Abstract
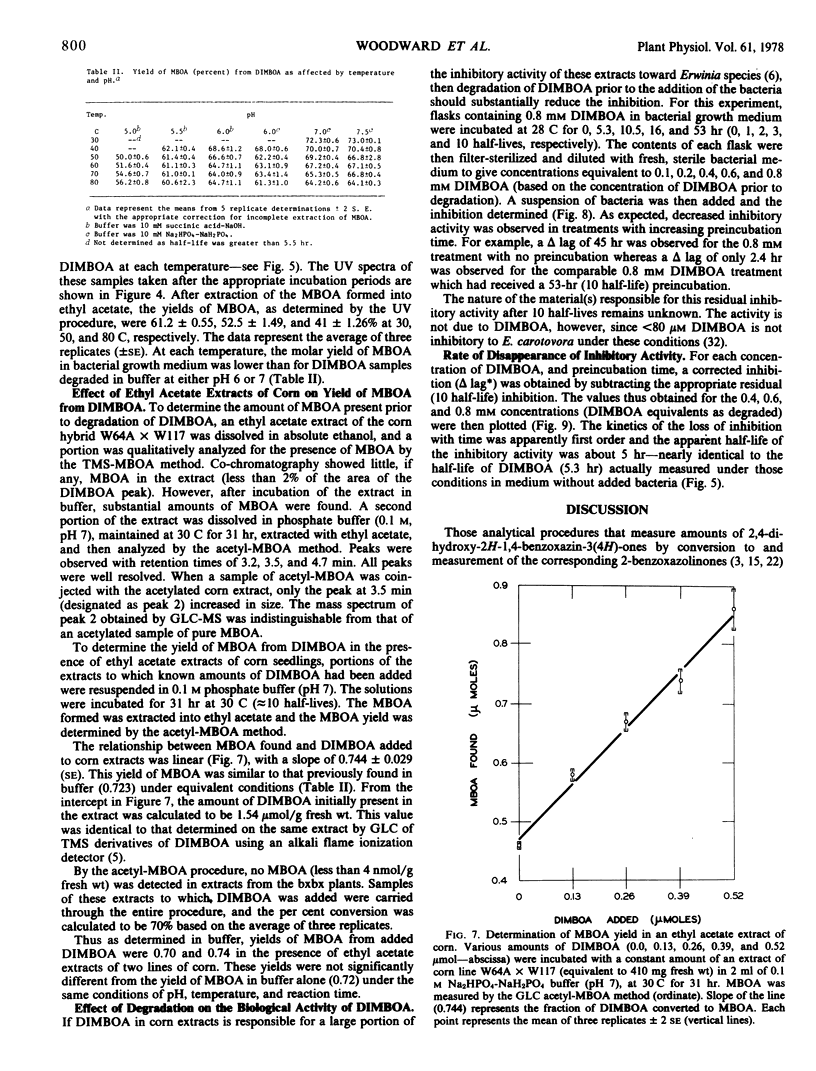
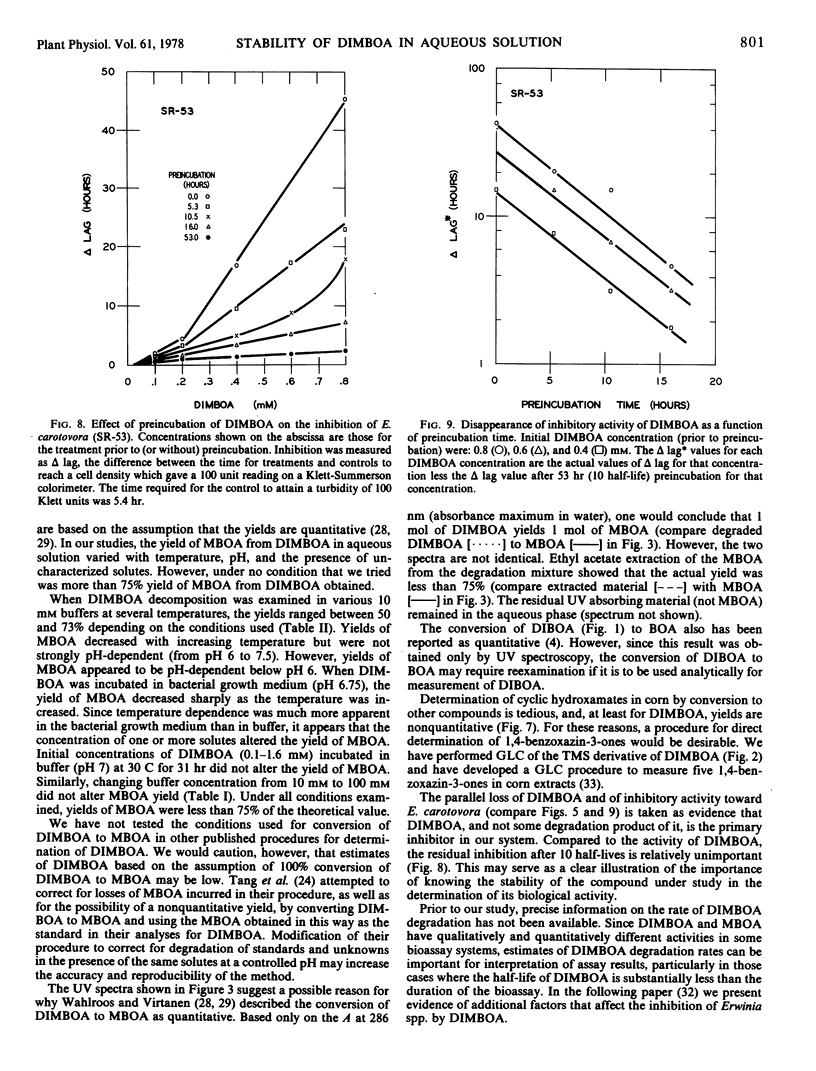
Cyclic hydroxamic acids present in some species of Gramineae have been reported to be important in resistance of these plants to fungi and insects. Since the nonglucosylated forms of these acids are unstable in aqueous solution, in vitro methods for the measurement of their antibiotic properties have been difficult. Kinetics of the decomposition of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA), the major hydroxamate in corn (Zea mays L.) extracts, were studied in buffered aqueous solutions from pH 5 to 7.5 at temperatures from 20 to 80 C. Kinetics were apparently first order under all conditions tested; energies of activation (24 to 28 kcal/mol) were nearly pH-independent. DIMBOA decomposed rapidly (half-life = 5.3 hours at 28 C, pH 6.75) relative to the time required for many procedures which have been used to demonstrate the biological activity of DIMBOA. The rate of disappearance of inhibitory activity of DIMBOA toward Erwinia carotovora was indistinguishable from the rate of decomposition of DIMBOA. Contrary to reports, yields of 6-methoxy-2-benzoxazolinone (MBOA) were not quantitative. Gas-liquid chromatography analytical procedures were developed for quantitation of trimethylsilyl and acetyl derivatives of MBOA. As measured by ultraviolet spectroscopy and/or gas-liquid chromatography, conversion of DIMBOA to MBOA ranged from 40 to 75% of theoretical in aqueous buffers, bacterial growth medium, and ethyl acetate extracts of corn tissue resuspended in buffer. Yields varied with temperature, pH, and constituents in the medium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corcuera L. J., Woodward M. D., Helgeson J. P., Kelman A., Upper C. D. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one, an Inhibitor from Zea mays with Differential Activity against Soft Rotting Erwinia Species. Plant Physiol. 1978 May;61(5):791–795. doi: 10.1104/pp.61.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scism A. J., BeMiller J. N., Caskey A. L. Determination of 2,4-dihydroxy-1,4(2h)-benzoxazin-3-one glucosides in corn (Zea mays L.). Anal Biochem. 1974 Mar;58(1):1–13. doi: 10.1016/0003-2697(74)90434-5. [DOI] [PubMed] [Google Scholar]
- VIRTANEN A. I., WAHLROOS O. Absence of 6-methoxybenzolinone in uninjured maize tissue. J Pharm Sci. 1963 Jul;52:713–714. doi: 10.1002/jps.2600520728. [DOI] [PubMed] [Google Scholar]
- Woodward M. D., Corcuera L. J., Helgeson J. P., Kelman A., Upper C. D. Factors That Influence the Activity of 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one on Erwinia Species in Growth Assays. Plant Physiol. 1978 May;61(5):803–805. doi: 10.1104/pp.61.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]



