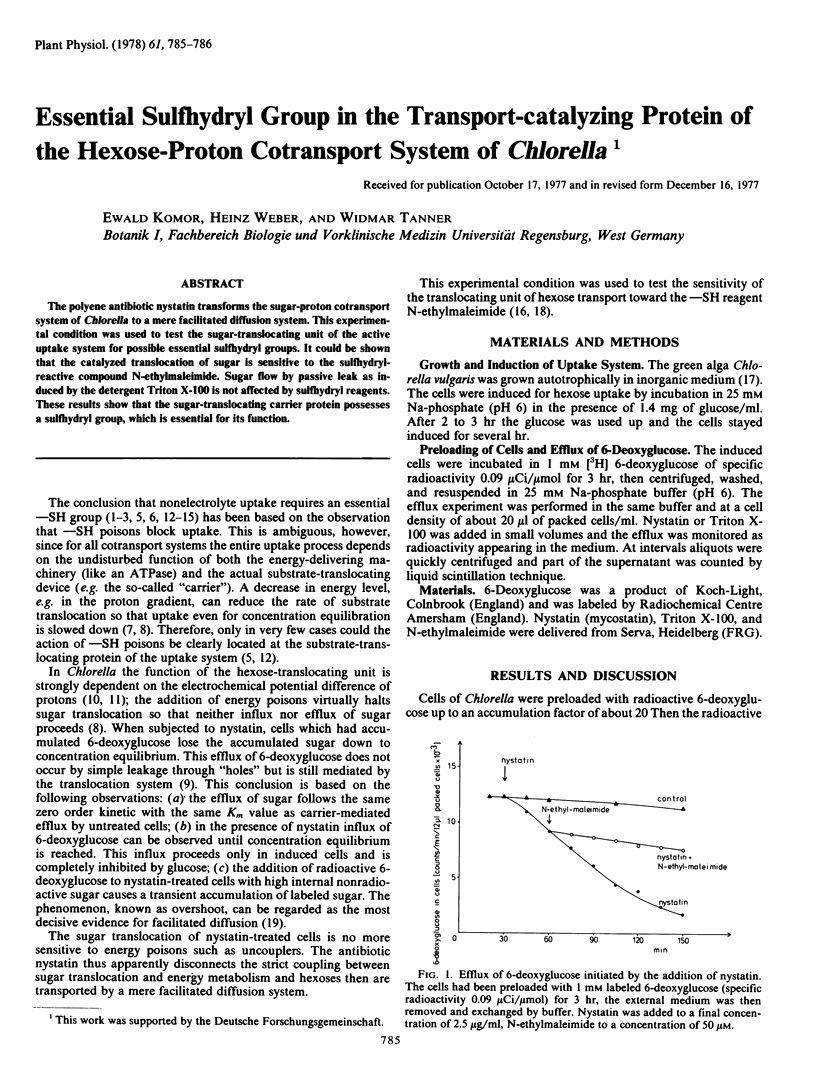
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bihler I., Cybulsky R. Sugar transport at the basal and lateral aspect of the small intestinal cell. Biochim Biophys Acta. 1973 Mar 16;298(2):429–436. doi: 10.1016/0005-2736(73)90370-2. [DOI] [PubMed] [Google Scholar]
- Bowen J. E. Sugar transport in immature internodal tissue of sugarcane: I. Mechanism and kinetics of accumulation. Plant Physiol. 1972 Jan;49(1):82–86. doi: 10.1104/pp.49.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Jr, Avruch J., Martin D. B. Glucose transport in plasma membrane vesicles from rat adipose tissue. J Biol Chem. 1972 May 10;247(9):2682–2688. [PubMed] [Google Scholar]
- Fenzl F., Decker M., Haass D., Tanner W. Characterization and partial purification of an inducible protein related to hexose proton cotransport of Chlorella vulgaris. Eur J Biochem. 1977 Feb;72(3):509–514. doi: 10.1111/j.1432-1033.1977.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Energy expenditure is obligatory for the downhill transport of galactosides. J Mol Biol. 1971 Aug 14;59(3):447–459. doi: 10.1016/0022-2836(71)90309-3. [DOI] [PubMed] [Google Scholar]
- Komor B., Komor E., Tanner W. Transformation of a strictly coupled active transport system into a facilitated diffusion system by nystatin. J Membr Biol. 1974 Jul 12;17(3):231–238. doi: 10.1007/BF01870184. [DOI] [PubMed] [Google Scholar]
- Komor E., Haass D., Tanner W. Unusual features of the active hexose uptake system of Chlorella vulgaris. Biochim Biophys Acta. 1972 Jun 20;266(3):649–660. doi: 10.1016/0006-3002(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem. 1976 Nov 1;70(1):197–204. doi: 10.1111/j.1432-1033.1976.tb10970.x. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem. 1974 May 2;44(1):219–223. doi: 10.1111/j.1432-1033.1974.tb03476.x. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- Nelson S. O., Glover G. I., Magill C. W. The essentiality of sulfhydryl groups to transport in Neurospora crassa. Arch Biochem Biophys. 1975 Jun;168(2):483–489. doi: 10.1016/0003-9861(75)90278-7. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Sugar transport in Mycoplasma gallisepticum. J Bacteriol. 1969 Feb;97(2):787–792. doi: 10.1128/jb.97.2.787-792.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAO T. C., BAILEY K. The extraction, purification and some chemical properties of actin. Biochim Biophys Acta. 1953 May;11(1):102–113. doi: 10.1016/0006-3002(53)90013-4. [DOI] [PubMed] [Google Scholar]
- WIDDAS W. F. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol. 1952 Sep;118(1):23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]


