Abstract
A radioimmunoassay, capable of detecting the Dolichos biflorus lectin at concentrations as low as 400 ng/ml, was developed and used to follow the distribution of this lectin in the plant during its life cycle.
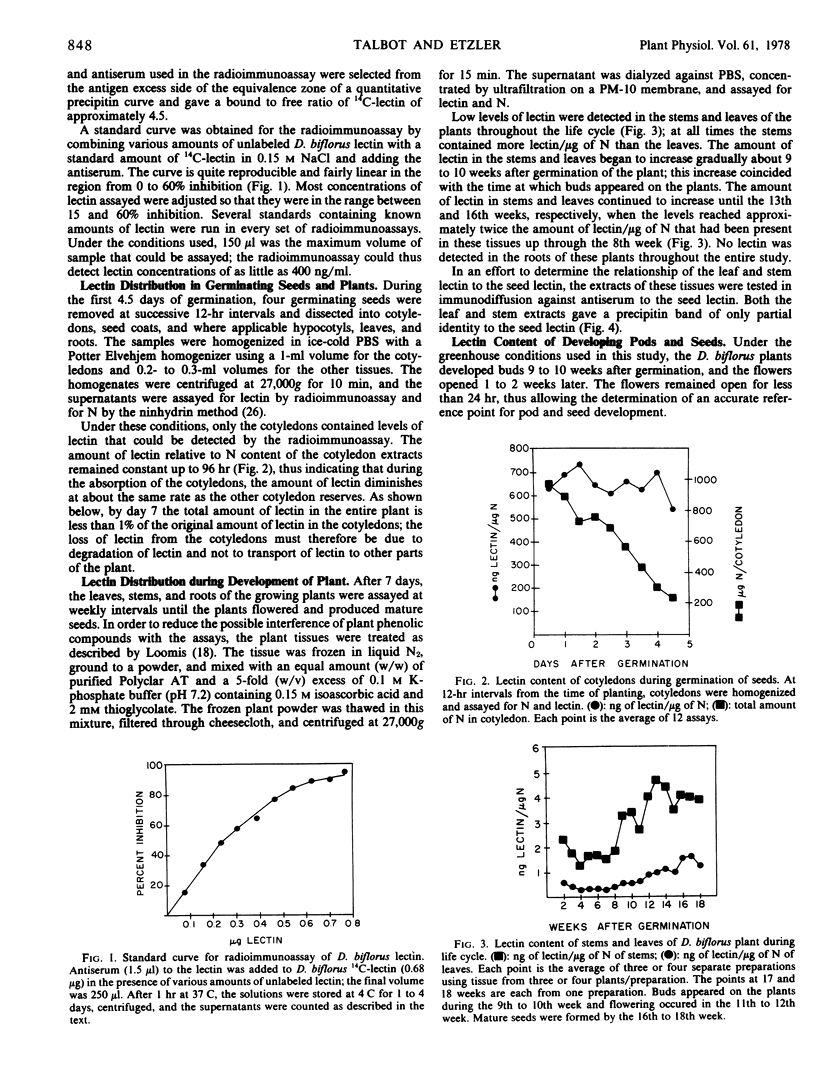
The lectin was first detected in the seeds of the plant 27 days after flowering and rapidly attained the high level of lectin present in the mature seed. The lectin content of the plant is highest in the seeds and cotyledons and decreases as the storage materials of the cotyledons decrease.
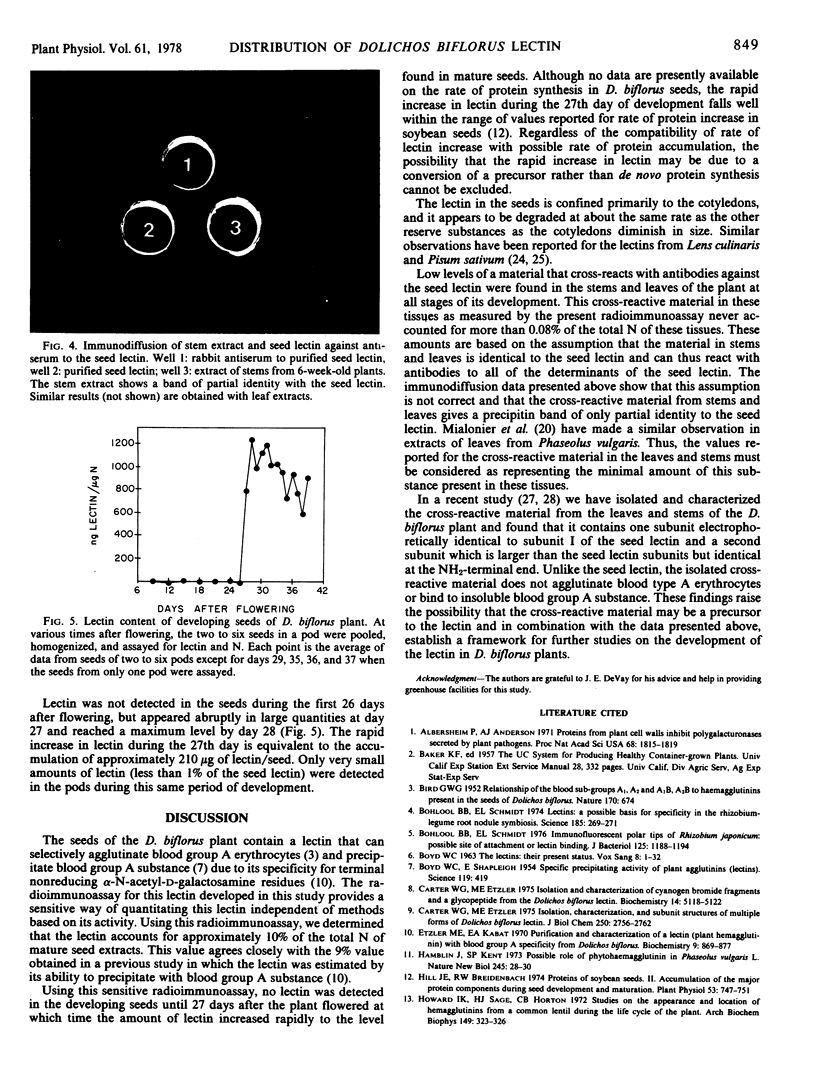
A low but measurable amount of material that reacts with antibodies to the seed lectin was detected in the leaves, stems, and pods of the plant. This material gives a precipitin band of only partial identity to the seed lectin when tested in immunodiffusion against antiserum to the seed lectin.
No lectin was detected by the radioimmunoassay in the roots of the plant at any stage of development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Anderson A. J. Proteins from plant cell walls inhibit polygalacturonases secreted by plant pathogens. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1815–1819. doi: 10.1073/pnas.68.8.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRD G. W. G. Relationship of the blood sub-groups A1, A2 and A1B, A2B to haemagglutinins present in the seeds of Dolichos biflorus. Nature. 1952 Oct 18;170(4329):674–674. doi: 10.1038/170674a0. [DOI] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Immunofluorescent polar tips of Rhizobium japonicum: possible site of attachment or lectin binding. J Bacteriol. 1976 Mar;125(3):1188–1194. doi: 10.1128/jb.125.3.1188-1194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Boyd W. C., Shapleigh E. Specific Precipitating Activity of Plant Agglutinins (Lectins). Science. 1954 Mar 26;119(3091):419–419. doi: 10.1126/science.119.3091.419. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Etzler M. E. Isolation and characterization of cyanogen bromide fragments and a glycopeptide from the Dolichos biflorus lectin. Biochemistry. 1975 Nov 18;14(23):5118–5122. doi: 10.1021/bi00694a015. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Etzler M. E. Isolation, characterization, and subunit structures of multiple forms of Dolichos biflorus lectin. J Biol Chem. 1975 Apr 10;250(7):2756–2762. [PubMed] [Google Scholar]
- Etzler M. E., Kabat E. A. Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry. 1970 Feb 17;9(4):869–877. doi: 10.1021/bi00806a022. [DOI] [PubMed] [Google Scholar]
- Hamblin J., Kent S. P. Possible role of phytohaemagglutinin in Phaseolus vulgaris L. Nat New Biol. 1973 Sep 5;245(140):28–30. doi: 10.1038/newbio245028a0. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Horton C. B. Studies on the appearance and location of hemagglutinins from a common lentil during the life cycle of the plant. Arch Biochem Biophys. 1972 Mar;149(1):323–326. doi: 10.1016/0003-9861(72)90328-1. [DOI] [PubMed] [Google Scholar]
- Janzen D. H., Juster H. B., Liener I. E. Insecticidal action of the phytohemagglutinin in black beans on a bruchid beetle. Science. 1976 May 21;192(4241):795–796. doi: 10.1126/science.1265481. [DOI] [PubMed] [Google Scholar]
- Kauss H., Glaser C. Carbohydrate-binding proteins from plant cell walls and their possible involvement in extension growth. FEBS Lett. 1974 Sep 1;45(1):304–307. doi: 10.1016/0014-5793(74)80867-7. [DOI] [PubMed] [Google Scholar]
- MAKELA O. Studies in hemagglutinins of leguminosae seeds. Ann Med Exp Biol Fenn. 1957;35(Suppl 11):1–133. [PubMed] [Google Scholar]
- Mirelman D., Galun E., Sharon N., Lotan R. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975 Jul 31;256(5516):414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]




